 Green and Sustainable Chemistry, 2011, 1, 132-148 doi:10.4236/gsc.2011.14022 Published Online November 2011 (http://www.SciRP.org/journal/gsc) Copyright © 2011 SciRes. GSC Poly Ethylene Glycols as Efficient Media for the Synthesis of β-Nitro Styrenes from α, β-Unsaturated Carboxylic Acids and Metal Nitrates under Conventional and Non-Conventional Conditions Kamatala Chinna Rajanna1, Kola Ramesh2, Soma Ramgopal1, Som ann agari Shylaja2, Pochampally Giridhar Reddy2, Pondichery Kuppuswamy Saiprakash1 1Department of Chemistry, Osmania University, Hyderabad, India 2Department of Chemistry, CBIT, Gandipet, Hyderabad, India E-mail: kcrajannaou@yahoo.com Received August 18, 2011; revised October 14, 2011; accepted October 26, 2011 Abstract Poly ethylene glycols (PEG-200, 400, 600, 4000 and 6000) supported reactions were conducted with certain α, β-unsaturated acids in presence of metal nitrates under solvent free (solid state) and mineral acid free con- ditions. The reactants were ground in a mortar with a pestle for about 30 minutes. The aromatic acids under- went nitro decarboxylation and afforded β-nitro styrene derivatives in very good yield while α, β-unsaturated aliphatic carboxylic acids gave corresponding nitro derivatives. Addition of PEG accelerated rate of the reac- tion enormously. Reaction times substantially decreased from several hours to few minutes followed by highly significant increase in the product yield. Among the several PEGs PEG-300 has been found to be much more effective than other PEGs. Keywords: Poly Ethylene Glycols (PEG), Rate Accelerations, α, β-Unsaturated Acids, Metal Nitrates, Solvent Free (Solid State), β-Nitro Styrene Derivatives, α, β-Unsaturated Aliphatic Acids, Nitro Derivatives 1. Introduction The use of non volatile solvents is an essential ingredient in a large number of organic synthesis protocols, which may be toxic, hazardous and also cause environmental pollution. Therefore the use of environmentally safe and non-toxic solvents and more specifically removal of or- ganic solvents in chemical synthesis are important in the drive towards benign chemical technologies. Solvent-free organic reactions make synthesis simpler, save energy, and prevent solvent wastes, hazards, and toxicity. The development of solvent-free organic synthetic methods has thus become an important and popular research area. Reports on solvent-free reactions between solids, gases and solids, solids and liquid, between liquids, and on solid inorganic supports have become increasingly fre- quent in recent years. A mortar and pestle is a tool used to crush, grind, and mix solid substances. Solvent less preparation of organic compounds in the solid state and via microwave irradiation has been the subject of interest for the past one decade which has the advantage of being eco-friendly, easy to handle, employ shorter reaction times and solvent less conditions. Reactions performed under solvent-free conditions have gained much attention be- cause of their enhanced selectivity, mild reaction con- ditions and associated ease of manipulation. The recent reviews and publications [1-6] in this field prove the importance of solvent free organic synthesis and high- lights that, this process is not only simple but also satis- fies both economical and environmental demands by replacing the toxic solvents. Since more than a decade our group is also actively working on exploiting the use of a variety of eco friendly materials such as metal ions and surfactants as catalysts and non-conventional energy sources (such as microwave and ultra sound) to assist organic transformations such as Vilsmeier-Haack [7-9], Hunsdiecker [10] and nitration reactions [11-13]. The classical Hunsdiecker-Borodin reaction [14,15] is an im-  133 K. C. RAJANNA ET AL. portant halo decarboxylation reaction, which is used for the synthesis of β-halo styrenes from α, β-unsaturated Cinnamic acid. This reaction has been modified by seve- ral workers with a view to overcome the toxicity factors arising from the use of molecular bromine and metal salt catalysts [16-25]. The use of solid acid catalysis has been found potentially more attractive because of the ease of removal and recycling of the catalyst and the possibility that the solid might influence the selectivity. In one of the recent reports Das and coworkers [26] reported that nitro styrenes can be achieved from α, β-unsaturated carboxylic acids using nitric acid (3 equiv) and catalytic amount of AIBN (2 mol%) in acetonitrile medium. In another report Rao et al. [27] enlightened the use of ceric ammonium nitrate (CAN) in nitro Hunsdiecker-Borodin reactions. Recently we have concentrated on developing new methodologies using non-conventional energy sou- rces and eco-friendly materials as catalysts in organic transformations, and reported a methodology in metal ion mediated nitration of organic compounds in presence of small amount of HNO3 under solvent free (solid state) conditions [28]. Polyethylene glycol (PEG-400) is a bio- logically acceptable inexpensive polymer and an eco- friendly reagent [29], which is widely used in many or- ganic reactions for conversion of oxiranes to thiiranes [30], asymmetric aldol reactions [31], cross-coupling rea- ctions [18], Baylis-Hillman reaction [32,33] and ring o- pening of epoxides [34]. Encouraged by these results, we want to explore, the use of Polyethylene glycols (PEGs) as efficient catalyst in this study. We have studied PEG triggered Hunsdiecker-Borodin reactions for the synthe- sis of β-nitro styrenes from α, β-unsaturated carboxylic acids under conventional and non-conventional (solvent free mortar-pestle and microwave) conditions. 2. Experimental Details Cinnamic acid, metal nitrates, nitric acid and polyethyle- ne glycols were obtained from SD Fine Chemicals or Loba. Substituted Cinnamic acid were prepared by Per- kins reaction as cited in literature [35]. 2.1. General Procedure for PEG Mediated Synthesis of β-Nitro Styrenes in MeCN Medium In a typical solid state synthesis, Cinnamic acid (0.01 mol), PEG (0.02 mmol) and metal nitrate (0.12 mmol) are placed in a clean two necked R. B. flask and stirred for certain time. Ground with a pestle for about 30 to 60 minutes until the mixture is homogeneous and particles are no longer getting smaller. Progress of the reaction is periodically monitored by TLC. After completion, the reaction mixture is treated with 2% sodium carbonate solution, followed by the addition dichloro methane (DCM) or dichloro ethane (DCE). The organic layer was separated, dried over Na2SO4 and the solvent is recollected by distillation using Rotavapor. The resul- tant compound is further purified with column chroma- togram-phy using ethyl acetate: hexane (3:7) as eluent to get pure product. Hexane and ethyl acetate are also separated using Rotavapor according to standard pro- cedures [35-37]. 2.2. General Procedure for the Synthesis of β-Nitro Styrenes in Acetonitrile Medium under Continuously Stirred Conditions In a typical synthesis, Cinnamic acid (0.01 mol), PEG (0.02 mmol) and metal nitrate (0.12 mmol) are placed in a clean mortar and ground with a pestle for about 30 to 60 minutes until the mixture is homogeneous, the parti- cles are no longer getting smaller. Progress of the reac- tion is periodically monitored by TLC. After completion, the reaction mixture is treated with 2% sodium bicarbo- nate solution, followed by the addition dichloro methane (DCM) or dichloro ethane (DCE). The organic layer was further treated in a similar manner discussed in the ear- lier section to get pure product. 2.3. General Procedure for the Synthesis of β-Nitro Styrenes under Microwave Irradiated Conditions Cinnamic acid (0.01 mol), PEG (0.02 mmol) and metal nitrate (0.12 mmol) were dissolved in minimum amount of MeCN, and mixed with silica gel (10 g) and the mix- ture was transfered into a test tube and subjected to mi- crowave irradiation (BPL make, BMO 700T, 650 W, power 80%) for a specified period. Reaction was moni- tored by TLC (hexane-ethyl acetate, 7:3). After comple- tion of the reaction, products are isolated as discussed in the above section. 2.4. General Procedure for the Synthesis of β-Nitro Styrenes under Solvent-Free Conditions A mortar was charged with Cinnamic acid (0.01 mol), PEG (0.02 mmol) and metal nitrate (0.12 mmol). The mixture was ground at room temperature with a pestle until TLC showed complete disappearance of the starting material. After completion, the reaction mixture is trea- ted with 2% sodium bicarbonate solution, followed by addition of dichloro methane (DCM) or dichloro ethane Copyright © 2011 SciRes. GSC  K. C. RAJANNA ET AL. Copyright © 2011 SciRes. GSC 134 (DCE). The organic layer was further treated in a similar manner discussed in the earlier section to get pure product. 3. Results and Discussion The α, β-unsaturated aromatic carboxylic acids such as Cinnamic acid afforded β-nitro styrenes when they are taken along with PEG in presence of metal nitrates in a mortar and ground with a pestle for about half a hour. The reactions afforded good yield of products with high regio selectivity. The yields of major products are com- piled in Tables 1-3. The products were characterized by Table 1. NMR and Mass Sp ectral data for selected reaction produ cts. Spectral data Entry Substrate Product m/z 1HNMR 1 CA β–Nitro Styrene 149 δ 6.4 (d 1H, β-CH), δ 7.3 - 7.65 (m 5H, Ar-H) δ 7.8(d 1H, α-CH) 2 4-ClCA 4-Chloro β–Nitro Styrene 184 δ 6.6 (d 1H, β-CH) δ 7.2 (d 2H, Ar-H) δ 7.6 (d 2H, Ar-H) δ 8.3 (d 1H, α-CH) 3 4-OMeCA 4-Methoxy β–Nitro Styrene 179 δ 3.8 (s 3H, OCH3) δ 6.4 (d 1H, β-CH) δ 7.32 - 7.7 (m 4H, Ar-H) δ 7.9 (d 1H, α-CH) 4 4-MeCA 4-Methyl β–Nitro Styrene 163 δ 3.0 (s 3H, CH3) δ 6.6 (d 1H, , β-CH) δ 7.4 - 7.7 (m 4H, Ar-H δ 7.9 (d 1H, α-CH) 5 4-NO2CA 4-Nitro β–Nitro Styrene 194 δ 6.6 (d 1H, β-CH) δ 7.4 (d 2H, Ar-H) δ 7.8 (d 2H, Ar-H) δ8.2 (s 1H, α-CH) 6 4-OHCA 4-Hydroxy β–Nitro Styrene 165 δ 6.5 (d 1H, β-CH) δ 7.3 (d 2H, Ar-H) δ 7.8 (d 2H, Ar-H) δ 8.1 (d 1H, α-CH) δ 10.5 (s 1H, Ar-OH) 7 AA 1–Nitro Ethene 73 δ 5.92 (d 1H, β-CH) δ 6.6 (d 1H, trans β-CH) δ 7.25 (q 1H, α-CH) 8 CRA 1–Nitro Propene 87 δ 2.12 (d 3H, CH3) δ 7.0 (d 1H, α-CH) δ 7.15 (m 1H, β-CH ) 9 3-PhCRA 3- Phenyl 1–Nitro Propene 163 δ 3.3 (d 2H, CH2) δ 7.23 - 7.33 (m 5H, Ar-H) δ 8.2 (d 1H, α-C-H) 10 2-ClCA 2-Chloro β–Nitro Styrene 183 δ 6.6 (d 1H, β-CH) δ 7.3 - 7.7 (m 4H, Ar-H) δ 8.2 (d 1H, α- C-H) 11 2-MeCA 2-Methyl β–Nitro Styrene 163 δ 2.9 (s 3H, CH3) δ 6.7 (d 1H, β-CH) δ 7.1 - 7.8 (m 4H, Ar-H) δ 8.2 (d 1H, α-CH) Table 2. Effect of different PEGs on nitro Hunsdiecker reactions (Solution phase) with Cinnamic acid. PEG-200 PEG-300 PEG-400 PEG-600 PEG-4000 PEG-6000 S.No Metal Nitrate RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1 Ni(NO3)2 1.75 75 1.75 88 1.75 85 1.75 80 2.75 85 2.75 81 2 Zn(NO3)2 1.75 80 1.75 85 1.75 86 1.75 85 2.75 87 2.75 86 3 ZrO(NO3)2 1.75 75 1.75 85 1.75 88 1.75 87 2.75 82 2.75 82 4 Cd(NO3)2 1.75 80 1.75 85 1.75 84 1.75 86 2.75 84 2.75 84 5 Hg(NO3)2 1.75 80 1.75 85 1.75 82 1.75 85 2.75 78 2.75 70 6 Mg(NO3)2 1.75 85 1.75 90 1.75 87 1.75 88 2.75 85 2.75 86 7 Sr(NO3)2 1.75 88 1.75 90 1.75 92 1.75 94 2.75 88 2.75 90 8 Al(NO3)2 1.75 83 1.75 89 1.75 85 1.75 86 2.75 83 2.75 83 9 UO2(NO3)2 1.75 88 1.75 90 1.75 90 1.75 91 2.75 88 2.75 89 10 Th(NO3)2 1.75 88 1.75 88 1.75 86 1.75 90 2.75 85 2.75 90 11 AgNO3 1.75 70 1.75 80 1.75 80 1.75 86 2.75 78 2.75 80 12 NH4(NO3)2 1.75 75 1.75 80 1.75 80 1.75 78 2.75 75 2.75 75 13 Ca(NO3)2 1.75 80 1.75 85 1.75 84 1.75 86 2.75 84 2.75 84 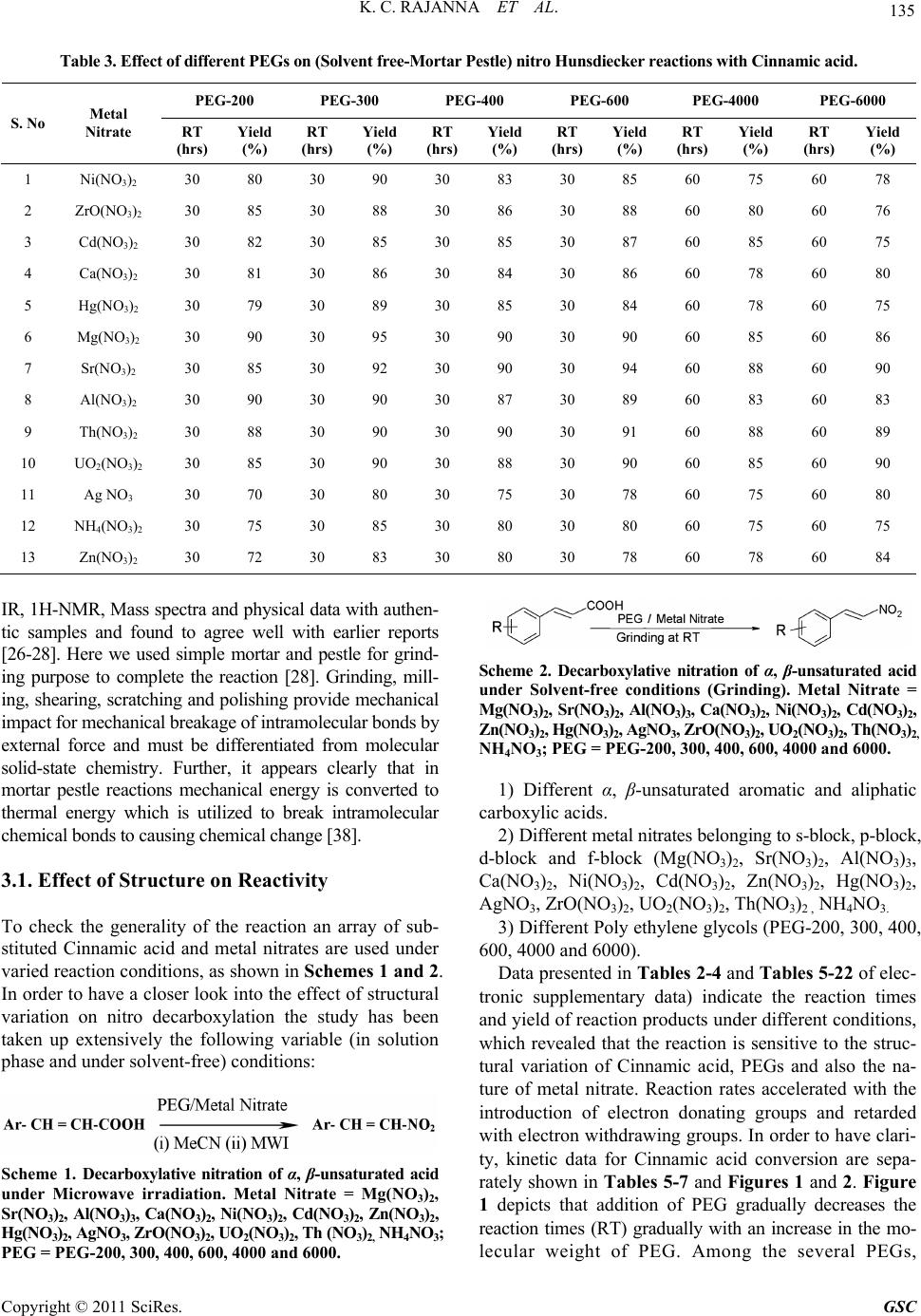 135 K. C. RAJANNA ET AL. Table 3. Effect of different PEGs on (Solvent free-Mortar Pestle) nitro Hunsdiecker reactions with Cinnamic acid. PEG-200 PEG-300 PEG-400 PEG-600 PEG-4000 PEG-6000 S. No Metal Nitrate RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1 Ni(NO3)2 30 80 30 90 30 83 30 85 60 75 60 78 2 ZrO(NO3)2 30 85 30 88 30 86 30 88 60 80 60 76 3 Cd(NO3)2 30 82 30 85 30 85 30 87 60 85 60 75 4 Ca(NO3)2 30 81 30 86 30 84 30 86 60 78 60 80 5 Hg(NO3)2 30 79 30 89 30 85 30 84 60 78 60 75 6 Mg(NO3)2 30 90 30 95 30 90 30 90 60 85 60 86 7 Sr(NO3)2 30 85 30 92 30 90 30 94 60 88 60 90 8 Al(NO3)2 30 90 30 90 30 87 30 89 60 83 60 83 9 Th(NO3)2 30 88 30 90 30 90 30 91 60 88 60 89 10 UO2(NO3)2 30 85 30 90 30 88 30 90 60 85 60 90 11 Ag NO3 30 70 30 80 30 75 30 78 60 75 60 80 12 NH4(NO3)2 30 75 30 85 30 80 30 80 60 75 60 75 13 Zn(NO3)2 30 72 30 83 30 80 30 78 60 78 60 84 IR, 1H-NMR, Mass spectra and physical data with authen- tic samples and found to agree well with earlier reports [26-28]. Here we used simple mortar and pestle for grind- ing purpose to complete the reaction [28]. Grinding, mill- ing, shearing, scratching and polishing provide mechanical impact for mechanical breakage of intramolecular bonds by external force and must be differentiated from molecular solid-state chemistry. Further, it appears clearly that in mortar pestle reactions mechanical energy is converted to thermal energy which is utilized to break intramolecular chemical bonds to causing chemical change [38]. 3.1. Effect of Structure on Reactivity To check the generality of the reaction an array of sub- stituted Cinnamic acid and metal nitrates are used under varied reaction conditions, as shown in Schemes 1 and 2. In order to have a closer look into the effect of structural variation on nitro decarboxylation the study has been taken up extensively the following variable (in solution phase and under solvent-free) conditions: Scheme 1. Decarboxylative nitration of α, β-unsaturated acid under Microwave irradiation. Metal Nitrate = Mg(NO3)2, Sr(NO3)2, Al(NO3)3, Ca(NO3)2, Ni(NO3)2, Cd(NO3)2, Zn(NO3)2, Hg(NO3)2, AgNO3, ZrO(NO3)2, UO2(NO3)2, Th (NO3)2, NH4NO3; PEG = PEG-200, 300, 400, 600, 4000 and 6000. Scheme 2. Decarboxylative nitration of α, β-unsaturated acid under Solvent-free conditions (Grinding). Metal Nitrate = Mg(NO3)2, Sr(NO3)2, Al(NO3)3, Ca(NO3)2, Ni(NO3)2, Cd(NO3)2, Zn(NO3)2, Hg(NO3)2, AgNO3, ZrO(NO 3)2, UO2(NO3)2, Th(N O3)2, NH4NO3; PEG = PEG-200, 300, 400, 600, 4000 and 6000. 1) Different α, β-unsaturated aromatic and aliphatic carboxylic acids. 2) Different metal nitrates belonging to s-block, p-block, d-block and f-block (Mg(NO3)2, Sr(NO3)2, Al(NO3)3, Ca(NO3)2, Ni(NO3)2, Cd(NO3)2, Zn(NO3)2, Hg(NO3)2, AgNO3, ZrO(NO3)2, UO2(NO3)2, Th(NO3)2 , NH4NO3. 3) Different Poly ethylene glycols (PEG-200, 300, 400, 600, 4000 and 6000). Data presented in Tables 2-4 and Tables 5-22 of elec- tronic supplementary data) indicate the reaction times and yield of reaction products under different conditions, which revealed that the reaction is sensitive to the struc- tural variation of Cinnamic acid, PEGs and also the na- ture of metal nitrate. Reaction rates accelerated with the introduction of electron donating groups and retarded with electron withdrawing groups. In order to have clari- ty, kinetic data for Cinnamic acid conversion are sepa- rately shown in Tables 5-7 and Figures 1 and 2. Figure 1 depicts that addition of PEG gradually decreases the reaction times (RT) gradually with an increase in the mo- lecular weight of PEG. Among the several PEGs, Copyright © 2011 SciRes. GSC 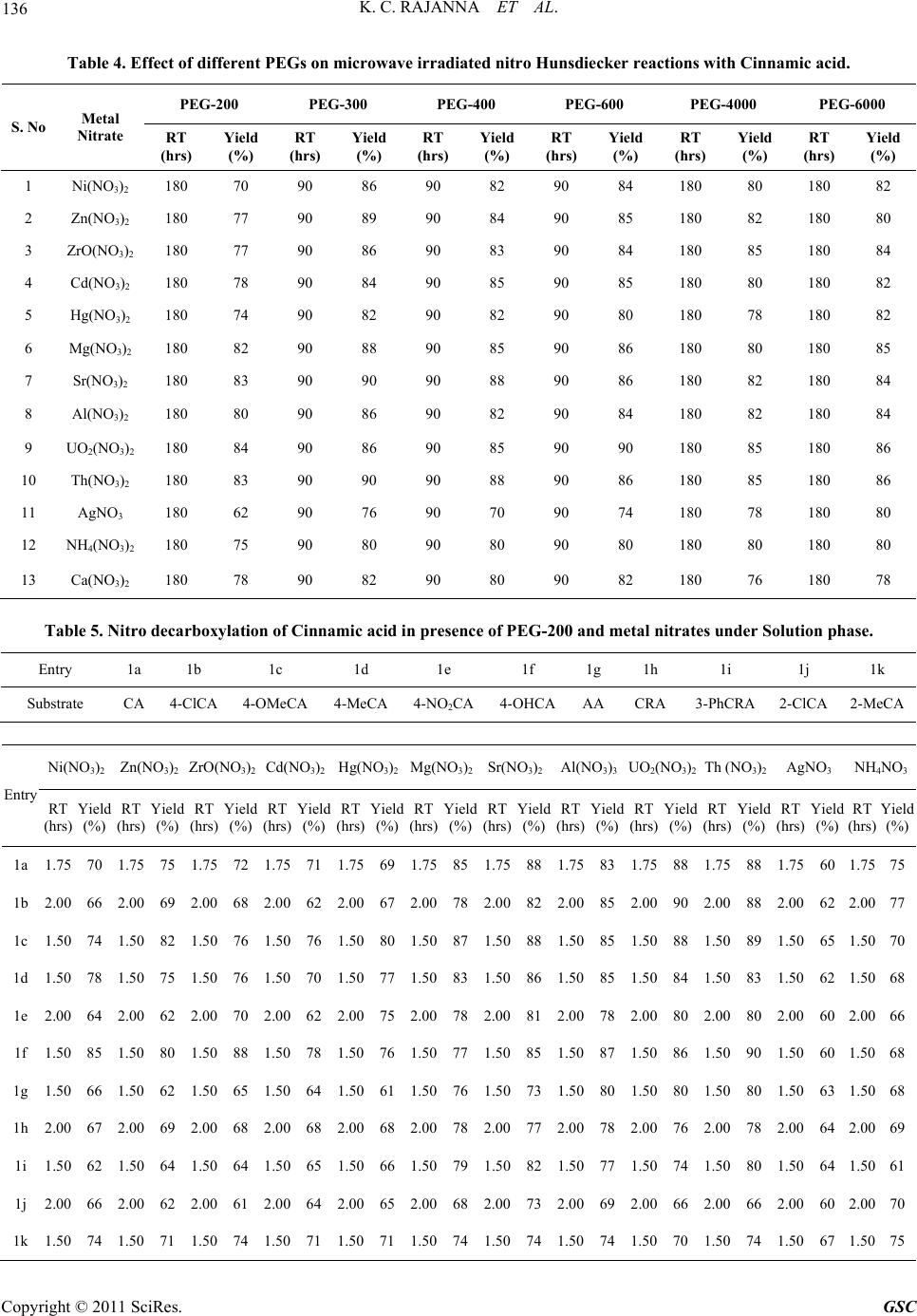 K. C. RAJANNA ET AL. 136 Table 4. Effect of different PEGs on microwave irradiated nitro Hunsdiecker reactions with Cinnamic acid. PEG-200 PEG-300 PEG-400 PEG-600 PEG-4000 PEG-6000 S. No Metal Nitrate RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1 Ni(NO3)2 180 70 90 86 90 82 90 84 180 80 180 82 2 Zn(NO3)2 180 77 90 89 90 84 90 85 180 82 180 80 3 ZrO(NO3)2 180 77 90 86 90 83 90 84 180 85 180 84 4 Cd(NO3)2 180 78 90 84 90 85 90 85 180 80 180 82 5 Hg(NO3)2 180 74 90 82 90 82 90 80 180 78 180 82 6 Mg(NO3)2 180 82 90 88 90 85 90 86 180 80 180 85 7 Sr(NO3)2 180 83 90 90 90 88 90 86 180 82 180 84 8 Al(NO3)2 180 80 90 86 90 82 90 84 180 82 180 84 9 UO2(NO3)2 180 84 90 86 90 85 90 90 180 85 180 86 10 Th(NO3)2 180 83 90 90 90 88 90 86 180 85 180 86 11 AgNO3 180 62 90 76 90 70 90 74 180 78 180 80 12 NH4(NO3)2 180 75 90 80 90 80 90 80 180 80 180 80 13 Ca(NO3)2 180 78 90 82 90 80 90 82 180 76 180 78 Table 5. Nitro decarboxylation of Cinnamic acid in presence of PEG-200 and metal nitrates under Solution phase. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1a 1.75 70 1.75 75 1.75 72 1.75 71 1.75 69 1.7585 1.75 88 1.75 83 1.7588 1.75 88 1.75 601.7575 1b 2.00 66 2.00 69 2.00 68 2.00 62 2.00672.00 78 2.00 822.00 852.00 902.00 88 2.00 622.0077 1c 1.50 74 1.50 82 1.50 76 1.50 76 1.50 80 1.5087 1.50 88 1.50 85 1.5088 1.50 89 1.50 651.5070 1d 1.50 78 1.50 75 1.50 76 1.50 70 1.50771.50 83 1.50 861.50 851.50 841.50 83 1.50 621.5068 1e 2.00 64 2.00 62 2.00 70 2.00 62 2.00 75 2.0078 2.00 81 2.00 78 2.0080 2.00 80 2.00 602.0066 1f 1.50 85 1.50 80 1.50 88 1.50 78 1.50761.50 77 1.50 851.5087 1.50861.50 90 1.50 601.5068 1g 1.50 66 1.50 62 1.50 65 1.50 64 1.50611.50 76 1.5073 1.5080 1.5080 1.50 80 1.50 631.5068 1h 2.00 67 2.00 69 2.00 68 2.00 68 2.00682.00 78 2.00 772.00 782.00 762.00 78 2.00 642.0069 1i 1.50 62 1.50 64 1.50 64 1.50 65 1.50 661.5079 1.50 821.50 771.50 741.50 80 1.50 641.5061 1j 2.00 66 2.00 62 2.00 61 2.00 64 2.00 652.0068 2.00 732.00 692.00 662.00 66 2.00 602.0070 1k 1.50 74 1.50 71 1.50 74 1.50 71 1.50711.50 74 1.50 741.50 741.50 701.50 74 1.50 671.5075 Copyright © 2011 SciRes. GSC 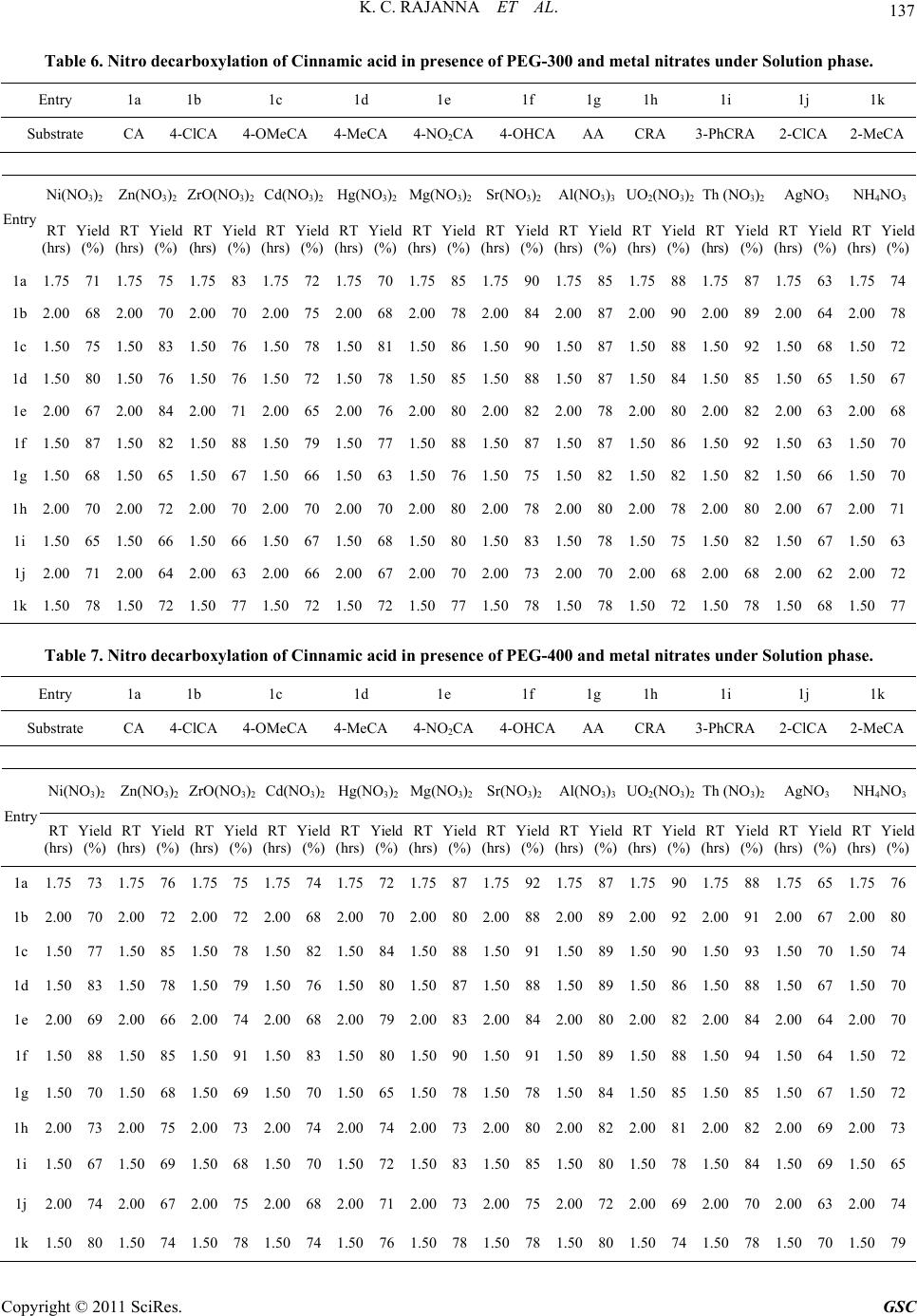 137 K. C. RAJANNA ET AL. Table 6. Nitro decarboxylation of Cinnamic acid in presence of PEG-300 and metal nitrates under Solution phase. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1a 1.75 71 1.75 75 1.75 83 1.75 72 1.75 701.75 851.75 901.75 851.75 88 1.75 87 1.75 63 1.7574 1b 2.00 68 2.00 70 2.00 70 2.00 75 2.00 682.00782.00842.0087 2.00902.00 89 2.00 642.0078 1c 1.50 75 1.50 83 1.50 76 1.50 78 1.50 811.50 861.50 901.50 871.50 88 1.50 92 1.50 68 1.5072 1d 1.50 80 1.50 76 1.50 76 1.50 72 1.50 78 1.50851.50881.5087 1.50841.50 85 1.50 651.5067 1e 2.00 67 2.00 84 2.00 71 2.00 65 2.00 762.00 802.00 822.00 78 2.0080 2.00 82 2.00 63 2.0068 1f 1.50 87 1.50 82 1.50 88 1.50 79 1.50 77 1.5088 1.5087 1.5087 1.50861.50 92 1.50 631.5070 1g 1.50 68 1.50 65 1.50 67 1.50 66 1.5063 1.50761.50751.5082 1.50821.50 82 1.50 661.5070 1h 2.00 70 2.00 72 2.00 70 2.00 70 2.0070 2.00802.00782.0080 2.00782.00 80 2.00 672.0071 1i 1.50 65 1.50 66 1.50 66 1.50 67 1.5068 1.5080 1.50831.5078 1.5075 1.50 82 1.50 67 1.5063 1j 2.00 71 2.00 64 2.00 63 2.00 66 2.0067 2.0070 2.00732.0070 2.00682.00 68 2.00 622.0072 1k 1.50 78 1.50 72 1.50 77 1.50 72 1.5072 1.50771.50781.5078 1.50721.50 78 1.50 681.5077 Table 7. Nitro decarboxylation of Cinnamic acid in presence of PEG-400 and metal nitrates under Solution phase. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1a 1.75 73 1.75 76 1.75 75 1.75 74 1.7572 1.75871.75 92 1.7587 1.75901.75 88 1.75 651.7576 1b 2.00 70 2.00 72 2.00 72 2.00 68 2.00 70 2.00802.00 882.00 89 2.00 92 2.00 91 2.00 67 2.0080 1c 1.50 77 1.50 85 1.50 78 1.50 82 1.5084 1.50881.5091 1.5089 1.50901.50 93 1.50 70 1.5074 1d 1.50 83 1.50 78 1.50 79 1.50 76 1.50 80 1.50871.50 881.50 89 1.50 86 1.50 88 1.50 67 1.5070 1e 2.00 69 2.00 66 2.00 74 2.00 68 2.0079 2.00832.00 84 2.0080 2.00822.00 84 2.00 642.0070 1f 1.50 88 1.50 85 1.50 91 1.50 83 1.50 80 1.50901.50 911.50 89 1.50 88 1.50 94 1.50 64 1.5072 1g 1.50 70 1.50 68 1.50 69 1.50 70 1.50651.50 78 1.5078 1.5084 1.50851.50 85 1.50 67 1.5072 1h 2.00 73 2.00 75 2.00 73 2.00 74 2.00 74 2.00732.00 802.00 82 2.00 81 2.00 82 2.00 69 2.0073 1i 1.50 67 1.50 69 1.50 68 1.50 70 1.5072 1.50831.50 85 1.5080 1.50781.50 84 1.50 691.5065 1j 2.00 74 2.00 67 2.00 75 2.00 68 2.0071 2.00732.00 75 2.0072 2.00692.00 70 2.00 632.0074 1k 1.50 80 1.50 74 1.50 78 1.50 74 1.50 76 1.50781.50 781.50 80 1.50 74 1.50 78 1.50 70 1.5079 Copyright © 2011 SciRes. GSC 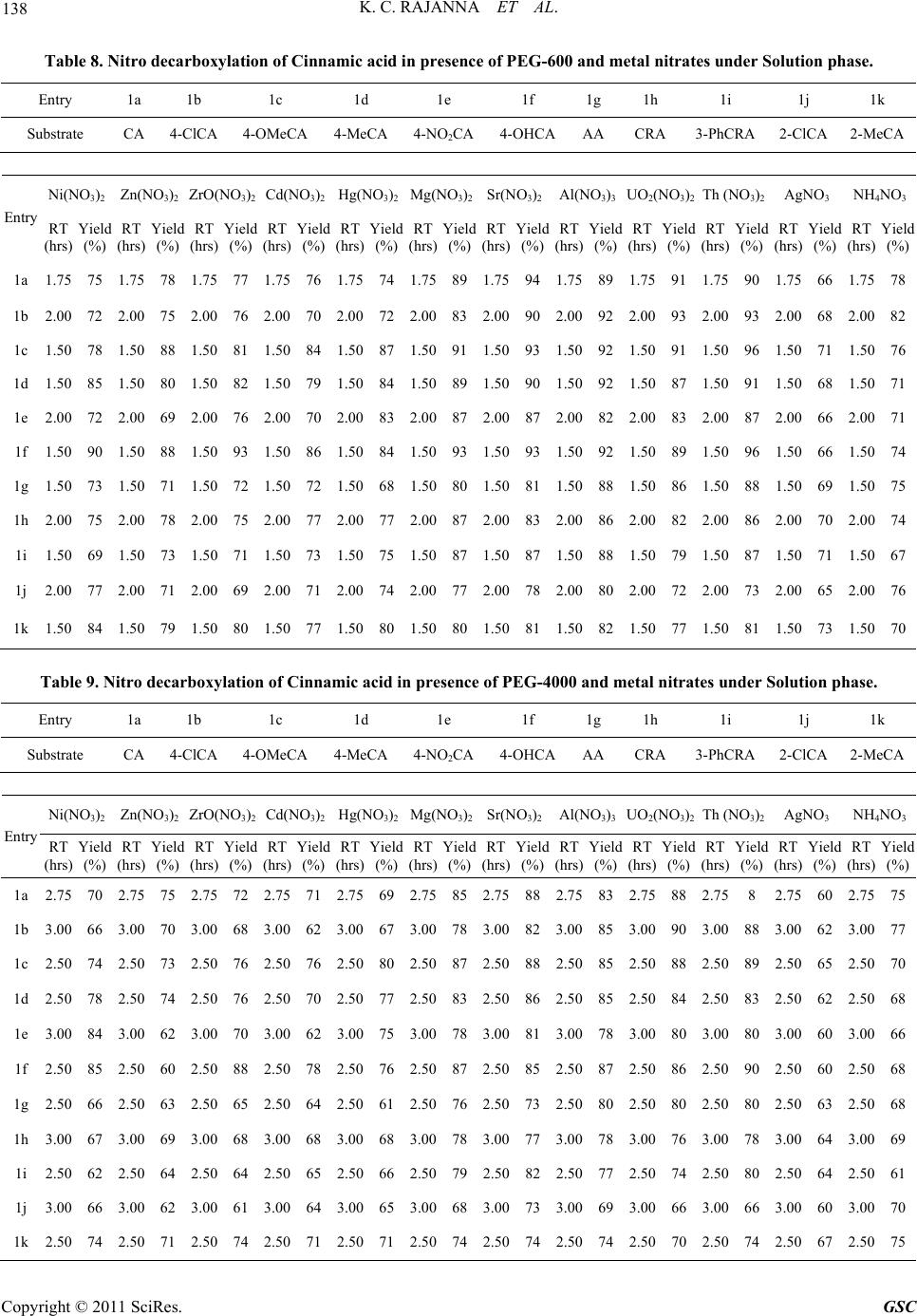 K. C. RAJANNA ET AL. 138 Table 8. Nitro decarboxylation of Cinnamic acid in presence of PEG-600 and metal nitrates under Solution phase. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (hrs) (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1a 1.75 75 1.75 78 1.75 77 1.75 76 1.7574 1.75 891.7594 1.7589 1.75911.75 90 1.75 66 1.7578 1b 2.00 72 2.00 75 2.00 76 2.00 70 2.00 72 2.00832.00 90 2.00 92 2.00 93 2.00 93 2.00 68 2.0082 1c 1.50 78 1.50 88 1.50 81 1.50 84 1.5087 1.50 911.5093 1.5092 1.50911.50 96 1.50 71 1.50 76 1d 1.50 85 1.50 80 1.50 82 1.50 79 1.50 84 1.50891.50 901.50 92 1.50 87 1.50 91 1.50 68 1.5071 1e 2.00 72 2.00 69 2.00 76 2.00 70 2.0083 2.00872.00 87 2.0082 2.00832.00 87 2.00 662.0071 1f 1.50 90 1.50 88 1.50 93 1.50 86 1.50 84 1.50931.50 931.50 92 1.50 89 1.50 96 1.50 66 1.5074 1g 1.50 73 1.50 71 1.50 72 1.50 72 1.50 68 1.50801.50 811.50 88 1.50 86 1.50 88 1.50 69 1.5075 1h 2.00 75 2.00 78 2.00 75 2.00 77 2.00 77 2.00872.00 832.00 86 2.00 82 2.00 86 2.00 70 2.0074 1i 1.50 69 1.50 73 1.50 71 1.50 73 1.50 751.50 87 1.50871.50 881.50 791.50 87 1.50 71 1.5067 1j 2.00 77 2.00 71 2.00 69 2.00 71 2.0074 2.00772.00 78 2.0080 2.00722.00 73 2.00 652.0076 1k 1.50 84 1.50 79 1.50 80 1.50 77 1.50 80 1.50801.50 811.50 82 1.50 77 1.50 81 1.50 73 1.5070 Table 9. Nitro decarboxylation of Cinnamic acid in presence of PEG-4000 and metal nitrates under Solution phase. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (hrs) (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1a 2.75 70 2.75 75 2.75 72 2.75 71 2.75692.75 85 2.7588 2.75832.75 88 2.75 8 2.75 60 2.7575 1b 3.00 66 3.00 70 3.00 68 3.00 62 3.0067 3.00783.0082 3.0085 3.00903.00 88 3.00 62 3.0077 1c 2.50 74 2.50 73 2.50 76 2.50 76 2.50802.50 87 2.5088 2.50852.50 88 2.50 89 2.50 65 2.5070 1d 2.50 78 2.50 74 2.50 76 2.50 70 2.5077 2.50832.5086 2.5085 2.50842.50 83 2.50 62 2.5068 1e 3.00 84 3.00 62 3.00 70 3.00 62 3.00753.00 78 3.0081 3.00783.00 80 3.00 80 3.00 60 3.0066 1f 2.50 85 2.50 60 2.50 88 2.50 78 2.5076 2.50872.50 85 2.50 87 2.5086 2.50 90 2.50 60 2.50 68 1g 2.50 66 2.50 63 2.50 65 2.50 64 2.5061 2.50 762.50 73 2.5080 2.50802.50 80 2.50 63 2.5068 1h 3.00 67 3.00 69 3.00 68 3.00 68 3.0068 3.00783.0077 3.0078 3.00763.00 78 3.00 64 3.0069 1i 2.50 62 2.50 64 2.50 64 2.50 65 2.50662.50 79 2.5082 2.50772.50 74 2.50 80 2.50 64 2.5061 1j 3.00 66 3.00 62 3.00 61 3.00 64 3.00653.00 68 3.0073 3.0069 3.00 663.00 66 3.00 60 3.0070 1k 2.50 74 2.50 71 2.50 74 2.50 71 2.5071 2.50742.5074 2.5074 2.50702.50 74 2.50 67 2.5075 Copyright © 2011 SciRes. GSC  139 K. C. RAJANNA ET AL. Table 10. Nitro decarboxylation of Cinnamic acid in presence of PEG-6000 and metal nitrates under Solution phase. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) RT (hrs) Yield (%) 1a 2.75 71 2.75 76 2.75 72 2.75 72 2.75 50 2.7586 2.7590 2.7583 2.7589 2.75 90 2.75 602.7575 1b 3.00 67 3.00 70 3.00 68 3.00 66 3.00 683.00 80 3.0084 3.0085 3.0091 3.00 90 3.00 623.0077 1c 2.50 75 2.50 73 2.50 76 2.50 78 2.50 80 2.50 89 2.50 90 2.50 85 2.50 81 2.50 81 2.50 65 2.5070 1d 2.50 79 2.50 75 2.50 77 2.50 72 2.50 782.50 85 2.5088 2.5085 2.5085 2.50 84 2.50 622.5088 1e 3.00 65 3.00 63 3.00 71 3.00 63 3.00 76 3.0080 3.0083 3.0078 3.0081 3.00 82 3.00 603.0066 1f 2.50 85 2.50 81 2.50 89 2.50 75 2.50 772.50 892.50 872.50 872.50 88 2.50 92 2.50 60 2.50 68 1g 2.50 66 2.50 64 2.50 86 2.50 65 2.50 632.50 78 2.5075 2.50 80 2.5082 2.50 84 2.50 632.5068 1h 3.00 67 3.00 69 3.00 69 3.00 69 3.00 703.00 80 3.0079 3.0078 3.0078 3.00 80 3.00 643.0069 1i 2.50 62 2.50 65 2.50 65 2.50 67 2.50 682.50 81 2.5083 2.5077 2.5076 2.50 82 2.50 642.5061 1j 3.00 66 3.00 63 3.00 62 3.00 66 3.00 663.00 70 3.0073 3.0069 3.0068 3.00 68 3.00 603.0070 1k 2.50 74 2.50 72 2.50 76 2.50 63 2.50 732.50 76 2.5074 2.5074 2.5072 2.50 76 2.50 672.5075 Table 11. Nitro decarboxylation of Cinnamic acid in presence of PEG-200 and metal nitrates under Solvent free conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) 1a 45 80 45 82 45 81 45 81 45 7845 85 45 86 45 83 4587 45 87 45 60 45 75 1b 60 76 60 75 60 72 60 77 60 7660 78 608060 85 6087 60 88 60 646074 1c 30 78 30 81 30 84 30 83 30 8230 86 30 84 30 84 3088 30 87 30 66 30 70 1d 30 80 30 78 30 84 30 82 30 8030 83 308230 85 3082 30 84 30 653068 1e 60 74 60 72 60 78 60 77 60 7860 79 60 78 60 78 6075 60 80 60 64 60 66 1f 30 88 30 84 30 90 30 90 30 8630 883085 30 87 30 8830 85 30 653068 1g 30 76 30 68 30 75 30 76 30 6930 76307030 81308030 80 30 663068 1h 60 74 60 70 60 78 60 83 60 7860 78 607560 80 6078 60 76 60 676070 1i 30 72 30 70 30 76 30 78 30 75 3079 30 80 30 78 30 7630 78 30 68 30 64 1j 60 78 60 68 60 68 60 76 60 76 6068 60 70 60 70 60 6560 66 60 66 60 72 1k 30 82 30 81 30 88 30 87 30 8430 76 307530 76 3073 30 76 30 783068 Copyright © 2011 SciRes. GSC  K. C. RAJANNA ET AL. 140 Table 12. Nitro decarboxylation of Cinnamic acid in presence of PEG-300 and metal nitrates under Solvent free conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) 1a 45 90 45 88 45 85 45 86 45 80 45 88 45 88 45 86 45 90 45 90 45 75 45 85 1b 60 86 60 80 60 76 60 80 60 7860 8360 8260 88 6088 60 88 60 74 6084 1c 30 88 30 86 30 88 30 88 30 84 30 87 30 85 30 86 30 88 30 86 30 78 30 80 1d 30 86 30 83 30 88 30 86 30 8230 8530 8430 88 3082 30 85 30 76 3078 1e 60 76 60 76 60 82 60 80 60 80 60 80 60 80 60 82 60 76 60 80 60 75 60 75 1f 30 92 30 90 30 90 30 90 30 88 30 8830 8630 90 30 8830 86 30 763078 1g 30 80 30 72 30 78 30 78 30 7130 7830 7230 84 3082 30 82 30 78 3076 1h 60 78 60 75 60 82 60 86 60 8060 7860 7660 84 6080 60 78 60 78 6078 1i 30 76 30 75 30 80 30 82 30 78 30 80 30 82 30 82 30 78 30 82 30 76 30 75 1j 60 82 60 72 60 72 60 76 60 78 60 70 60 72 60 77 60 70 60 65 60 76 60 76 1k 30 86 30 86 30 90 30 88 30 8630 7830 7630 80 3076 30 80 30 80 3080 Table 13. Nitro decarboxylation of Cinnamic acid in presence of PEG-400 and metal nitrates under Solvent free conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) 1a 45 84 45 85 45 82 45 84 45 85 45 86 45 90 45 84 45 90 45 88 45 70 45 80 1b 60 82 60 77 60 73 60 78 60 7860 8160 8060 86 6086 60 86 60 70 6080 1c 30 84 30 83 30 85 30 86 30 82 30 85 30 82 30 83 30 85 30 85 30 74 30 75 1d 30 82 30 80 30 84 30 84 30 8030 8230 8030 85 3080 30 82 30 72 3072 1e 60 72 60 73 60 80 60 78 60 82 60 78 60 82 60 80 60 72 60 78 60 70 60 70 1f 30 88 30 87 30 86 30 86 30 88 30 8630 8530 90 30 8630 83 30 723073 1g 30 76 30 70 30 75 30 76 30 7030 7630 7030 82 3080 30 80 30 73 3071 1h 60 75 60 72 60 80 60 84 60 8060 7560 7460 80 6078 60 76 60 74 6073 1i 30 72 30 72 30 78 30 80 30 78 30 78 30 80 30 80 30 76 30 80 30 72 30 70 1j 60 78 60 68 60 78 60 74 60 78 60 68 60 70 60 74 60 68 60 62 60 72 60 74 1k 30 82 30 82 30 88 30 85 30 8630 7630 7530 76 3074 30 78 30 76 3075 Copyright © 2011 SciRes. GSC  141 K. C. RAJANNA ET AL. Table 14. Nitro decarboxylation of Cinnamic acid in presence of PEG-600 and metal nitrates under Solvent free conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) 1a 45 85 45 86 45 84 45 84 45 80 45 88 45 88 45 85 45 90 45 88 45 74 45 82 1b 60 83 60 78 60 74 60 78 60 7360 8260 7860 86 6084 60 85 60 72 6082 1c 30 85 30 84 30 84 30 84 30 78 30 84 30 80 30 84 30 86 30 84 30 75 30 76 1d 30 83 30 80 30 85 30 82 30 7530 8230 7830 86 3080 30 82 30 72 3074 1e 60 73 60 74 60 82 60 78 60 78 60 78 60 80 60 80 60 74 60 78 60 70 60 72 1f 30 88 30 86 30 86 30 86 30 85 30 8630 8230 90 30 8630 84 30 753075 1g 30 77 30 72 30 76 30 76 30 6830 7730 7030 82 3080 30 80 30 73 3072 1h 60 76 60 74 60 82 60 84 60 7560 7660 7260 82 6078 60 76 60 74 6073 1i 30 73 30 74 30 80 30 80 30 76 30 78 30 80 30 80 30 74 30 80 30 72 30 70 1j 60 78 60 68 60 78 60 76 60 76 60 70 60 72 60 75 60 68 60 64 60 74 60 74 1k 30 82 30 82 30 88 30 86 30 8430 7830 7430 76 3075 30 78 30 80 3076 Table 15. Nitro decarboxylation of Cinnamic acid in presence of PEG-4000 and metal nitrates under Solvent free conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) 1a 90 82 90 84 90 85 90 80 9078 90 82 90 84 90 83 90 86 90 85 90 75 9078 1b 120 81 120 76 120 74 120 74 1207112078 120 74 12084 120 80 120 82 120 73120 78 1c 60 82 60 82 60 83 60 80 6076 60 78 60 76 60 82 60 82 60 80 60 76 6072 1d 60 81 60 80 60 84 60 80 60 7560 7660756082607660 80 60 73 60 70 1e 120 70 120 72 120 82 120 74 120 76 120 70 120 76 120 78 120 70 120 72 120 71 120 68 1f 60 86 60 85 60 86 60 84 60 82608060796088 608260 80 60 76 6072 1g 60 74 60 70 60 75 60 76 60 686071606560 80607660 78 60 74 6070 1h 120 75 120 72 120 80 120 80 1207212070 120 78 12080 120 74 120 75 120 75120 71 1i 60 71 60 72 60 80 60 78 60 75 60 68 60 78 60 7860 70 60 78 60 73 60 70 1j 120 76 120 68 120 78 120 72 1207612065 120 68 12072 120 65 120 62 120 75120 75 1k 60 80 60 80 60 86 60 84 60 8260 7060706072607260 75 60 80 60 77 Copyright © 2011 SciRes. GSC  K. C. RAJANNA ET AL. 142 Table 16. Nitro decarboxylation of Cinnamic acid in presence of PEG-6000 and metal nitrates under Solvent free conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) RT (min) Yield (%) 1a 90 78 90 80 90 82 90 80 90 78 90 86 90 85 90 84 90 86 90 86 90 80 90 78 1b 120 77 120 72 120 71 120 74 120 7212080 120 74120 8412080 120 83 120 7812076 1c 60 78 60 80 60 80 60 82 60 76 60 80 60 76 60 82 60 82 60 82 60 78 60 74 1d 60 78 60 78 60 82 60 82 6074 60 78 6076 6080 6076 60 80 60 7660 72 1e 120 68 120 70 120 80 120 75 120 76 120 72 120 78 120 76 120 72 120 74 120 76 120 68 1f 60 85 60 82 60 83 60 84 6082 6082 60 8060 886082 60 81 60 78 6074 1g 60 72 60 68 60 72 60 75 6068 6075 60 66 608260 7660 78 60 75 6072 1h 120 72 120 70 120 78 120 80 120 7412072 120 78120 8212075 120 76 120 7612072 1i 60 68 60 70 60 78 60 78 60 76 60 70 60 80 60 78 60 72 60 78 60 76 60 74 1j 120 73 120 65 120 80 120 74 120 76 12068120 68120 74 12066120 64 120 7812076 1k 60 80 60 78 60 85 60 82 6082 60 72 6072 6076 6074 60 76 60 8060 78 Table 17. Nitro decarboxylation of Cinnamic acid in presence of PEG-200 and metal nitrates under microwave conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) 1a 180 70 180 77 180 77 180 78 180 74 180 82 18083 180 80 180 84 180 83 180 62 180 75 1b 180 66 180 70 180 68 180 73 180 72 180 7518077 180 82 180 84180 85 180 64 18074 1c 180 88 180 66 180 80 180 79 180 78 180 82 18081 180 81 180 85 180 82 180 66 180 70 1d 180 70 180 73 180 80 180 78 180 76 180 8018079 180 82 180 78180 81 180 65 18068 1e 180 64 180 67 180 74 180 73 180 74 180 75 18075 180 75 180 72 180 78 180 64 180 66 1f 120 78 120 80 120 86 120 85 120 82120 85 120 82 120 84120 85 120 82 120 6612070 1g 180 66 180 63 180 71 180 73 180 65180 73 180 66 180 78180 78 180 77 180 6618068 1h 200 64 200 65 200 74 200 80 200 74 200 7520072 200 78 200 75200 73 200 68 20070 1i 180 62 180 65 180 72 180 74 180 72180 76 180 76 180 75180 73 180 75 180 6818065 1j 240 68 240 63 240 64 240 72 240 72240 65 240 67 240 67240 64 240 64 240 6524072 1k 200 72 200 68 200 84 200 82 200 80 200 7320072 200 73 200 70200 75 200 78 20070 Copyright © 2011 SciRes. GSC  143 K. C. RAJANNA ET AL. Table 18. Nitro decarboxylation of Cinnamic acid in presence of PEG-300 and metal nitrates under microwave conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) 1a 90 86 90 89 90 86 90 84 90 82 90 88 90 90 90 86 90 86 90 90 90 76 90 80 1b 120 82 120 80 120 76 120 80 120 78 120 84120 84 120 88 120 85 120 86 120 7412082 1c 90 84 90 85 90 86 90 86 90 82 90 85 90 82 90 86 90 86 90 84 90 78 90 78 1d 60 82 60 84 60 88 60 86 6080 608660 856086 60 80 60 82 60 75 6076 1e 90 72 90 76 90 84 90 82 90 82 90 80 90 80 90 84 90 78 90 78 90 75 90 72 1f 60 90 60 90 60 90 60 90 6088608660 856090 60 8860 86 60 78 6076 1g 120 78 120 74 120 78 120 78 120 70 120 78120 72 120 84 120 80 120 80 120 7612074 1h 120 76 120 75 120 80 120 86 120 80 120 76120 75 120 80 120 78 120 76 120 7812075 1i 120 76 120 75 120 80 120 80 120 78 120 8012082 120 82 120 76 120 80 120 7612072 1j 180 82 180 70 180 72 180 76 180 76 180 7218074 180 76 180 72 180 68 180 7618070 1k 120 86 120 85 120 86 120 88 120 85 120 78120 78 120 78 120 78 120 80 120 8012076 Table 19. Nitro decarboxylation of Cinnamic acid in presence of PEG-400 and metal nitrates under microwave conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) 1a 90 82 90 84 90 83 90 85 90 82 90 85 90 88 90 82 90 85 90 88 90 70 90 80 1b 120 80 120 76 120 72 120 78 120 78120 82 120781208412082120 85 120 70 12082 1c 90 82 90 82 90 84 90 85 90 80 90 84 90 80 90 81 90 81 90 84 90 74 90 75 1d 60 80 60 80 60 82 60 84 60826081 6078 6083 607660 80 60 726074 1e 90 70 90 72 90 78 90 78 90 78 90 76 90 80 90 78 90 68 90 78 90 70 90 70 1f 60 88 60 86 60 85 60 86 60 88 6084 60 84 608860 8460 84 60 74 6076 1g 120 74 120 68 120 74 120 75 120 70120 78 120701208012076120 80 120 73 12071 1h 120 72 120 70 120 78 120 82 120 82120 76 120741207812075120 75 120 74 12073 1i 120 70 120 70 120 76 120 80 120 7812078 120801208012072120 80 120 72 12070 1j 180 76 180 66 180 75 180 75 180 7618068 180701807218066180 64 180 70 18072 1k 120 80 120 80 120 84 120 84 120 85120 78 120761207412075120 78 120 76 12076 Copyright © 2011 SciRes. GSC  K. C. RAJANNA ET AL. 144 Table 20. Nitro decarboxylation of Cinnamic acid in presence of PEG-600 and metal nitrates under microwave conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) 1a 90 84 90 85 90 84 90 85 90 80 90 86 90 86 90 84 90 90 90 86 90 74 90 80 1b 120 82 120 76 120 75 120 78 120 72 12080 120 76 12085 120 82 120 83 120 7612082 1c 90 83 90 82 90 84 90 82 90 78 90 82 90 78 90 82 90 84 90 82 90 75 90 78 1d 60 82 60 79 60 86 60 80 60 7460 78 60 766084 60 80 60 80 60 7260 76 1e 90 74 90 72 90 80 90 78 90 78 90 76 90 78 90 80 90 74 90 76 90 70 90 72 1f 60 88 60 85 60 86 60 86 60 8460 83 60 806090 60 85 60 84 60 7660 78 1g 120 76 120 70 120 78 120 76 120 68 120 76120 68 12080 120 80 120 78 120 7312072 1h 120 75 120 72 120 82 120 82 120 75 12075 120 70 12082 120 78 120 75 120 7512073 1i 120 74 120 72 120 80 120 80 120 76120 76 120 78120 78 12075 120 78 120 72120 70 1j 180 76 180 66 180 76 180 75 180 78180 72 180 70180 74 18068 180 66 180 74180 78 1k 120 80 120 80 120 86 120 84 120 82 12076 120 76 12075 120 76 120 78 120 8012080 Table 21. Nitro decarboxylation of Cinnamic acid in presence of PEG-4000 and metal nitrates under microwave conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al(NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) 1a 180 80 180 82 180 85 180 80 180 78 180 80 180 82 180 82 180 85 180 85 180 78 180 80 1b 180 78 180 74 180 74 180 74 1807218076 180 72 18082 180 80 180 80 180 74180 78 1c 180 80 180 80 180 80 180 79 180 76 180 78 180 74 180 80 180 82 180 82 180 75 180 75 1d 120 78 120 78 120 82 120 81 1207512075 120 72 12079 120 76 120 78 120 72120 70 1e 180 72 180 70 180 80 180 74 180 76 180 70 180 75 180 78 180 70 180 74 180 70 180 68 1f 120 85 120 86 120 82 120 85 120 80 120 80 120 78120 86120 82120 80 120 78120 74 1g 180 72 180 68 180 72 180 75 1806818069 180 65 18078 180 76 180 78 180 74180 70 1h 220 74 220 70 220 78 220 80 2207222070 220 76 22080 220 74 220 75 220 75220 71 1i 180 72 180 72 180 80 180 78 1807518068 180 78 18078 180 70 180 78 180 74180 70 1j 240 74 240 70 240 76 240 74 2407024066 240 68 24075 240 68 240 68 240 75240 76 1k 220 80 220 80 220 82 220 82 2208022070 220 74 22076 220 74 220 76 220 80220 78 Copyright © 2011 SciRes. GSC  K. C. RAJANNA ET AL. Copyright © 2011 SciRes. GSC 145 Table 22. Nitro decarboxylation of Cinnamic acid in presence of PEG-6000 and metal nitrates under microwave conditions. Entry 1a 1b 1c 1d 1e 1f 1g 1h 1i 1j 1k Substrate CA 4-ClCA 4-OMeCA 4-MeCA4-NO2CA4-OHCAAACRA 3-PhCRA 2-ClCA2-MeCA Ni(NO3)2 Zn(NO3)2 ZrO(NO3)2 Cd(NO3)2 Hg(NO3)2Mg(NO3)2Sr(NO3)2Al (NO3)3UO2(NO3)2 Th (NO3)2 AgNO3NH4NO3 Entry RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) RT (sec) Yield (%) 1a 180 82 180 80 180 84 180 82 180 82 180 85 180 84 180 84180 86 180 86 180 80 180 80 1b 180 77 180 76 180 73 180 72 180 74 18082 180 73 18084 180 81 180 84 180 7518076 1c 180 80 180 82 180 82 180 80 180 78 180 80 180 75 180 80180 84 180 82 180 78 180 78 1d 120 78 120 78 120 80 120 80 120 7612078 120 76120 78 120 76120 80 120 76120 72 1e 180 68 180 72 180 78 180 76 180 75 180 74 180 78 180 75180 74 180 75 180 74 180 68 1f 120 85 120 84 120 85 120 86 120 82 12085 120 80 12088 120 84 120 82 120 7812080 1g 180 72 180 68 180 70 180 75 180 70 18076 180 68 18080 180 76 180 78 180 7518072 1h 220 72 220 70 220 78 220 80 220 74 22074 220 78 22082 220 75 220 76 220 7622074 1i 180 68 180 72 180 78 180 78 180 75180 70 180 80180 78 18074 180 78 180 76 180 76 1j 240 73 240 68 240 80 240 76 240 78240 72 240 70240 75 24068 240 68 240 78240 76 1k 220 82 220 82 220 83 220 80 220 80 22075 220 76 22080 220 78 220 80 220 8022078 Figure 2. Effect of different Metal nitrates on RT versus Yield (%) in presence of PEG-300. Figure 1. Effect of different Poly ethylene glycols (PEG) on RT versus Yield (%) in presence of Nickel Nitrate. PEG-300 has been found to be much more effective than other PEGs. The catalytic activity was found to be in the increasing order: PEG-300 > PEG-400 > PEG-600 > PEG-200. The plot of RT versus PEG type indicated that reaction time decreases with an increase in molecular weight of PEG as could be seen from the data presented in Figure 1. A comparative data profile given in Figure 2 clearly shows remarkable rate enhancements in presence of a variety of metal nitrates. However, the metal nitrates belonging to s and p -blocks such as Mg(NO3)2, Sr(NO3)2, Al(NO3)3, found to be much more reactive than other metal nitrates, which could be attributed to their hardness compa- red to d- and f-block metal nitrate species. Similar trends are shown in other systems. When PEG is added to the reaction system Metal nitrate is capable to form PEG bound Metal nitrates due to complexation accor- ding to the following reaction. The species thus formed could act as an effecttive catalyst to accelerate the re- action by generating nitronium ion. Nitronium thus fo- rmed converts Cinnamic acid into beta nitro styrene as shown in the following sequence of steps shown in Scheme 3.  K. C. RAJANNA ET AL. 146 R-CH=CH-COOH R C H = C HCO O [H-(OCH2-CH2)n -O - M( NO3)x-1] + M(NO3)x-1 H-(OCH2-CH2)n -O + NO2+ NO2+ C O 2 R C H= C H N O2 + N O3fast W here R = Alky l (or ) Aryl group ; MNO3 = Metal fast + NO3 [H-(OCH2-CH2)n -O - M( NO3)x-1] + + NO2+ [H-(OCH2-CH2)n -O - M( NO3)x-1] H+ H+MNO3 [H-(OCH2-CH2)n -O - M(NO3)x-1] ++ PEG H2O 2HNO3 H-(OCH2-CH2)n -OH+ [M(NO3)x] K (PEG) (Metal Nitrate) [H-(OCH2-CH2)n -O - M( NO3)x-1] +HNO3 [PEG-Metal Nitrate} Scheme 3. The formation of Nitronium converts Cinnamic acid into beta nitro styrene. 4. Conclusions Poly ethylene glycols (PEG-200, 300, 400, 600, 4000 and 6000) supported reactions were conducted with cer- tain α, β-unsaturated acids in presence of metal nitrates under mineral acid free and solvent free microwave irra- diated (MWI) and grinding (mortar –pestle) conditions. The aromatic acids underwent nitro decarboxylation and afforded β-nitro styrene derivatives in very good yield while α, β-unsaturated aliphatic carboxylic acids gave corresponding nitro derivatives. Addition of PEG accele- rated the rate of the reaction enormously. Reaction times substantially decreased from several hours to few minu- tes followed by highly significant increase in the produ- ct yield. Among the several PEGs, PEG-300 has been found to be much more effective than other PEGs. 5. Electronic Supplementary Material Elaborated data pertains to nitro decarboxylation of cer- tain α, β-unsaturated acids in presence of metal nitrates under mineral acid free and solvent free MWI and grinding (mortar-pestle) conditions are presented sepa- rately in Tables 5-22, which is furnished as supplemen- tary data. 6. References [1] P. Anastas and J. Warner, “Green Chemistry: Theory and Practice,” Oxford University Press, New York, 1998. [2] L. El Kaïm, L. Gautier, L. Grimaud, L. M. Harwood and V. Michaut, “The Mannich Reaction of Hydrazones: Im- proved Reactivity under Solvent-Free Conditions,” Green Chemistry, Vol. 5, No. 4, 2003, pp. 477-479. doi:10.1039/b306242b [3] G. R. Desiraju and B. S. Goud, “Reactivity of Solids: Pre- sent, Past and Future,” In: V. V. Boldyrev, Ed., Blackwell Sciences, London, 1995, p. 223. [4] K. Tanaka, “Solvent-Free Organic Synthesis,” Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2003. doi:10.1016/S0040-4039(02)01333-3 [5] J. D. Lou and Z. N. Xu, “Selective Oxidation of Primary Alcohols with Chromium Trioxide under Solvent Free Conditions,” Tetrahedron Letters, Vol. 43, No. 35, 2002, pp. 6095-6097. [6] C. L.Raston and J. L. Scott, “Solvent-Free Synthesis of 3- Carboxycoumarins,” Green Chemistry, Vol. 49, No. 5, 2000, pp. 245-247. [7] M. M. Ali, Tasneem, K. C. Rajanna and P. K. Saiprakash, “An Efficient and Facile Synthesis of 2-Chloro-3-formyl Quinolines from Acetanilides in Micellar Media by Vils- meier-HaackCyclisation,” Synlett, Vol. 2, 2001, pp. 251- 253. [8] K. C. Rajanna, M. M. Ali, S. Sana, Tasneem andP. K. Saiprakash, “Ultrasonically Accelerated Vilsmeier-Haack Cyclisation and Formylation Reactions,” Synthetic Comu- nications, Vol. 132, No. 9, 2002, pp. 1351-1356. [9] K. C. Rajanna, M. Moazzam Ali, S. Sana, Tasneem and P. K. Saiprakash, “Vilsmeier Haack Acetylation in Micellar Media: An Efficient One Pot Synthesis of 2-Chloro, 3- acetyl Quinolines,” Journal of Dispersion Science and Technology, Vol. 25, No. 1, 2004, pp. 17-21. doi:10.1081/DIS-120027663 [10] K. C. Rajanna, N. Maasi-Reddy, M. Rajender-Reddy and P. K. Saiprakash, “Micellar Mediated Halo decarboxyla- tion of α, β-Unsaturated Aliphatic and Aromatic Carbox- Copyright © 2011 SciRes. GSC  147 K. C. RAJANNA ET AL. ylic Acids—A Novel Green Hunsdiecker–Borodin Reac- tion,” Journal of Dispersion Science and Technology, Vol. 28, No. 4, 2007, pp. 613-616. doi:10.1080/01932690701282690 [11] S. Sana, Tasneem, M. Moazzam-Ali, K. C. Rajanna and P. K. Saiprakash, “Efficient and Facile Method for the Ni- tration of Aromatic Compounds by Nitric Acid in Mi- cellar Media,” Synthetic Comunications, Vol. 39, No. 10, 2009, pp. 2949-2953. doi:10.1080/00397910802711318 [12] K. C. Rajanna, M. M. Ali, S. Sana and P. K. Saiprakash, “Mild, Efficient and Selective Nitration of Anilides, Non- Activated and Moderately Activated Aromatic Com- pounds with Ammonium Molybdate and Nitric Acid as a New Nitrating Agent,” Chemical Letters, Vol. 1, 2000, pp. 48-49. [13] K. C. Rajanna, M. Moazzam-Ali, S. Sana, Tasneem and P. K. Saiprakash, “Ammonium Nickel Sulphate Mediated Nitration of Aromatic Compounds with Nitric Acid,” Synthetic Comunications, Vol. 31, No. 7, 2001, pp. 1123- 1127. doi:10.1081/SCC-100103545 [14] C. Hunsdiecker and H. Hunsdiecker, “Über den Abbau der Salze aliphatischer Säuren durch Brom Ber,” Chemis- che Berichte, Vol. 75, No. 3, 1942, pp. 291-297. [15] A. Borodin, “Ueber Bromvaleriansäure und Brombutter- säure”. Justus Liebigs Annalen der Chemie, Vol. 119, No. 1, 1861, pp. 121-123. [16] R. G. Johnson and R. K. Ingham, “The Degradation Of Carboxylic Acid Salts By Means Of Halogen The Huns- diecker Reaction”, Chemical Reviews, Vol. 56, 1956, pp. 219-269. doi:10.1021/cr50008a002 [17] D. Naskar and S. Roy, “Catalytic Hunsdiecker Reaction and One-Pot Catalytic Hunsdiecker-Heck Strategy: Syn- thesis of α, β-Unsaturated Aromatic Halides, α-(Dihalome- thyl)benzenemethanols, 5-Aryl-2,4-pentadienoic acids, Die- noates and Dienamides,” Tetrahedron, Vol. 56, No. 10, 2000, pp. 1369-1377. doi:10.1016/S0040-4020(99)01035-2 [18] D. Naskar and S. Roy, “Synthesis of α-Bromo-β-lactam via a Novel Catalytic Hunsdiecker Like Protocol,” Jour- nal of the Chemical Society Perkin Transactions, Vol. 1, No. 17, 1999, pp. 2435-2436. doi:10.1039/a904515e [19] D. Naskar, S. Roy, S. Das, K. L. Giribabu and B. G. Maiya, “Novel Catalytic Hunsdiecker−Heck (CHH) Stra- tegy toward All-E Stereocontrolled Ferrocene-Capped Conjugated Push-Pull Polyenes,” Oraganometallics, Vol. 19, No. 8, 2000, pp. 1464-1469. doi:10.1021/om000020+ [20] S. Roy, C. Guin and G. Maiti, “A Mild and Efficient Me- thod for Oxidative Halodecarboxylation of α, β-Unsatu- rated Aromatic Acids Using Lithium Bromide/Chloride and Ceric Ammonium Nitrate,” Tetrahedron Letters, Vol. 42, No. 52, 2001, pp. 9253-9255. doi:10.1016/S0040-4039(01)01936-0 [21] F. Homsi and G. Rousseau, “Chemistry of Hydrazinopep- tides: A New Hydroperoxydeamination Process,” Tetra- hedron Letters, Vol. 40, No. 8, 1999, pp. 1491-1494. doi:10.1016/S0040-4039(99)00020-9 [22] S. J. Cristol and W. C. Firth, Jr., “A Convenient Synthesis of Alkyl Halides from Carboxylic Acids,” Journal of Organic Chemistry, Vol. 26, No. 1, 1961, pp. 280-280. doi:10.1021/jo01060a628 [23] C. Kuang, H. Senboku and M. Tokuda, “Stereoselective Synthesis of (E)-β-Arylvinyl Halides by Microwave-In- duced Hunsdiecker Reaction,” Synlett, No. 10, 2000, pp. 1439-1442. [24] G. V. Ramanarayanan, V. G. Shukla and K. G. Akamanchi, “A Novel and One Step Procedure for Preparation of α- Bromo-α, β-unsaturated Carbonyl Compounds,” Synlett, No. 12, 2002, pp. 2059-2061. [25] V. N. Telvekar, N. D. Arote and O. P. Herlekar, “Mild and Efficient Method for Decarboxylative Bromination of α, β-Unsaturated Carboxylic Acids with Dess-Martin Pe- riodinane,” Synlett, No. 16, 2005, pp. 2495-2497. doi:10.1055/s-2005-917077 [26] J. P. Das, P. Sinha and S. Roy, “A Nitro-Hunsdiecker Re- action: From Unsaturated Carboxylic Acids to Nitrosty- renes and Nitroarenes,” Organic Letters, Vol. 4, No. 18, 2002, pp. 3055-3058. doi:10.1021/ol0262901 [27] J. M. Rao, A. S. Rao, P. V. Srinivas and K. Suresh-Babu, “An Efficient Synthesis of Conjugated Nitro-Olefins Us- ing Ceric Ammonium Nitrate,” Tetrahedron Letters, Vol. 46, No. 47, 2005, pp. 8141-8143. doi:10.1016/j.tetlet.2005.09.126 [28] S. Ramgopal, K. Ramesh, A. Chakradhar, N. Maasi-Re- ddy and K. C. Rajanna, “Metal Nitrate Driven Nitro Hunsdiecker Reaction with α, β-Unsaturated Carboxylic Acids under Solvent-Free Conditions,” Tetrahedron Let- ters, Vol. 48, No. 23, 2007, pp. 4043-4045. doi:10.1016/j.tetlet.2007.04.026 [29] T. J. Dickerson, N. N. Reed and K. D. Janda, “Soluble Polymers as Scaffolds for Recoverable Catalysts and Re- agents”, Chemical Reviews, Vol. 102, No. 10, 2002, pp. 3325-3344. doi:10.1021/cr010335e [30] B. Das, V. S. Reddy and M. Krishnaiah, “An Efficient Catalyst-Free Synthesis of Thiiranes from Oxiranes Us- ing Polyethylene Glycol as the Reaction Medium,” Tetra- hedron Letters, Vol. 47, No. 48, 2006, pp. 8471-8473. doi:10.1016/j.tetlet.2006.09.153 [31] S. Chandrasekhar, N. R. Reddy, S. S. Sultana, C. Nar- sihmulu and K. V. Reddy, “l-Proline Catalysed Asym- metric Aldol Reactions in PEG-400 as Recyclable Me- dium and Transfer Aldol Reactions,” Tetrahedron, Vol. 62, No. 2-3, 2006, 334-338. doi:10.1016/j.tet.2005.09.122 [32] J. -H. Li, X. -C. Hu, Y. Liang and Y. -X. Xie, “PEG-400 Promoted Pd(OAc)2/DABCO-Catalyzed Cross-Coupling Reactions in Aqueous Media,” Tetrahedron, Vol. 62, No. 1, 2006, pp. 31-38. doi:10.1016/j.tet.2005.09.138 [33] S. Chandrasekhar, C. Narsihmulu, B. Saritha and S. S. Sulthana, “Poly(ethyleneglycol) (PEG): A Rapid and Re- cyclable Reaction Medium for the DABCO-Catalyzed Baylis-Hillman Reaction,” Tetrahedron Letters, Vol. 45, No. 30, 2004, pp. 5865-5867. doi:10.1016/j.tetlet.2004.05.153 [34] B. Das, M. Krishnaiah, P. Thirupathi and K. Laxminara- yana, “An Efficient Catalyst-Free Regio- and stereoselec- Copyright © 2011 SciRes. GSC  K. C. RAJANNA ET AL. Copyright © 2011 SciRes. GSC 148 tive Ring-Opening of Epoxides with Phenoxides Using Polyethylene Glycol as the Reaction Medium,” Tetrahe- dron Letters, Vol. 48, No. 24, 2007, pp. 4263-4265. doi:10.1016/j.tetlet.2007.04.062 [35] A. I. Vogel, “Text Book of Practical Organic Chemistry,” 4th Edition, Longman, London and New York, 1986. [36] M. J. Thompson and P. Zeegers, “A Theoretical Study on the Two-Phase Nitration of Phenols,” Tetrahedron, Vol. 45, No. 1, 1989, pp. 191-202. doi:10.1016/0040-4020(89)80046-8 [37] D. Gaudea, R. Le Goallera and J. L. Pierrea, “Nitration of Phenols with Nitrates in a Two-Phase System,” Synthetic Comunications, Vol. 16, No. 1, 1986, pp. 63-68. doi:10.1080/00397918608057689 [38] G. Kaupp, “Mechanochemistry: The Varied Applications of Mechanical Bond-Breaking,” CrystEngCommunity, Vol. 11, No. 3, 2009, pp. 388-403. doi:10.1039/b810822f
|