 American Journal of Plant Sciences, 2011, 2, 629-635 doi:10.4236/ajps.2011.25074 Published Online November 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS 629 Genetic Variability in Germplasm Accessions of Capsicum annuum L Shrilekha Misra1*, Raj Kishori Lal2, Mahendra Pandurang Darokar2, Suman Preet Singh Khanuja2 1Banasthali University, Rajasthan, India; 2Central Institute of Medicinal and Aromatic Plants, Lucknow, India. Email: *shreelekha2@rediffmail.com Received December 13th, 2010; revised March 14th, 2011; accepted May 1st, 2011. ABSTRACT Capsicum annuum is the most widely cultivated species of peppers (chilies) in the world. For culinary purposes, its fruits are used for pungency (capsaicin) and also color (capsanthin). Capsaicin is also used for medicinal purposes particularly in anti-inflammatory formulations. Genetic divergence among 38 accessions collected from diverse loca- tions in India (28 from Uttar Pradesh, 5 from Assam, 3 from Maharash tra and 2 from Uttaranc hal), was estimated from the data pooled over 3 consecutive years for 15 morphological, growth and chemotypic characters that included days to first and second flowering, fruit onset, plant height, primary, secondary and tertiary branches, leaf surface area, fruit length and diameter, fruit surface area, fresh and dry fruit weight, capsaicin and capsanthin content. Based on this characterization the plants could be grouped into 7 clusters wherein substantial diversity among accessions was indi- cated by the wide range of 2 D values (752.901 - 1918683.00). Accessions with distinct identity were marked, which are likely to be quite suitable for breeding through hybridization combining desirable traits. The accessions labeled number 38, 27, 26, 14 and 24 to high capsaicin content (%); 35, 23, 3, 16, 29 and 11 for high capsanthin content (%) and 26 and 27 for dual purpose had characteristics de sirable. Above accessions could be utilized in hybridiza tion pro- gramme for C. annuum crop improvement. Keywords: Genetic Diversity, Capsicum Annuum, Capsaicin and Capsanthin Content, Recombination Breeding, Geographic al Di st ri but i o n 1. Introduction Capsicum is the unique genus of the family Solanaceae finding diverse uses from nutritional and culinary to pharmaceutical uses. In this genus, native to the Ameri- cas, more than 30 species have been described, but only five of them, Capsicum annuum var. annuum, C. chi- nense, C. frutescens, C. baccatum var. pendulum, and C. pubescens are considered to be domesticated (Pandey, Pozzobon et al., 2006; Moscone et al., 2007) [1-3]. Pun- gent peppers commonly known as chilies in India be- long to the species C. annuum L. which is the most widely cultivated species in the world. Both hot and sweet peppers have been reported to have originated from C. annuum [4]. The pungent chemical principle of Capsicum is cap- saicin, which is synthesized and accumulated specifically in fruits. The capsaicin has found use in a wide range of pain problems, including post-mastectomy syndrome, urticaria, psoriasis, diabetic neuropathy, arthritis, pruritis, contact allergy, post-surgical neuromas etc. [5]. The sweet pepper on the other hand is one of the richest sources of carotenoids. The ketoxanthophylls, capsanthin and cap- sorubin are the major pigments contributing to the red color of Capsicum, while β-carotene and zeaxanthin are responcible for the yellow-orange colors [6]. The characterization and the evaluation of the Capsi- cum domesticated species are particularly interesting for gene bank curators, since a wide variability, not yet fully known and exploited, is available in these species (Guz- mán et al. [7], 2005; Sudré et al. [8], 2006; Ince et al. [9], 2009). In this study, genetic variability for diverse traits in available germplasm collections assembled from dif- ferent places in India were evaluated as a prelude to crop improvement. 2. Materials and Methods Genetic divergence among 38 indigenous accessions of Capsicum annuum collected from various geographical locations (Tabl e 1) in India was studied. The plants were grown in randomized block design at the research farm  Genetic Variability in Germplasm Accessions of Capsicum annuum L 630 Table 1. Accessions/collections of Capsicum annuum and their geographic origin. Accession No. Pl ace of collection/acquisition 1 Lucknow, U.P. 2 " 3 " 4 " 5 " 6 " 7 " 8 " 9 " 10 " 11 " 12 " 13 " 14 " 15 " 16 " 17 " 18 Ramnagar, Uttranchal. 19 Pantnagar, Uttranchal. 20 Lucknow, U.P. 21 " 22 " 23 Nagpur, Maharashtra. 24 Assam. 25 Assam. 26 Assam. 27 Assam. 28 Assam. 29 Lucknow, U.P. 30 " 31 " 32 " 33 " 34 " 35 " 36 " 37 Pune, Maharashtra. 38 Pune Maharashtra. of CIMAP, Lucknow, India during 1998, 1999 and 2000 with two replicates in each year. Each treatment con- sisted of single row 45 cm long and 60 cm apart. The plant received normal intercultural operations, irrigations and fertilizer applications (20 kg N, 30 kg P2O5 and 30 kg K2O per hectare). Observations were recorded for the 15 characters for days to first flowering, days to second flowering, days to fruit initiation, plant height, primary branches/plant, secondary branches/primary branch, tertiary branches/ secondary branch, leaf surface area, fruit length, fruit diameter, fruit surface area, fruit weight fresh and dry, capsaicin and capsanthin content. Mean values for all the characters (pooled over three years) were subjected to D2 and canonical analysis of genetic divergence based on the procedures outlined by Mahalanobis [10] and Rao [4]. 3. Results Highly significant differences (P < 0.01) among the col- lection of C. annuum for all the studied 15 characters indicated the presence of considerable genetic diversity. Tremendous variation in shape and size of fruits were observed (Table 2, for the mean, ranges and C.D.). Hence D2 values for 703 pairs of genotypes were deve- loped. Based on these values, all 38 accessions/genotypes could be grouped into seven clusters such that the geno- type within the clusters had smaller 2 D-values among themselves than those belonging to different clusters (Table 3). Divergence for the pooled characters (in terms of D2 values) ranged from 752.901 to 1918683 and clus- ter mean (for 164 to 241 days—first flowering, 000 - 243 days to second flowering, 259 - 336 days of fruit initia- tion, 50 - 106 cm plant height, 5 - 8 primary branches, 7 - 10 secondary branches, 5 - 9 tertiary branches, 20.06 - 45.39 cm2 leaf surface area, 2.332 - 9.960 cm fruit length, 0.802 - 1.830 cm fruit diameter, 4.312 - 27.400 cm2 fruit surface area, 0.76 - 4.17 mg fruit weight fresh, 0.20 - 0.75 mg fruit weight dry, 0.000% - 0.0869% capsaicin content and 0.063% - 0.517% capsanthin content). It is evident from the results, Table 2 and Figure 1 (in Fig- ure 1, D = 2 D), That the mean inter-cluster 2 D - values and the cluster mean indicated the highest diver- gence between clusters designated III and VII (2 D= 1665293.0), corroborating the proposed variation of the germplasm into different gene complexes based on cyto- genetic aspects and botanical characters. Divergence was also noted between clusters designated I and VII (2 D = 1661982.0). II and VII (2 D = 1167484.0) and between IV and VII (2 D = 1119514.0). The lowest inter-cluster distance was noticed in between cluster I and cluster III (2 D = 76941.01) (Table 3). Considerable differences in economic traits, growth and other parameters were noted (Tables 2 and 4). The comparative characteristic features of Capcicum annuum plant collected from various places Copyright © 2011 SciRes. AJPS  Genetic Variability in Germplasm Accessions of Capsicum annuum L Copyright © 2011 SciRes. AJPS 631 Table 2. Mean performance (X), ranges and critical difference of 38 accessions of Capsicum annuum. Accession No. Days to first flowering Days to second flowering Days to fruit initiation Plant height (cm) No. of primary branches/ plant No. of secondary branch es/ primary branch No. of tertiary branch es/ secondary branch Leaf surface area (cm2) Fruit length (cm) Fruit diameter (cm) Fruit surface area (cm2) Fruit weight fresh (g) Fruit weight dry (g) Capsaicin (%) Capsanthin (%) 1 173 233 271 100 4 9 5 29.98 5.98 0.66 6.65 0.81 0.23 0.018 0.036 2 180 243 275 103 7 7 6 34.66 3.01 1.09 5.18 2.81 0.50 0.036 0.052 3 156 000 207 53 7 6 5 11.41 2.23 0.65 2.64 0.48 0.16 0.090 0.307 4 184 237 267 63 8 7 6 19.68 5.18 0.81 7.52 0.93 0.32 0.102 0.046 5 177 242 272 106 5 8 6 24.00 2.62 1.84 10.94 3.81 0.88 0.002 0.015 6 177 241 253 91 8 9 6 18.26 2.16 0.68 3.23 0.54 0.23 0.049 0.008 7 178 237 253 79 5 9 8 40.19 3.90 1.35 10.56 4.44 0.96 0.002 0.140 8 144 000 174 41 8 8 6 10.62 1.61 1.08 3.58 0.72 0.24 0.004 0.186 9 174 237 275 91 9 9 6 16.59 4.38 0.68 5.35 0.74 0.17 0.075 0.183 10 178 238 267 96 8 8 7 23.04 4.19 0.67 4.67 1.26 0.23 0.043 0.071 11 180 244 261 82 8 8 6 14.8802.56 0.71 3.04 0.36 0.14 0.011 0.204 12 168 221 251 98 8 7 4 16.63 4.18 0.77 4.69 0.53 0.23 0.014 0.062 13 175 232 255 92 7 8 6 14.19 3.11 0.78 3.32 0.45 0.15 0.086 0.106 14 181 237 256 90 10 7 7 24.79 4.74 0.76 5.52 1.21 0.25 0.140 0.042 15 189 240 281 92 11 6 8 38.75 3.06 0.79 4.28 0.53 0.20 0.100 0.079 16 178 241 267 84 9 10 6 13.10 3.75 0.74 5.21 0.85 0.25 0.089 0.291 17 179 240 266 81 11 10 7 8.53 3.96 0.97 6.32 0.52 0.19 0.049 0.067 18 152 000 179 58 8 10 8 8.83 4.52 0.82 6.60 1.00 0.25 0.045 0.035 19 168 000 207 50 6 7 5 14.18 3.50 0.81 4.07 0.61 0.21 0.060 0.078 20 166 237 279 85 8 8 6 37.37 3.24 1.51 8.42 2.26 0.49 0.036 0.149 21 175 242 250 98 8 6 6 27.69 4.06 1.31 9.75 2.05 0.44 0.082 0.101 22 171 248 310 91 9 10 6 19.55 3.95 1.21 8.31 1.83 0.42 0.083 0.145 23 176 240 297 79 8 7 7 25.27 3.72 1.21 9.52 2.55 0.54 0.044 0.334 24 183 237 303 110 10 10 5 22.50 1.62 1.35 4.98 1.21 0.20 0.128 0.043 25 161 241 268 96 8 11 6 9.40 2.13 1.03 4.01 2.56 0.51 0.082 0.017 26 179 237 302 111 11 10 5 43.28 1.45 1.35 4.97 1.31 0.32 0.144 0.191 27 183 240 265 72 7 7 5 6.62 2.65 0.74 3.84 0.48 0.20 0.145 0.145 28 180 237 305 42 9 9 5 44.01 2.78 0.76 4.13 0.77 0.22 0.131 0.045 29 157 000 187 59 8 10 6 17.66 3.80 0.76 4.24 0.69 0.23 0.011 0.214 30 177 232 266 50 5 7 5 23.23 5.84 1.43 14.98 2.01 0.49 0.001 0.051 31 179 244 278 74 8 7 5 29.07 3.32 0.74 4.38 0.80 0.22 0.001 0.111 32 159 224 252 83 7 9 5 20.77 2.97 1.02 4.28 0.65 0.25 0.068 0.180 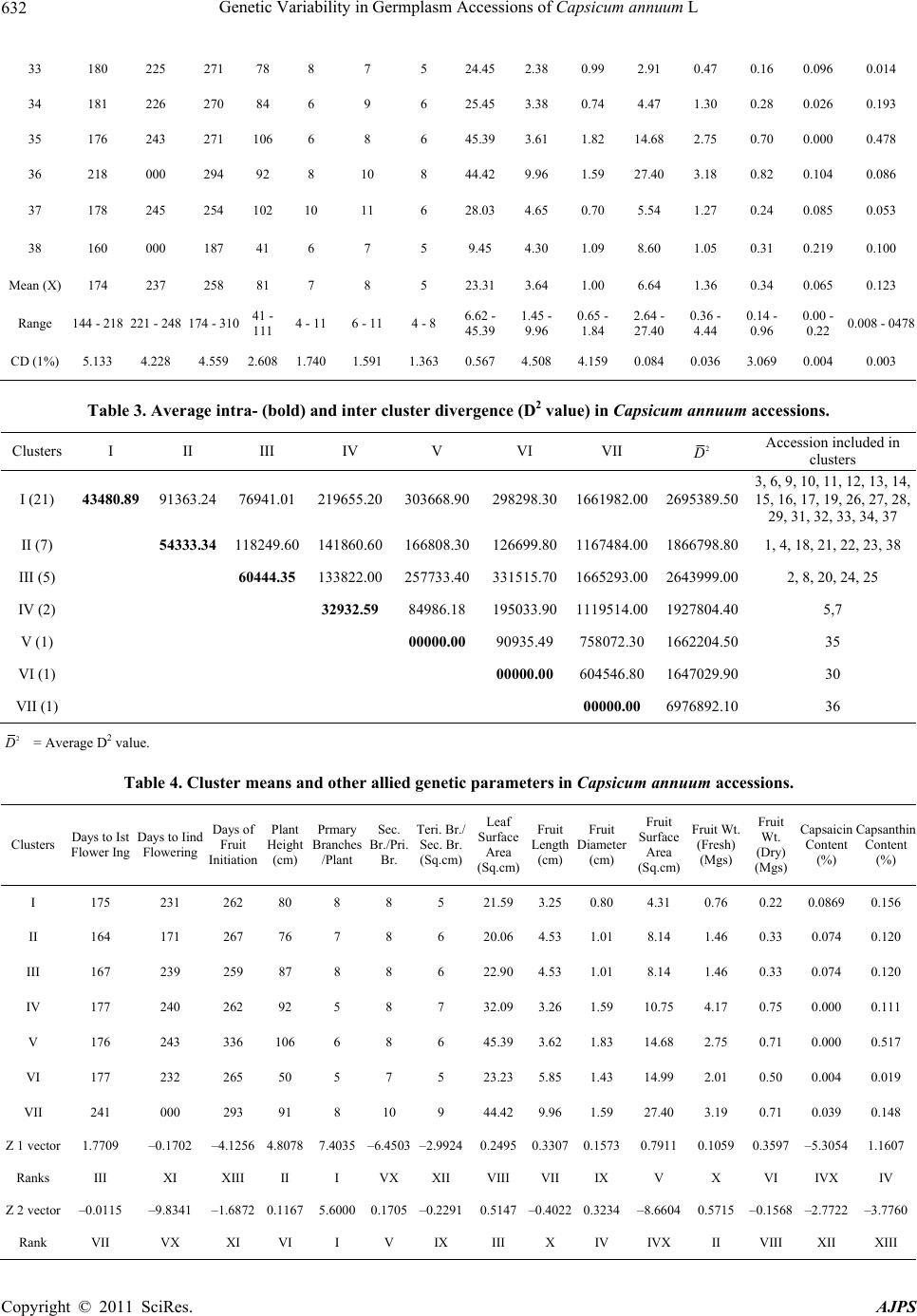 Genetic Variability in Germplasm Accessions of Capsicum annuum L 632 33 180 225 271 78 8 7 5 24.45 2.38 0.99 2.91 0.47 0.16 0.096 0.014 34 181 226 270 84 6 9 6 25.45 3.38 0.74 4.47 1.30 0.28 0.026 0.193 35 176 243 271 106 6 8 6 45.39 3.61 1.82 14.68 2.75 0.70 0.000 0.478 36 218 000 294 92 8 10 8 44.42 9.96 1.59 27.40 3.18 0.82 0.104 0.086 37 178 245 254 102 10 11 6 28.03 4.65 0.70 5.54 1.27 0.24 0.085 0.053 38 160 000 187 41 6 7 5 9.45 4.30 1.09 8.60 1.05 0.31 0.219 0.100 Mean (X) 174 237 258 81 7 8 5 23.31 3.64 1.00 6.64 1.36 0.34 0.065 0.123 Range 144 - 218 221 - 248 174 - 31041 - 111 4 - 11 6 - 114 - 8 6.62 - 45.39 1.45 - 9.96 0.65 - 1.84 2.64 - 27.40 0.36 - 4.44 0.14 - 0.96 0.00 - 0.22 0.008 - 0478 CD (1%) 5.133 4.228 4.559 2.608 1.740 1.591 1.363 0.567 4.508 4.159 0.084 0.036 3.069 0.004 0.003 Table 3. Average intra- (bold) and inter cluster divergence (D2 value) in Capsicum annuum acce ssions. Clusters I II III IV V VI VII 2 D Accession included in clusters I (21) 43480.89 91363.24 76941.01 219655.20 303668.90298298.301661982.002695389.50 3, 6, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 26, 27, 28, 29, 31, 32, 33, 34, 37 II (7) 54333.34 118249.60 141860.60166808.30126699.801167484.001866798.80 1, 4, 18, 21, 22, 23, 38 III (5) 60444.35 133822.00257733.40331515.701665293.002643999.00 2, 8, 20, 24, 25 IV (2) 32932.59 84986.18 195033.901119514.001927804.40 5,7 V (1) 00000.00 90935.49 758072.301662204.50 35 VI (1) 00000.00 604546.801647029.90 30 VII (1) 00000.00 6976892.10 36 2 D = Average D2 value. Table 4. Cluster means and other allied genetic parameters in Capsicum annuum accessions. Clusters Days to Ist Flower Ing Days to Iind Flowering Days of Fruit Initiation Plant Height (cm) Prmary Bran ches /Plant Sec. Br./Pri. Br. Teri. Br./ Sec. Br. (Sq.cm) Leaf Surface Area (Sq.cm) Fruit Length (cm) Fruit Diameter (cm) Fruit Surface Area (Sq.cm) Fruit Wt. (Fresh) (Mgs) Fruit Wt. (Dry) (Mgs) Capsaicin Content (%) Capsanthin Content (%) I 175 231 262 80 8 8 5 21.59 3.250.80 4.31 0.76 0.22 0.08690.156 II 164 171 267 76 7 8 6 20.06 4.531.01 8.14 1.46 0.33 0.074 0.120 III 167 239 259 87 8 8 6 22.90 4.531.01 8.14 1.46 0.33 0.074 0.120 IV 177 240 262 92 5 8 7 32.09 3.261.59 10.75 4.17 0.75 0.000 0.111 V 176 243 336 106 6 8 6 45.39 3.621.83 14.68 2.75 0.71 0.000 0.517 VI 177 232 265 50 5 7 5 23.23 5.851.43 14.99 2.01 0.50 0.004 0.019 VII 241 000 293 91 8 10 9 44.42 9.961.59 27.40 3.19 0.71 0.039 0.148 Z 1 vector 1.7709 –0.1702 –4.1256 4.8078 7.4035 –6.4503–2.99240.24950.33070.15730.79110.1059 0.3597 –5.30541.1607 Ranks III XI XIII II I VX XII VIII VII IX V X VI IVX IV Z 2 vector –0.0115 –9.8341 –1.6872 0.1167 5.6000 0.1705–0.22910.5147–0.40220.3234–8.66040.5715 –0.1568 –2.7722–3.7760 Rank VII VX XI VI I V IX III X IV IVX II VIII XII XIII Copyright © 2011 SciRes. AJPS 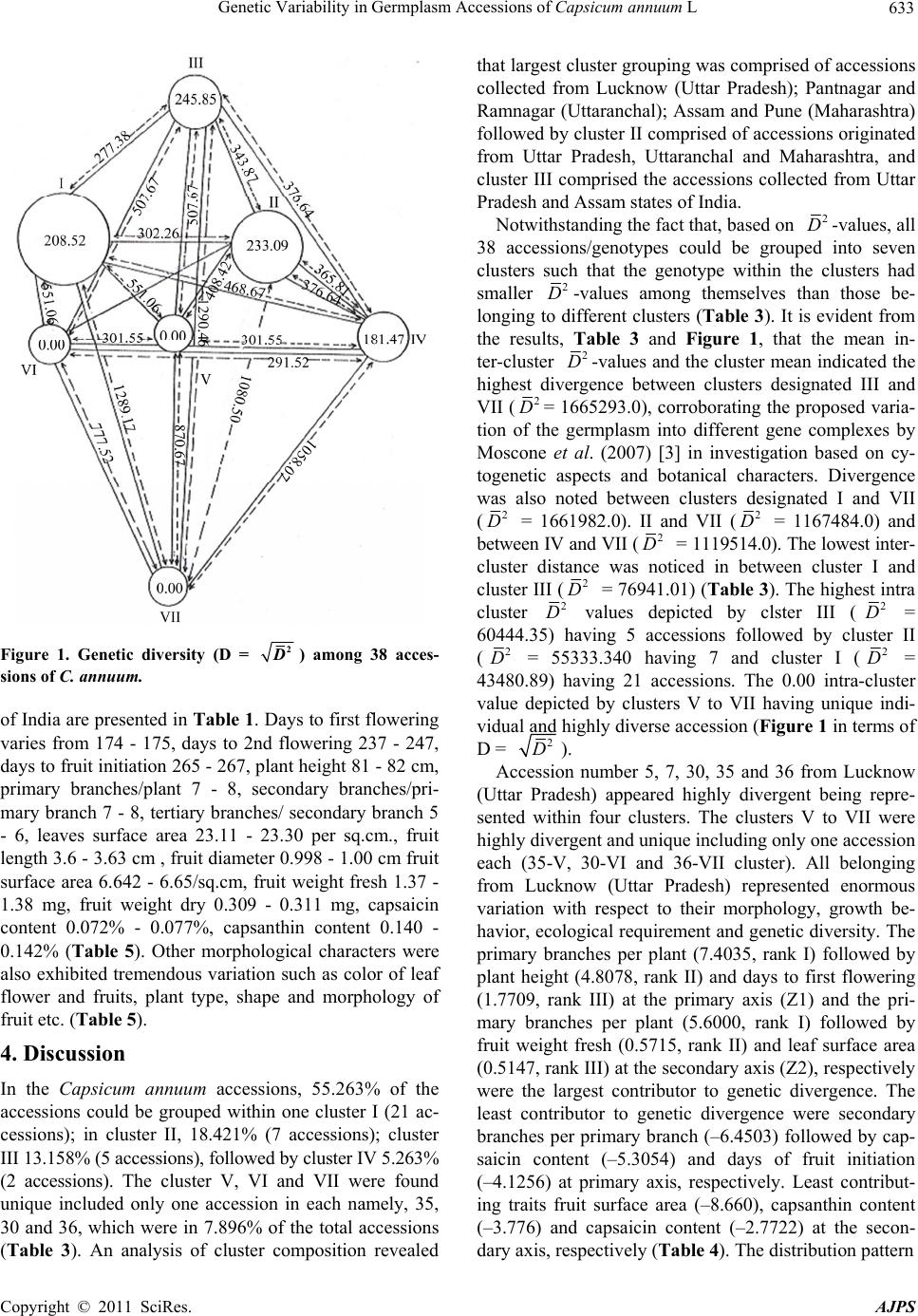 Genetic Variability in Germplasm Accessions of Capsicum annuum L Copyright © 2011 SciRes. AJPS 633 that largest cluster grouping was comprised of accessions collected from Lucknow (Uttar Pradesh); Pantnagar and Ramnagar (Uttaranchal); Assam and Pune (Maharashtra) followed by cluster II comprised of accessions originated from Uttar Pradesh, Uttaranchal and Maharashtra, and cluster III comprised the accessions collected from Uttar Pradesh and Assam states of India. Notwithstanding the fact that, based on 2 D-values, all 38 accessions/genotypes could be grouped into seven clusters such that the genotype within the clusters had smaller 2 D-values among themselves than those be- longing to different clusters (Table 3). It is evident from the results, Table 3 and Figure 1, that the mean in- ter-cluster 2 D-values and the cluster mean indicated the highest divergence between clusters designated III and VII (2 D= 1665293.0), corroborating the proposed varia- tion of the germplasm into different gene complexes by Moscone et al. (2007) [3] in investigation based on cy- togenetic aspects and botanical characters. Divergence was also noted between clusters designated I and VII (2 D = 1661982.0). II and VII (2 D = 1167484.0) and between IV and VII (2 D = 1119514.0). The lowest inter- cluster distance was noticed in between cluster I and cluster III (2 D = 76941.01) (Table 3). The highest intra cluster 2 D values depicted by clster III (2 D = 60444.35) having 5 accessions followed by cluster II (2 D = 55333.340 having 7 and cluster I (2 D = 43480.89) having 21 accessions. The 0.00 intra-cluster value depicted by clusters V to VII having unique indi- vidual and highly diverse accession (Figure 1 in terms of D = 2 D). Figure 1. Genetic diversity (D = 2 ) among 38 acces- sions of C. annuum. of India are presented in Table 1. Days to first flowering varies from 174 - 175, days to 2nd flowering 237 - 247, days to fruit initiation 265 - 267, plant height 81 - 82 cm, primary branches/plant 7 - 8, secondary branches/pri- mary branch 7 - 8, tertiary branches/ secondary branch 5 - 6, leaves surface area 23.11 - 23.30 per sq.cm., fruit length 3.6 - 3.63 cm , fruit diameter 0.998 - 1.00 cm fruit surface area 6.642 - 6.65/sq.cm, fruit weight fresh 1.37 - 1.38 mg, fruit weight dry 0.309 - 0.311 mg, capsaicin content 0.072% - 0.077%, capsanthin content 0.140 - 0.142% (Table 5). Other morphological characters were also exhibited tremendous variation such as color of leaf flower and fruits, plant type, shape and morphology of fruit etc. (Table 5). Accession number 5, 7, 30, 35 and 36 from Lucknow (Uttar Pradesh) appeared highly divergent being repre- sented within four clusters. The clusters V to VII were highly divergent and unique including only one accession each (35-V, 30-VI and 36-VII cluster). All belonging from Lucknow (Uttar Pradesh) represented enormous variation with respect to their morphology, growth be- havior, ecological requirement and genetic diversity. The primary branches per plant (7.4035, rank I) followed by plant height (4.8078, rank II) and days to first flowering (1.7709, rank III) at the primary axis (Z1) and the pri- mary branches per plant (5.6000, rank I) followed by fruit weight fresh (0.5715, rank II) and leaf surface area (0.5147, rank III) at the secondary axis (Z2), respectively were the largest contributor to genetic divergence. The least contributor to genetic divergence were secondary branches per primary branch (–6.4503) followed by cap- saicin content (–5.3054) and days of fruit initiation (–4.1256) at primary axis, respectively. Least contribut- ing traits fruit surface area (–8.660), capsanthin content (–3.776) and capsaicin content (–2.7722) at the secon- dary axis, respectively (Table 4). The distribution pattern 4. Discussion In the Capsicum annuum accessions, 55.263% of the accessions could be grouped within one cluster I (21 ac- cessions); in cluster II, 18.421% (7 accessions); cluster III 13.158% (5 accessions), followed by cluster IV 5.263% (2 accessions). The cluster V, VI and VII were found unique included only one accession in each namely, 35, 30 and 36, which were in 7.896% of the total accessions (Table 3). An analysis of cluster composition revealed  Genetic Variability in Germplasm Accessions of Capsicum annuum L 634 Table 5. Average intra and inter cluster D value in Capsicum annuum accessions. Clusters I II III IV V VI VII I (21) 208.52 302.26 277.38 468.67 551.06 546.16 1289.17 II (7) 283.09 343.87 376.64 408.42 355.94 1080.50 III (5) 245.85 365.81 507.67 575.77 1290.46 IV (2) 181.47 291.52 441.62 1058.07 V (1) 000.00 301.55 870.67 VI (1) 000.00 777.52 VII (1) 000.00 Where D = 2 D. of accessions of diverse origin in the clusters indicates that genetic diversity observed within C. annuum was not related to geographic origin. Noted difference in plant characters probably occurred over time with free move- ment of plant material from location to location, mutation, hybridization, gene recombination and selection. Our findings were also in consonance of results of Pandey and Dobhal [1] and Varalakshmi and Haribabu [11] in C. annuum crop. Selection of promising accessions based on genetic divergence and capsaicin and capsanthin con- tent quality are useful in chili crop improvement. The accessions labeled number 38, 27, 26, 14 and 24 to high capsaicin content (%); 35, 23, 3, 16, 29 and 11 for high capsanthin content (%) and 26 and 27 for dual purpose had characteristics desirable. Among the above, the ac- cession no. 35 (0.000% capsaicin content) should be utilized for the study of genetics of capsaicin content, making hybrids between the parents 0.000 X high cap- saicin content. Mainly due to the versatility of application, C. annuum plants have become important from an economic point of view in several countries since their fruits can be used for different purposes, such as in cooking, in industry (e.g., production of “pepper spray”) and for medicinal and or- namental purposes (Pickersgill, [12] 1971; [13] Moscone et al. [3], 2007). In Brazil this vegetable is of great im- portance, ranking second in the generation of foreign currency among exported vegetables (Embrapa, [6,14] 2009). Therefore, the above listed accessions of the C. annuum could be utilized, as parents for hybridization in recombination breeding programme followed by repeated selection to obtain maximum gain. 5. Acknowledgements This research work was supported by a grant from CSIR (Council of Scientific and Industrial Research) and con- ducted at CIMAP (Central Institute of medicinal and Aromatic Plants) Lucknow, India. REFERENCES [1] G. Pandey and V. K. Dobhal, “Multivariate Analysis in Chilli,” Journal of Spices and Aromatic Crops, Vol. 2, No. 1-2, 1993, pp. 71-74. [2] M. T. P. Schifino-Wittmann and L. B. Bianchetti, “Chromosome Numbers in Wild and Semidomesticated Brazilian Capsicum L (Solanaceae) Species Do x = 12 and x = 13 Represent to Evolutionary Lines?” Biological Journal of the Linnean Society, Vol. 151, No. 2, 2006, pp. 259- 269. [3] E. A. Moscone, M. A. Scaldaferro, M. Grabiele, N. M. Cecchini, et al., “The Evolution of Chili Peppers (Capsi- cum Solanaceae) a Cytogenetic Perspective. VI Interna- tional Solanaceae Conference: Genomics Meets Biodi- versity,” Acta Horticulturae, Vol. 745, 2007, pp. 137- 170. [4] C. R. Rao, “Advanced Statistical Methods in Biometric Research,” John Wiley and Sons, New York, 1952. [5] D. Palevitch and L. E. Craker, “Nutritional and Medicinal Importance of Red Pepper (Capsicum spp.),” Journal of Herbs, Spices and Medicinal Plants, Vol. 3, No. 2, 1995, pp. 55-83. doi:10.1300/J044v03n02_08 [6] B. Pickersgill, “Chromosome and Evolution in Capsicum. In S. Lantiri, 1991. Lack of Karyotype Class and Skewed Chromosome Segregation in Two Backcross Progenies of Capsicum,” Journal of Genetics and Breeding, Vol. 45, 1977, pp. 51-58. [7] F. A. Guzmann, H. A. C. Azurdia, M. C. Duque and M. C. de Vicente, “AFLP Assessment of Genetic Diversity of Capsicum Genetic Resources in Guatemala: Home Gar- dens as an Option for Conservation,” Crop Science, Vol. 45, 2005, pp. 363-370. [8] C. P. Sudre, C. D. Cruz, R. Rodrigues, E. M. Riva, et al., “Variaveis Multicategoricas na Determinacao da Diver- encia Enetica Entre Acessos de Pimento e Pimentao,” Hortic Bras, Vol. 24, No. 1, 2006, pp. 88-93. doi:10.1590/S0102-05362006000100018 [9] A. G. Ince, M. Karaca and A. N. Onus, “Development and Utilization of Diagnostic DAMD-PCR Markers for Capsicum Accessions,” Genetic Resources and Crop Copyright © 2011 SciRes. AJPS  Genetic Variability in Germplasm Accessions of Capsicum annuum L635 Evolution, Vol. 56, No. 2, 2009, pp. 211-221. doi:10.1007/s10722-008-9356-4 [10] P. C. Mahalanobis, “On the Generalized Distance in Sta- tistics,” Proceedings. National Institute of Sciences (In- dia), Vol. 2, 1936, pp. 49-55. [11] B. Varalakshmi and K. Haribabu, “Genetic Divergence, Heritability and Genetic Advance in Chilli,” Indian jour- nal of Genetics, Vol. 51, No. 2, 1991, pp.174-178. [12] B. Pickersgill, “Relationship between Weedy and Culti- vated Forms in Some Species of Chili Peppers (Genus Capsicum),” Evolution, Vol. 25, No. 4, 1971, pp. 683-691. doi:10.2307/2406949 [13] J. Singh, “Improvement of Chilies,” Advances in Horti- culture, Vol. 5, 1993, pp. 69-86. [14] Embrapa, “Exportecoes Brasileiras de Hortelicas 2000-2007, ” 2009. http.//www.cnph.embrapa.br/paginas/hortalicas_em_num eros/exportecoes_brasileiras_hortalicas_2000_2007.pdf Copyright © 2011 SciRes. AJPS
|