Phytochemical Study of Glycosmis Mauritiana
658
H-3') and 10.80 (1H, H-7) [17-20].
The 1H-NMR demonstrated two one-proton doublets
at δ 7.82 (1H, d, J = 2.2 Hz) and δ 7.34 (1H, d, J = 8.3 Hz)
and one double doublet δ 7.74 (1H, dd, J = 2.2, 8.3 Hz)
assignable to H-2', H-5' and H-6' protons respectively.
The 1H NMR displayed one proton singlet at δ 6.74
could be assigned to H-3 proton [19]. In addition, the
methine carbon signal at δc 105.41 was attributed to C-3
in the 13C NMR spectrum, indicating a 3', 5, 7-trihydroxy
flavone [21].
The 1H NMR spectrum of the compound showed two
meta-coupled doublets at δ 6.49(1H, d, J = 2.2 Hz) and
6.26(1H, d, J = 2.2 Hz) each integrating for one proton,
were assigned to H-8 and H-6, respectively of ring A of 5,
7-dihydroxy flavone. The 1H NMR studies of the com-
pound showed it to be flavonoid with sugar moieties i.e.
glactose and rhamnose in a trisaccharide fashion. In the
1H NMR spectra the resonances of the anomeric protons
observed in the low-field region at δ 5.58 (1H, d, J = 7.9
Hz, H-1"), 5.46 (1H, d, J = 3.6 Hz, H-1'"), and 5.41 (1H,
d, J = 3.1 Hz, H-1"") applicable for three sugar anomeric
protons suggesting the presence of triglycoside linkage
[17-19]. The anomeric proton signals were consistent
with the β-configuration of one glactose and α-configu-
ration of two rhamnose moieties.
The downfield chemical shift of C-2" and C-6" and
slight upfield shift of C-1' and C-5'of galactopyranosyl
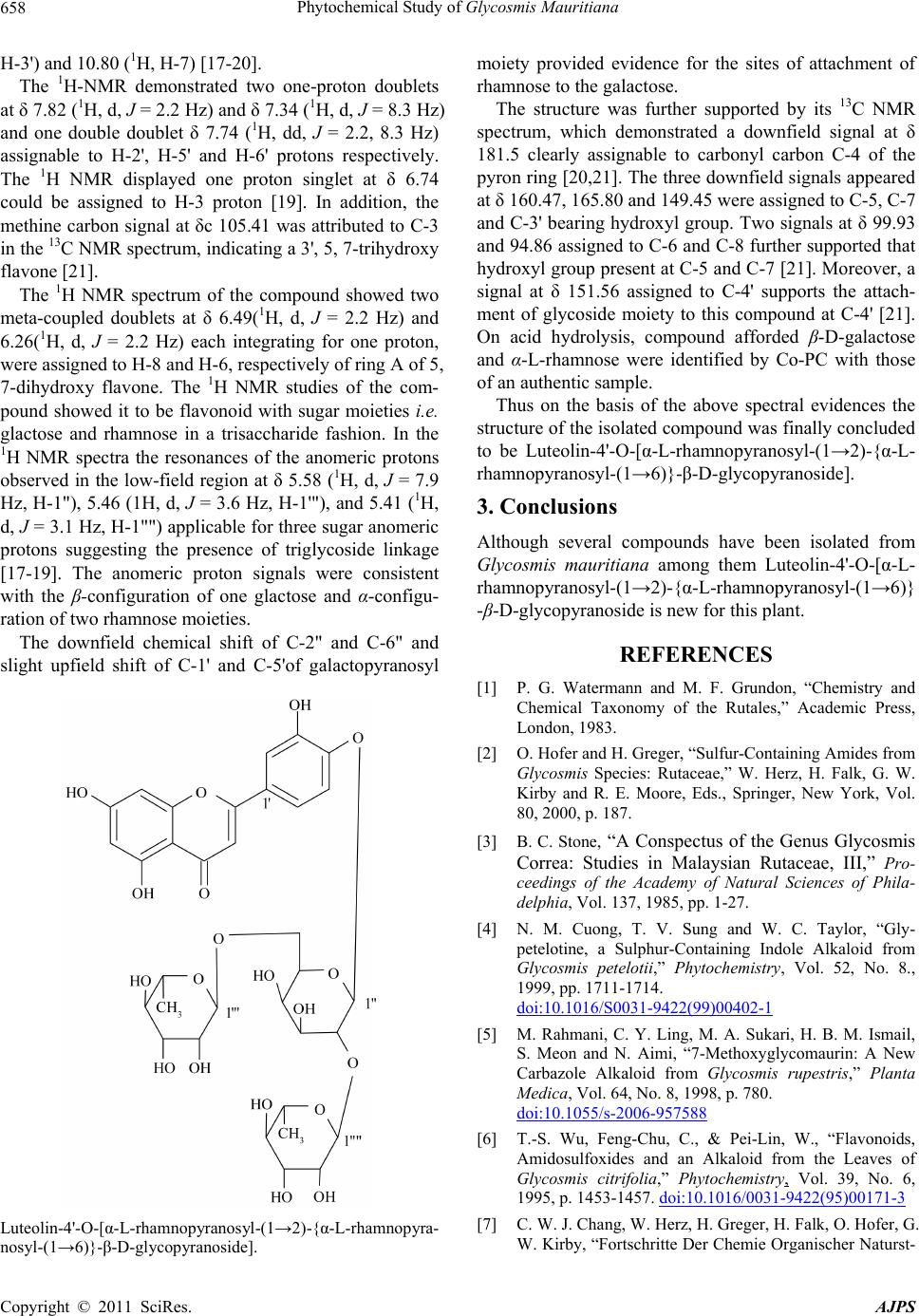
Luteolin-4'-O-[α-L-rhamnopyranosyl-(1→2)-{α-L-rhamnopyra-
nosyl-(1→6)}-β-D-glycopyranoside].
moiety provided evidence for the sites of attachment of
rhamnose to the galactose.
The structure was further supported by its 13C NMR
spectrum, which demonstrated a downfield signal at δ
181.5 clearly assignable to carbonyl carbon C-4 of the
pyron ring [20,21]. The three downfield signals appeared
at δ 160.47, 165.80 and 149.45 were assigned to C-5, C-7
and C-3' bearing hydroxyl group. Two signals at δ 99.93
and 94.86 assigned to C-6 and C-8 further supported that
hydroxyl group present at C-5 and C-7 [21]. Moreover, a
signal at δ 151.56 assigned to C-4' supports the attach-
ment of glycoside moiety to this compound at C-4' [21].
On acid hydrolysis, compound afforded β-D-galactose
and α-L-rhamnose were identified by Co-PC with those
of an authentic sample.
Thus on the basis of the above spectral evidences the
structure of the isolated compound was finally concluded
to be Luteolin-4'-O-[α-L-rhamnopyranosyl-(1→2)-{α-L-
rhamnopyranosyl-(1→6)}-β-D-glycopyranoside].
3. Conclusions
Although several compounds have been isolated from
Glycosmis mauritiana among them Luteolin-4'-O-[α-L-
rhamnopyranosyl-(1→2)-{α-L-rhamnopyranosyl-(1→6)}
-β-D-glycopyranoside is new for this plant.
REFERENCES
[1] P. G. Watermann and M. F. Grundon, “Chemistry and
Chemical Taxonomy of the Rutales,” Academic Press,
London, 1983.
[2] O. Hofer and H. Greger, “Sulfur-Containing Amides from
Glycosmis Species: Rutaceae,” W. Herz, H. Falk, G. W.
Kirby and R. E. Moore, Eds., Springer, New York, Vol.
80, 2000, p. 187.
[3] B. C. Stone, “A Conspectus of the Genus Glycosmis
Correa: Studies in Malaysian Rutaceae, III,” Pro-
ceedings of the Academy of Natural Sciences of Phila-
delphia, Vol. 137, 1985, pp. 1-27.
[4] N. M. Cuong, T. V. Sung and W. C. Taylor, “Gly-
petelotine, a Sulphur-Containing Indole Alkaloid from
Glycosmis petelotii,” Phytochemistry, Vol. 52, No. 8.,
1999, pp. 1711-1714.
doi:10.1016/S0031-9422(99)00402-1
[5] M. Rahmani, C. Y. Ling, M. A. Sukari, H. B. M. Ismail,
S. Meon and N. Aimi, “7-Methoxyglycomaurin: A New
Carbazole Alkaloid from Glycosmis rupestris,” Planta
Medica, Vol. 64, No. 8, 1998, p. 780.
doi:10.1055/s-2006-957588
[6] T.-S. Wu, Feng-Chu, C., & Pei-Lin, W., “Flavonoids,
Amidosulfoxides and an Alkaloid from the Leaves of
Glycosmis citrifolia,” Phytochemistry, Vol. 39, No. 6,
1995, p. 1453-1457. doi:10.1016/0031-9422(95)00171-3
[7] C. W. J. Chang, W. Herz, H. Greger, H. Falk, O. Hofer, G.
W. Kirby, “Fortschritte Der Chemie Organischer Naturst-
Copyright © 2011 SciRes. AJPS