 Advances in Biological Chemistry, 2011, 1, 93-102 doi:10.4236/abc.2011.13011 Published Online November 2011 (http://www.SciRP.org/journal/abc/ ABC ). Published Online November 2011 in SciRes. http://www.scirp.org/journal/ABC Garlic (Allium sativum) modulates the expression of angiotensin II AT2 receptor in adrenal and renal tissues of streptozotocin-induced diabetic rats Mohamed H. Mansour*, Khaled Al-Qa tta n, Marth a Thomson, Musli m Ali Department of Biological Sciences, Faculty of Science, Kuwait University, Kuwait City, Kuwait. Email: *mohamed.shaker@ku.edu.kw Received 14 September 2011; revised 19 October 2011; accepted 27 October 2011. ABSTRACT The loss of balance between the antagonistic activities of angiotensin II AT1/AT2 receptors has been impli- cated as a major mediator in the development of hy- pertension and progressive nephropathy in experi- mental diabetes. The present study was designed to investigate the potential of garlic to modulate the level of expression of the AT2 receptor in the adrenal and renal tissues of diabetic rats. Three groups of rats were studied after 8 weeks following diabetes induction: normal, streptozotocin-induced diabetic (control diabetic), and garlic-treated diabetic rats. A polyclonal antibody of proven specificity to the AT2 receptor, as verified by Western blotting and emplo- yed in immunohistochemical assays, indicated that compared to normal rats, the highest adrenocortical AT2 receptor expression was significantly shifted from the zona glomerulosa to the zona fasciculate/ reticu- laris, and was significantly reduced in adrenomedul- lary chromaffin cells of control diabetic rats. In the kidney, STZ treatments were associated with a signi- ficant decrease in AT2 receptor expression throughout glomeruli and all cortical and medullary tubular segments. Compared to control diabetic rats, the la- beling of the AT2 receptor in the garlic-treated dia- betic group was restored among adrenocortical zona glomerulosa cells and adrenomedullary chromaffin cells and significantly reduced in the zona fasiculata, and was also restored in glomeruli and throughout renal cortical and medullary tubular segments, to le- vels comparable to those observed in normal rats. The capacity of garlic to modulate diabetes-induced AT2 receptor down-regulation may be implicated in restoring the recuperative processes mediated by AT2 receptors, which interfere with the development of hypertensi on and nephropathy. Keywords: AT 2 Receptor; Garlic; Streptozotocin-Induced Diabetes; Immunohistochemistry 1. INTRODUCTION The renin-angiotensin-aldosterone system (RAAS) is a major regulator of blood pressure and sodium and water homeostasis [1]. The octapeptide angiotensin II (Ang II) is the primary mediator of the RAAS effects. Ang II in- duces its effects by binding to two major receptor types, AT1 and AT2 [2]. The AT1 receptors are ubiquitously ex- pressed in adult tissues and mediate Ang II-induced vasoconstriction, sodium reabsorption, aldosterone se- cretion, and cell growth and proliferation [3-5]. The AT2 receptors are expressed abundantly during fetal devel- opment, decline after birth and become minimally rep- resented, in comparison with AT1 receptors, in various adult tissues [6]. In these tissues, AT2 receptors target Ang II-induced vasodilatation, apoptosis, antiprolifera- tion, natriuresis and fluid/sodium homeostasis [7-9] and, therefore, can be considered as functional antagonists to the AT1 receptors [10]. Compelling evidence suggest that the alteration in the expression and function of either Ang II receptor type may be the site of regulation that affects the initiation and progression of tissue remodel- ing in pathophysiological conditions [11-12]. As manifested in diabetic patients and animal models, the hyperglycemia-induced AT1 receptor up-regulation in the adrenal, kidney and other tissues mediates the in- crease of Ang II local effects in stimulating aldosterone production and over-activating renal sodium transporters causing sodium retention and hypertension [12-14]. Within the kidney, the up-regulated AT1 receptor signal- ing increases vascular resistance, glomerular capillary pressure and mechanical stretch-induced glomerular injury, and stimulates production of reactive oxygen species and extracellular matrix in the mesangium and tubulo-interstitium [2,14-16], which collectively perpe- trates the development of diabetic nephropathy. Interest- ingly, early diabetes exerts an opposite influence on AT2 receptor and significantly decreases its expression in glomeruli and tubular segments of the kidney [17]. The  M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 94 down-regulation of intrarenal AT2 receptor expression implies the subsequent decrease in AT2-mediated me- chanisms that may oppose the detrimental effects of AT1 receptor signaling, including inhibition of cell growth [18,19] as well as NO/cGMP-dependent vasodilatation and inhibition of renal sodium transporters [20-22]. This could result in an amplification of AT1-mediated effects on vasoconstriction, glomerulosclerosis or cell hyper- trophy, and thus contribute further to progressive injury in diabetic nephropathy [17,23]. Current views suggest that pharmacologically active blockers of the RAAS may be beneficial in the man- agement of diabetic complications including hyperten- sion and nephropathy [24,25]. Angiotensin receptor block- ers and angiotensin-converting enzyme inhibitors are used to treat hypertension and to improve renal function in diabetes [26]. Based on the suggestion that a balance between AT1- and AT2-receptor-mediated cell-signaling events may be a determinant of progression rate in dia- betic nephropathy [17], a debate of preferential use of angiotensin receptor blockers over angiotensin-conver- ting enzyme inhibitors have been initiated. The basis of such preference lies in that angiotensin-converting en- zyme inhibitors lower Ang II production, leading to re- duction in the functions of AT1 and AT2 receptors, whereas angiotensin receptor blockers selectively block AT1 receptors and leave the AT2 receptors unopposed to function [26]. Apparently, restoration of AT2 receptor activity, in early diabetes, may add more therapeutic efficacy in protecting against the development of renal morphologic and functional changes seen during the pro- gression of hypertension and nephropathy. Currently, the reliance on natural products is gaining popularity in combating various physiological threats, including diabetic complications, associated with the dysregulation of the RAAS [27]. In experimental diabe- tes, garlic, used either as a whole raw extract or as iso- lated organosulphur constituents, is one of the best stud- ied herbal remedies with documented anti-diabetic ac- tivities [28-32]. These activities include lowering serum glucose and cholesterol levels [30,31,33], inhibiting Ang II and promoting vasodilatation [29], preventing adrenal hypertrophy [34], attenuating oxidative stress [35], in- hibiting the formation of advanced glycation endpro- ducts [36], ameliorating hypertension and delaying the progression of diabetic nephropathy [31,32,35,36]. No- netheless, the potential of garlic in targeting the level of expression of Ang II receptors is, as yet, not fully ex- plored. In a recent study, we reported on the efficacy of an aqueous extract of raw garlic in modulating the up- regulated expression of Ang II AT1 receptors in the ad- renal and renal tissues in early diabetes [37]. In the pre- sent study, immunohistochemical evidence is presented for the capacity of garlic treatments to modulate the down-regulated expression of Ang II AT2 receptors in adrenal and renal tissues of streptozotocin-induced dia- betic rats. 2. MATERIALS AND METHODS 2.1. Antibodies and Reagents Except where noted, all chemicals were reagent grade and purchased from Sigma Chemical Company (St. Louis, MO, USA). Polyclonal anti-AT2 antibody (sc- 7421, rabbit IgG specific to an epitope mapping within the N-terminal extracellular domain of the human AT2 polypeptide) and peroxidase-conjugated goat anti-rabbit IgG antibody were purchased from Santa Cruz Biotech- nology, Inc. (Santa Cruz, Ca, USA). Gel electrophoresis reagents, peroxidase-conjugated molecular weight stan- dards and nitrocellulose membranes (0.45 ) were ob- tained from BioRad (Richmond, CA, USA). An eight amino acid peptide, corresponding to amino acids 10 - 17 (Thr-Ser-Arg-Asn-Ile-Thr-Ser-Ser) of the first extra- cellular domain, as deduced from the published AT2 re- ceptor cDNA sequence [38], was synthesized manually on the base-labile linker 4-(hydroxymethyl)-benzoyloxy- methyl and supplied by The Protein/DNA Technology Center (The Rockefeller University, NY, USA). The oc- tapeptide was conjugated to bovine serum albumin (BSA) and coupled to CNBr-activated Sepharose 4B as descri- bed previously [39]. An aqueous garlic extract (500 mg/ ml) was prepared from locally purchased, peeled garlic cloves by homogenization in cold, sterile 0.9% NaCl for 12 min followed by filtration and centrifugation at 200 g for 10 min to remove insolubles as previously described [30]. 2.2. Animals Adult male Sprague-Dawley (SD) rats (England) weigh- ing 150 - 200 gm were bred and raised at the animal house of the Department of Biological Sciences, Kuwait University, and used in the present investigation. Rats were given standard laboratory chow (170 mMol Na+/kg) and water ad libitum and kept under standard conditions (23˚C ± 2˚C, 12 h light, 12 h darkness). Diabetic rats, induced by injecting streptozotocin (STZ, 60 mg/kg) intraperitoneally into overnight fasting rats, were deter- mined to be diabetic if they had elevated plasma glucose concentrations ≥ 300 mg/dL five days post-injection, as described previously [40]. STZ-induced diabetic rats were divided into two groups (n = 8): group 1, the con- trol diabetic group, received daily intra-peritoneal injec- tions with saline, and group 2, the garlic-treated group, received daily intraperitoneal injections with 500 mg/kg of the garlic extract. All experiments were carried out at 8 weeks after diabetes induction. All animal procedures C opyright © 2011 SciRes. ABC  M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 95 were performed according to the guidelines for animal experimentation of Kuwait University, Faculty of Sci- ence. 2.3. Solubilization of Cell-Membranes and Endoglycosidase Tr eatments Left adrenal glands and kidneys, excised from anesthe- tized normal rats, were individually homogenized, solu- bilized and extracted in 10 mM Tris/HCl, pH 8.0 con- taining 2 mM phenylmethylsulfonyl fluoride and 2% deoxycholate by an automatic homogenizer followed by sonic disruption and stirring at room temperature for 2 h and three cycles of freezing at –20˚C and thawing at room temperature. Solubilized cell-membrane lysates were recovered in supernatants, following centrifugation at 100.000 g for 1 h, and their protein content deter- mined [41] using bovine serum albumin (BSA) in the same buffer as a standard. Cell-membrane lysates of both organs (120 μg protein) were separately precipi- tated with 20% trichloroacetic acid and ice-cold acetone for 1 h at –20˚C, washed for 1 h with acetone at –20˚C and reconstituted in 50 μl of 100 mM sodium phosphate, pH 6.1, 50 mM EDTA, 1% Nonidet P-40 containing 20 mU of Endo-F (Endo-ß-N-acetylglucosaminidase F, from Flavobacterium meningosepticum, 600 U/mg, Sigma Chem. Comp., St. Louis, MO). Samples were incubated for 18 h at 37˚C, precipitated with equal volume of 20% trichloroacetic acid, washed with cold acetone and dried under nitrogen gas before analysis by polyacrylamide gel electrophoresis. Control samples were similarly trea- ted but in the absence of Endo-F. 2.4. We s tern Blotting Aliquots of solubilized cell-membrane lysates (120 g protein) collected from adrenal and/or kidney tissues of normal SD rats, and either kept untreated or treated with Endo-F, were individually precipitated, reconstituted in sample buffer and resolved with 10% resolving gels, under non-reducing or reducing conditions, by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SD- S-PAGE) as described by Laemmli [42]. Resolved pro- tein samples were electrophoretically transblotted to 0.45 nitrocellulose membranes at 100 V (constant voltage) for 1.5 h at 4˚C in electrophoretic transfer buffer, pH 8.3 using a Bio Rad Mini Trans-Blot electrophoretic transfer cell. Nitrocellulose membranes were washed 3 times with 200 mM PBS, pH 7.2 containing 0.05% Tween 40 (PBS-Tween buffer), each for 15 min with constant agitation and nonspecific binding sites blocked by incubation for 1 h in blocking buffer (3% BSA in PBS-Tween buffer, pH 7.2). Membranes were then washed thrice in PBS-Tween buffer, pH 7.2 and subse- quently probed by the anti-AT2 receptor antibody (di- luted to 1:500 in PBS-Tween buffer, pH 7.2) by incuba- tion for 2 h at room temperature and then overnight at 4˚C with constant agitation. Control blots were prepared by substituting the specific antibody with an equal vol- ume of the anti-AT2 antibody preabsorbed with an AT2 octapeptide/BSA complex-coated CNBr-activated Sep- harose 4B beads. Following the incubation time, mem- branes were washed thrice in PBS-Tween buffer, pH 7.2, treated for 1 h at room temperature with peroxidase-con- jugated goat anti-rabbit IgG antibody (diluted to 1:1000 in PBS-Tween buffer, pH 7.2) and the reactions visualized by treatments with metal-enhanced 3,3-diaminobenzidine (DAB) tablets reconstituted in distilled water. Mem- branes were allowed to air-dry, photographed and rela- tive molecular weights estimated using peroxidase-con- jugated Bio Rad broad range molecular weight standards, which were analyzed under identical conditions in par- allel to the protein samples. 2.5. Preparation of Tissue Sections Left adrenal glands and kidneys of anesthetized normal, control diabetic and/or garlic-treated diabetic rats were individually excised and placed in vials containing 3 ml of Bouin’s fixative for 24 h - 48 h at room temperature. The tissues were carried through a routine paraffin em- bedding technique, which included dehydration through a series of ethanol concentrations 50%, 70%, 90% and 100%, clearing in toluene, embedding in paraffin wax, and finally sectioning of the paraffin blocks into 3 m - 4 m thick sections on a rotary microtome. The sections were picked up on clean slides after spreading them in a water bath at 40˚C. The slides were air-dried to be used for subsequent staining. 2.6. Labeling of Tissue Sections with Anti-AT2 Antibody Tissue sections were examined for AT2 receptor distribu- tion by an indirect immunohistochemical labeling tech- nique. Tissue sections were de-waxed in xylene, hy- drated with a series of 90%, 75% and 60% ethanol and washed with PBS, pH 7.2. Sections were quenched by the addition of 10% normal goat serum and 0.3% hy- drogen peroxide in PBS, pH 7.2 for 1 h and then indi- vidually labeled for 45 min in a humidified chamber with 300 l of the anti-AT2 receptor antibody (diluted to 1:100 with PBS, pH, 7.2). After several washes with 200 l PBS, pH 7.2, the sections were incubated for 45 min with 300 l of peroxidase-conjugated goat anti-rabbit IgG antibody (diluted to 1:200 in PBS, pH 7.2), followed by a 10-min treatment with 400 l of fast DAB recon- stituted in water. All sections were counter stained with 100 l haematoxylin (Gill #1) for 1 min and examined by light microscopy for positive labeling of cells ex- pressing AT2 receptors. Control sections were identically stained by replacing the specific antibody with anti-AT2 C opyright © 2011 SciRes. ABC 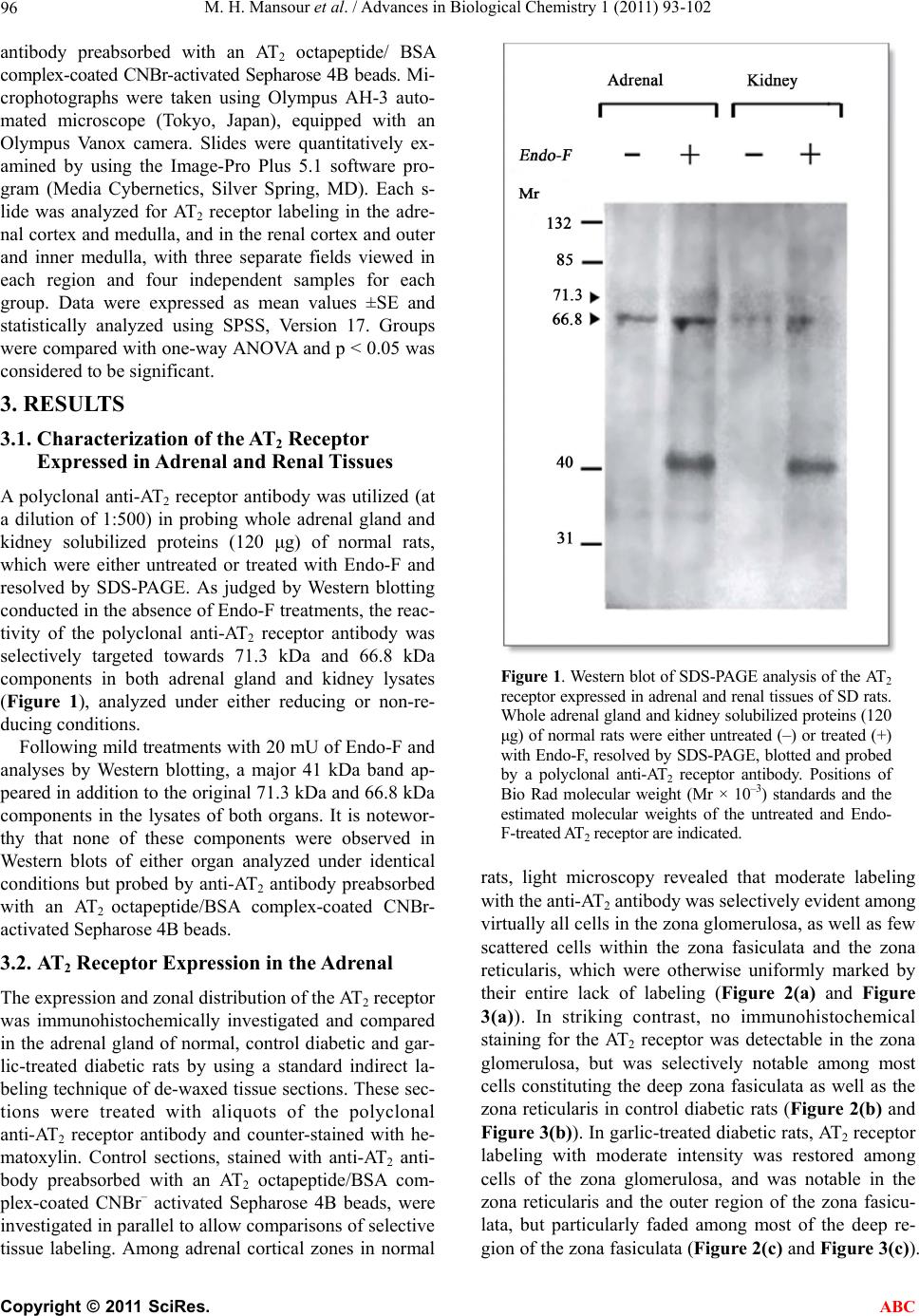 M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 96 antibody preabsorbed with an AT2 octapeptide/ BSA complex-coated CNBr-activated Sepharose 4B beads. Mi- crophotographs were taken using Olympus AH-3 auto- mated microscope (Tokyo, Japan), equipped with an Olympus Vanox camera. Slides were quantitatively ex- amined by using the Image-Pro Plus 5.1 software pro- gram (Media Cybernetics, Silver Spring, MD). Each s- lide was analyzed for AT2 receptor labeling in the adre- nal cortex and medulla, and in the renal cortex and outer and inner medulla, with three separate fields viewed in each region and four independent samples for each group. Data were expressed as mean values ±SE and statistically analyzed using SPSS, Version 17. Groups were compared with one-way ANOVA and p < 0.05 was considered to be significant. 3. RESULTS 3.1. Characterization of the AT2 Receptor Expressed in Adrenal and Renal Tissues A polyclonal anti-AT2 receptor antibody was utilized (at a dilution of 1:500) in probing whole adrenal gland and kidney solubilized proteins (120 μg) of normal rats, which were either untreated or treated with Endo-F and resolved by SDS-PAGE. As judged by Western blotting conducted in the absence of Endo-F treatments, the reac- tivity of the polyclonal anti-AT2 receptor antibody was selectively targeted towards 71.3 kDa and 66.8 kDa components in both adrenal gland and kidney lysates (Figure 1), analyzed under either reducing or non-re- ducing conditions. Following mild treatments with 20 mU of Endo-F and analyses by Western blotting, a major 41 kDa band ap- peared in addition to the original 71.3 kDa and 66.8 kDa components in the lysates of both organs. It is notewor- thy that none of these components were observed in Western blots of either organ analyzed under identical conditions but probed by anti-AT2 antibody preabsorbed with an AT2 octapeptide/BSA complex-coated CNBr- activated Sepharose 4B beads. 3.2. AT2 Receptor Expression in the Adrenal The expression and zonal distribution of the AT2 receptor was immunohistochemically investigated and compared in the adrenal gland of normal, control diabetic and gar- lic-treated diabetic rats by using a standard indirect la- beling technique of de-waxed tissue sections. These sec- tions were treated with aliquots of the polyclonal anti-AT2 receptor antibody and counter-stained with he- matoxylin. Control sections, stained with anti-AT2 anti- body preabsorbed with an AT2 octapeptide/BSA com- plex-coated CNBr– activated Sepharose 4B beads, were investigated in parallel to allow comparisons of selective tissue labeling. Among adrenal cortical zones in normal Figure 1. Western blot of SDS-PAGE analysis of the AT2 receptor expressed in adrenal and renal tissues of SD rats. Whole adrenal gland and kidney solubilized proteins (120 μg) of normal rats were either untreated (–) or treated (+) with Endo-F, resolved by SDS-PAGE, blotted and probed by a polyclonal anti-AT2 receptor antibody. Positions of Bio Rad molecular weight (Mr × 10–3) standards and the estimated molecular weights of the untreated and Endo- F-treated AT2 receptor are indicated. rats, light microscopy revealed that moderate labeling with the anti-AT2 antibody was selectively evident among virtually all cells in the zona glomerulosa, as well as few scattered cells within the zona fasiculata and the zona reticularis, which were otherwise uniformly marked by their entire lack of labeling (Figure 2(a) and Figure 3(a)). In striking contrast, no immunohistochemical staining for the AT2 receptor was detectable in the zona glomerulosa, but was selectively notable among most cells constituting the deep zona fasiculata as well as the zona reticularis in control diabetic rats (Figure 2(b) and Figure 3(b)). In garlic-treated diabetic rats, AT2 receptor labeling with moderate intensity was restored among cells of the zona glomerulosa, and was notable in the zona reticularis and the outer region of the zona fasicu- lata, but particularly faded among most of the deep re- gion of the zona fasiculata (Figure 2(c) and Figure 3(c)). C opyright © 2011 SciRes. ABC 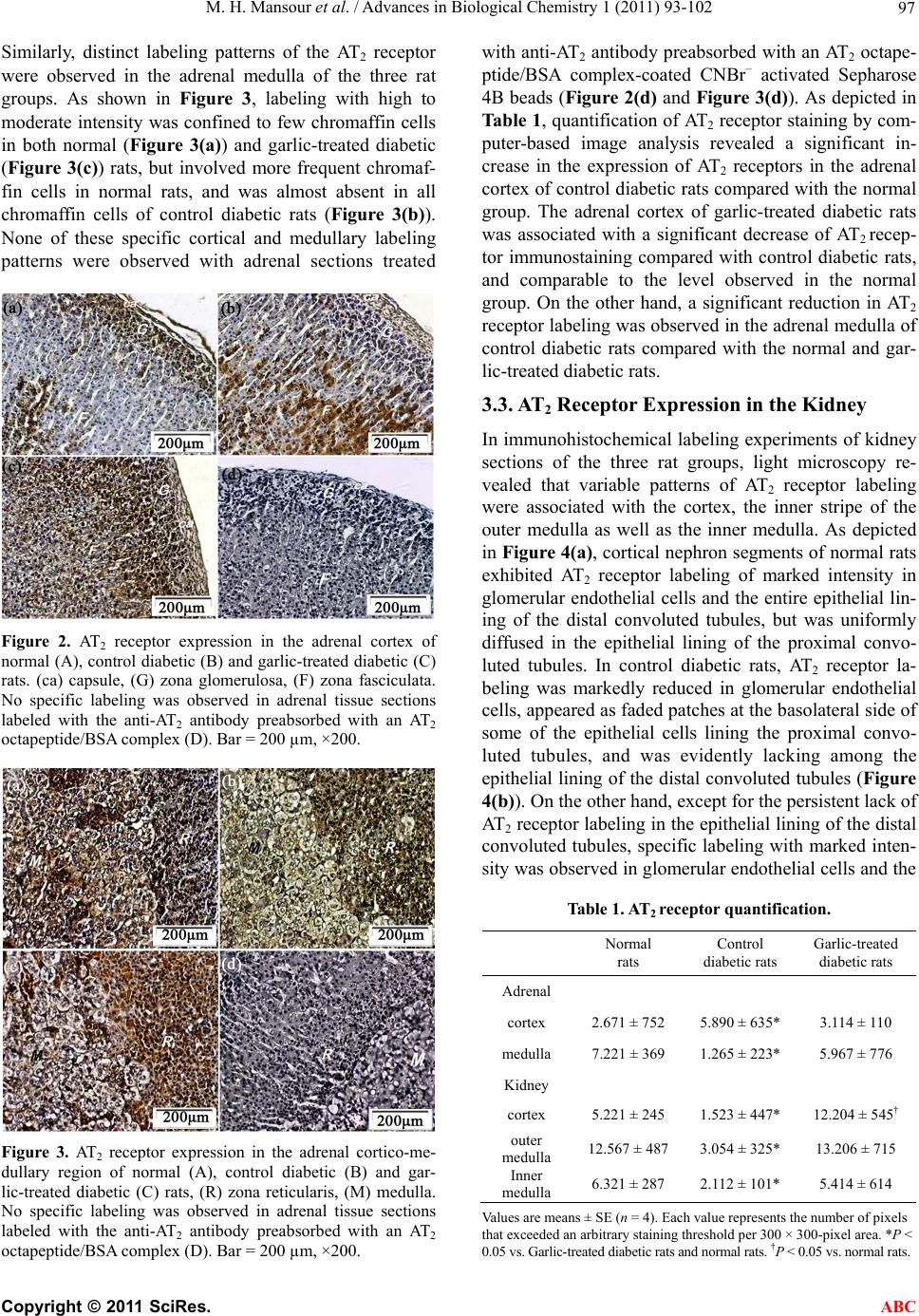 M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 97 Similarly, distinct labeling patterns of the AT2 receptor were observed in the adrenal medulla of the three rat groups. As shown in Figure 3, labeling with high to moderate intensity was confined to few chromaffin cells in both normal (Figure 3(a)) and garlic-treated diabetic (Figure 3(c)) rats, but involved more frequent chromaf- fin cells in normal rats, and was almost absent in all chromaffin cells of control diabetic rats (Figure 3(b)). None of these specific cortical and medullary labeling patterns were observed with adrenal sections treated Figure 2. AT2 receptor expression in the adrenal cortex of normal (A), control diabetic (B) and garlic-treated diabetic (C) rats. (ca) capsule, (G) zona glomerulosa, (F) zona fasciculata. No specific labeling was observed in adrenal tissue sections labeled with the anti-AT2 antibody preabsorbed with an AT2 octapeptide/BSA complex (D). Bar = 200 µm, ×200. Figure 3. AT2 receptor expression in the adrenal cortico-me- dullary region of normal (A), control diabetic (B) and gar- lic-treated diabetic (C) rats, (R) zona reticularis, (M) medulla. No specific labeling was observed in adrenal tissue sections labeled with the anti-AT2 antibody preabsorbed with an AT2 octapeptide/BSA complex (D). Bar = 200 µm, ×200. with anti-AT2 antibody preabsorbed with an AT2 octape- ptide/BSA complex-coated CNBr– activated Sepharose 4B beads (Figure 2(d) and Figure 3(d)). As depicted in Table 1, quantification of AT2 receptor staining by com- puter-based image analysis revealed a significant in- crease in the expression of AT2 receptors in the adrenal cortex of control diabetic rats compared with the normal group. The adrenal cortex of garlic-treated diabetic rats was associated with a significant decrease of AT2 recep- tor immunostaining compared with control diabetic rats, and comparable to the level observed in the normal group. On the other hand, a significant reduction in AT2 receptor labeling was observed in the adrenal medulla of control diabetic rats compared with the normal and gar- lic-treated diabetic rats. 3.3. AT2 Receptor Expression in the Kidney In immunohistochemical labeling experiments of kidney sections of the three rat groups, light microscopy re- vealed that variable patterns of AT2 receptor labeling were associated with the cortex, the inner stripe of the outer medulla as well as the inner medulla. As depicted in Figure 4(a), cortical nephron segments of normal rats exhibited AT2 receptor labeling of marked intensity in glomerular endothelial cells and the entire epithelial lin- ing of the distal convoluted tubules, but was uniformly diffused in the epithelial lining of the proximal convo- luted tubules. In control diabetic rats, AT2 receptor la- beling was markedly reduced in glomerular endothelial cells, appeared as faded patches at the basolateral side of some of the epithelial cells lining the proximal convo- luted tubules, and was evidently lacking among the epithelial lining of the distal convoluted tubules (Figure 4(b)). On the other hand, except for the persistent lack of AT2 receptor labeling in the epithelial lining of the distal convoluted tubules, specific labeling with marked inten- sity was observed in glomerular endothelial cells and the Table 1. AT2 receptor quantification. Normal rats Control diabetic rats Garlic-treated diabetic rats Adrenal cortex 2.671 ± 752 5.890 ± 635* 3.114 ± 110 medulla 7.221 ± 369 1.265 ± 223* 5.967 ± 776 Kidney cortex 5.221 ± 245 1.523 ± 447* 12.204 ± 545† outer medulla 12.567 ± 487 3.054 ± 325* 13.206 ± 715 Inner medulla 6.321 ± 287 2.112 ± 101* 5.414 ± 614 Values are means ± SE (n = 4). Each value represents the number of pixels that exceeded an arbitrary staining threshold per 300 × 300-pixel area. *P < 0.05 vs. Garlic-treated diabetic rats and normal rats. †P < 0.05 vs. normal rats. C opyright © 2011 SciRes. ABC 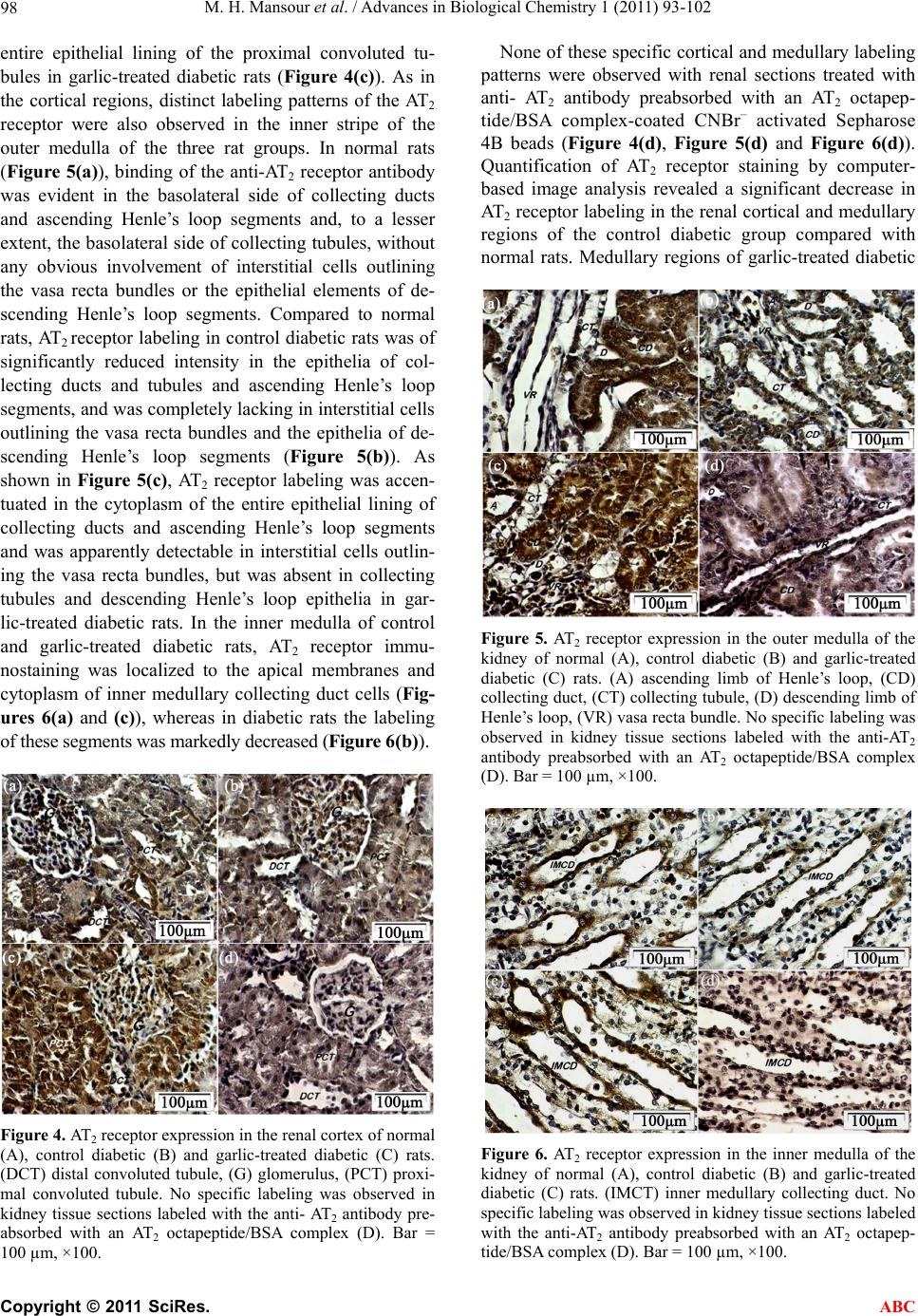 M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 98 entire epithelial lining of the proximal convoluted tu- bules in garlic-treated diabetic rats (Figure 4(c)). As in the cortical regions, distinct labeling patterns of the AT2 receptor were also observed in the inner stripe of the outer medulla of the three rat groups. In normal rats (Figure 5(a)), binding of the anti-AT2 receptor antibody was evident in the basolateral side of collecting ducts and ascending Henle’s loop segments and, to a lesser extent, the basolateral side of collecting tubules, without any obvious involvement of interstitial cells outlining the vasa recta bundles or the epithelial elements of de- scending Henle’s loop segments. Compared to normal rats, AT2 receptor labeling in control diabetic rats was of significantly reduced intensity in the epithelia of col- lecting ducts and tubules and ascending Henle’s loop segments, and was completely lacking in interstitial cells outlining the vasa recta bundles and the epithelia of de- scending Henle’s loop segments (Figure 5(b)). As shown in Figure 5(c), AT2 receptor labeling was accen- tuated in the cytoplasm of the entire epithelial lining of collecting ducts and ascending Henle’s loop segments and was apparently detectable in interstitial cells outlin- ing the vasa recta bundles, but was absent in collecting tubules and descending Henle’s loop epithelia in gar- lic-treated diabetic rats. In the inner medulla of control and garlic-treated diabetic rats, AT2 receptor immu- nostaining was localized to the apical membranes and cytoplasm of inner medullary collecting duct cells (Fig- ures 6(a) and (c)), whereas in diabetic rats the labeling of these segments was markedly decreased (Figure 6(b)). Figure 4. AT2 receptor expression in the renal cortex of normal (A), control diabetic (B) and garlic-treated diabetic (C) rats. (DCT) distal convoluted tubule, (G) glomerulus, (PCT) proxi- mal convoluted tubule. No specific labeling was observed in kidney tissue sections labeled with the anti- AT2 antibody pre- absorbed with an AT2 octapeptide/BSA complex (D). Bar = 100 µm, ×100. None of these specific cortical and medullary labeling patterns were observed with renal sections treated with anti- AT2 antibody preabsorbed with an AT2 octapep- tide/BSA complex-coated CNBr– activated Sepharose 4B beads (Figure 4(d), Figure 5(d) and Figure 6(d)). Quantification of AT2 receptor staining by computer- based image analysis revealed a significant decrease in AT2 receptor labeling in the renal cortical and medullary regions of the control diabetic group compared with normal rats. Medullary regions of garlic-treated diabetic Figure 5. AT2 receptor expression in the outer medulla of the kidney of normal (A), control diabetic (B) and garlic-treated diabetic (C) rats. (A) ascending limb of Henle’s loop, (CD) collecting duct, (CT) collecting tubule, (D) descending limb of Henle’s loop, (VR) vasa recta bundle. No specific labeling was observed in kidney tissue sections labeled with the anti-AT2 antibody preabsorbed with an AT2 octapeptide/BSA complex (D). Bar = 100 µm, ×100. Figure 6. AT2 receptor expression in the inner medulla of the kidney of normal (A), control diabetic (B) and garlic-treated diabetic (C) rats. (IMCT) inner medullary collecting duct. No specific labeling was observed in kidney tissue sections labeled with the anti-AT2 antibody preabsorbed with an AT2 octapep- tide/BSA complex (D). Bar = 100 µm, ×100. C opyright © 2011 SciRes. ABC  M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 99 rats were marked by levels of AT2 receptor labeling that were significantly higher than the control diabetic group and comparable to those observed in normal rats. In the cortical region, AT2 receptor labeling of garlic-treated diabetic rats was significantly higher than the normal and control diabetic groups (Table 1). 4. DISCUSSION Garlic has a wide range of therapeutic applications. In experimental diabetes, sulfur-containing and non-sulfur constituents of garlic work synergistically to exhibit an- tithrombotic, antioxidant, hypocholesterolaemic, hypo- glycaemic as well as hypotensive potentials, which col- lectively ameliorate the development and progression of diabetic complications, including nephropathy and hy- pertension [28-36]. Nonetheless, neither the precise mechanisms that underlie the anti-diabetic potentials of garlic nor the nature of the key receptor system(s) tar- geted, are fully understood. In hypertensive diabetic rats, altered gene and protein expression of the Ang II AT1 and AT2 receptor types and the loss of balance between their antagonistic activities, in renal and other tissues, have been directly implicated in the development of early changes associated with diabetic nephropathy [2,4, 5,12,14,17,21]. In a recent report, the remarkable capac- ity of garlic treatments to modulate the upregulated ex- pression of AT1 receptors, associated with hypersecretion of aldosterone and the impairment of renal vascular and tubular functions in early diabetes, has been demon- strated [37]. The present study was designed to investi- gate the level of expression of the AT2 receptor in the adrenal and renal tissues of normal, control diabetic and garlic-treated diabetic rats. In the present study, AT2 receptor expression in adre- nal and renal tissues was investigated using a polyclonal anti-AT2 antibody of proven specificity to an epitope mapping within the N-terminal extracellular domain of the AT2 polypeptide [43]. In Western blots, this antibody was selectively targeted towards 71.3 kDa and 66.8 kDa components in both adrenal gland and kidney lysates, which were in direct agreement with the estimated mo- lecular weight of glycosylated AT2 receptors previously detected in rat and other mammalian tissues [17,44,45]. Also consistent with the established presence of five highly-conserved N-linked glycosylation sites along the AT2 receptor sequence [45], mild enzymatic deglycosy- lation treatments resulted in the detection of a lower molecular weight component of 41 KDa in both organs, which corresponded exactly to the predicted mass of the deglycosylated protein back-bone deduced from the cDNA sequence of the AT2 receptor [38,46]. In normal rats, the binding of the anti-AT2 antibody was immuno- histochemically demonstrable in the zona glomerulosa of the adrenal cortex and scattered chromaffin cells of the adrenal medulla and in glomeruli and proximal and dis- tal convoluted tubules in the renal cortex, collecting tu- bules and ducts and ascending Henle’s loop segments in the inner stripe of the outer renal medulla, as well as inner renal medullary collecting ducts. These selective labeling patterns, observed in adrenal and renal tissues, were all in accordance with AT2 receptor distribution in both organs as previously determined by immunohisto- chemical, in vitro autoradiographic localization and in situ hybridization techniques [6,17,47,48]. None of the glycosylated or deglycosylated forms of the receptor observed in Western blots or the selective immunohisto- chemical labeling patterns were detectable when the antibody was preabsorbed with an AT2 octapeptide, cor- responding to amino acids 10 - 17 of the first extracellu- lar domain of the receptor polypeptide, thus establishing the specificity of the polyclonal antibody in selectively binding the AT2 receptor in adrenal and renal tissues. Confirming the reported loss of balance in the levels of AT1 and AT2 receptor expression in early diabetes [2, 4,5,12,14,17,21], STZ treatments in the present study were paralleled by quantitative and qualitative altera- tions in the pattern of AT2 receptor expression in both the adrenal gland and the kidney. Compared to normal rats, the highest adrenocortical AT2 receptor expression was significantly shifted from the zona glomerulosa to the zona fasciculate/reticularis, and was significantly re- duced in adrenomedullary chromaffin cells of STZ- treated, control diabetic rats. In this rat group, the reduc- tion of AT2 receptor expression in the zona glomerulosa was in direct contrast to the increase in AT1 receptor ex- pression observed in this zone in an earlier report [37] and may imply a decrease in AT2-mediated inhibition of cell growth [18,19] and the amplification of AT1-medi- ated effects on cell hypertrophy and aldosterone synthe- sis and release [2-5], leading to electrolyte imbalance and the development of hypertension. The significant reduction of AT2 receptor expression in adrenomedullary chromaffin cells may be also implicated in a possible loss of the synergistic AT1/AT2 receptor cross-talk sug- gested to regulate basal and stress-induced tyrosine hy- droxylase transcription rates [49], and thereby leading to dysregulation of adrenomedullary catecholamine secre- tions. Interestingly, the up-regulatory shift in AT2 recep- tor expression in the zona fasiculata/reticularis, which was also observed earlier for AT1 receptor expression [37], has been consistently observed with either receptor type under various abnormal physiological and patho- physiological conditions including dietary sodium re- striction, sodium loading, stimulated levels of aldoster- one or Ang II, and water deprivation [47,50-52]. Al- though the exact functional significance of AT1/AT2 re- ceptor perturbations in these zones is still to be eluci- dated, their altered expression may represent a signifi- C opyright © 2011 SciRes. ABC  M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 100 cant adaptive factor in adrenal tissue remodeling under various pathological conditions, including early diabetes. In the kidney, the present study also revealed that STZ treatments were associated with a significant decrease in AT2 receptor expression throughout all nephronal seg- ments including glomeruli and proximal and distal con- voluted tubules in the cortex, collecting tubules and ducts and ascending Henle’s loop segments in the inner stripe of the outer medulla, as well as inner medullary collecting ducts. The observed decrease in total intrare- nal expression of AT2 receptors is in direct accordance with previous observations in the same model of ex- perimental diabetes [17], and contrasts the documented effects of STZ treatments in upregulating intrarenal ex- pression of AT1 receptors [1,2,4,11,12,24,26,37]. Thus, in early diabetes, the expected decline in AT2-mediated mechanisms may potentiate the pathological augmenta- tion of intrarenal AT1-mediated activities promoting so- dium retention and hypertension, glomerulosclerosis and proteinuria, tubulo-interstitial cell hypertrophy and hy- perplasia associated with stimulation of extracellular matrix production, which are the hallmark of diabetic nephropathy [2,12-16]. A major observation of the present investigation was the significant modulation of the AT2 receptor expressed in adrenal and renal tissues of garlic-treated diabetic rats. Compared to the control diabetic rats, AT2 receptor ex- pression was restored among adrenocortical zona glomerulosa cells and adrenomedullary chromaffin cells and significantly reduced in the zona fasiculata of gar- lic-treated diabetic rats. In the renal tissues of this rat group, AT2 receptor expression was also significantly restored in glomeruli and throughout renal cortical and medullary tubular segments, to levels comparable to those observed in normal rats. Along with the reported efficacy of garlic treatments in reducing diabetes-in- duced AT1 receptor up-regulation [37], the capacity of garlic in modulating diabetes-induced AT2 receptor down- regulation may imply not only reversing the detrimental consequences of excessive AT1 receptor signaling, which is pivotal in the dysregulation of adrenal secretions and alterations in renal hemodynamic and tubular functions, but also restoring the recuperative processes mediated by AT2 receptors in both organs. This notion may be sup- ported by the documented AT2-mediated activities in inhibiting aldosterone hypersecretion and regulating catecholamine levels in the adrenal gland [1,7-9,49], inhibiting the sodium pump [53] and Na+-, K+-ATPase activity [20-22] in renal proximal tubules thereby pro- moting natriuresis/diuresis and hypotension, and inhib- iting vasoconstriction and cell hypertrophy [7-9,18,19] thereby interfering with excessive renal glomerular and tubular remodeling. In this regard, our observations may lend support to the documented efficacy of garlic treat- ments in ameliorating diabetic complications in STZ- treated rats [28-36] and further suggest that garlic may alleviate diabetic-induced hypertension and nephropathy by restoring the diabetic-induced loss of balance in AT1/AT2 receptor expression in key target organs. Future studies on the significance of constituent garlic metabo- lites, acting individually or in concert, may clarify the mechanisms that underlie its beneficial capacity in mo- dulating the expression of selective receptor molecules implicated in diabetes-associated disorders. 5. ACKNOWLEDGEMENTS The reported work was supported by Research Project # 2007-1302-04, funded by Kuwait Foundation for the Advancement of Science, and Research Project # SL 06/08, funded by Kuwait University. REFERENCES [1] Atlas, S.A. (2007) The renin-angiotensin aldosterone system: Pathophysiological role and pharmacologic Inhi- bition. Journal of Managed Care Pharmacy, 13, S9-S20. [2] Siragy, H.M. (2004) AT1 and AT2 receptor in the kidney: role in health and disease. Seminars in Nephrology, 24, 93-100. doi:10.1016/j.semnephrol.2003.11.009 [3] De Gasparo, M., Catt, K.J., Inagami, T., et al. (2000) International union of pharmacology. XXIII. The angio- tensin II receptors. Pharmacological Reviews, 52, 415- 742. [4] Kobori, H., Nangaku, M., Navar, L.G. and Nishiyama, A. (2007) The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kid- ney disease. Pharmacological Reviews, 59, 251-287. doi:10.1124/pr.59.3.3 [5] Kaschina, E. and Unger, T. (2003) Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Pres- sure, 12, 70-88. doi:10.1080/08037050310001057 [6] Ozono, R., Wang, Z., Moore, A.F., Inagami, T., et al. (1997) Expression of the subtype 2 angiotensin (AT2) re- ceptor protein in rat kidney. Hypertension, 30, 1238- 1246. [7] Millat, L.J., Abdel-Rahman, E. and Siragy, H.M. (1999) Angiotensin II and nitric oxide: A question of balance. Regulatory Peptides, 81, 1-10. doi:10.1016/S0167-0115(99)00027-0 [8] Carey, R.M., Howell, N.L., Jin, X.H. and Siragy, H.M. (2001) Angiotensin type 2 receptor-mediated hypoten- sion in angiotensin type-1 receptor-blocked rats. Hyper- tension, 38, 1272-1277.doi:10.1161/hy1201.096576 [9] Carey, R.M. (2005) Updates on the role of the AT2 re- ceptor. Current Opinion in Nephrology and Hypertension, 14, 67-71. doi:10.1097/00041552-200501000-00011 [10] Carey, R., Wang, Z. and Siragy, H. (2000) Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension, 35, 155-163. [11] Kalinyak, J.E., Sechi, L.A., Griffin, C.A., et al. (1993) The renin-angiotensin system in streptozotocin-induced diabetes mellitus in the rat. Journal of the American So- ciety of Nephrology, 4, 1337-1345. [12] Brown, L., Wall, D., Marchant, C. and Sernia, C. (1997) C opyright © 2011 SciRes. ABC  M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 101 Tissue-specific changes in angiotensin II receptors in streptozotocin-diabetic rats. Journal of Endocrinology, 154, 355-362. doi:10.1677/joe.0.1540355 [13] Wolf, G., Sharma, K., Chen, Y., et al. (1992) High glu- cose-induced proliferation in mesangial cells is reversed by autocrine TGF-β. Kidney International, 41, 369-402. 19. [14] Banday, A.A. and Lokhandwala, M.F. (2008) Oxidative stress-induced renal angiotensin AT1 receptor upregula- tion causes increased stimulation of sodium transporters and hypertension. American Journal of Physiology— Renal Physiology, 295, F698-F706. doi:10.1152/ajprenal.90308.2008 [15] Wolf, G., Ziyadeh, F.N. and Stahl, R.A. (1999) Angio- tensin II stimulates expression of transforming growth factor beta receptor type II in cultured mouse proximal tubular cells. Journal of Molecular Medicine, 77, 556- 564. doi:10.1007/s001099900028 [16] Siragy, H.M., Awad, A.A., Abadir, P.M. and Webb, R. (2003) The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-α in diabetic rats. Endocrinology, 144, 2229-2233. doi:10.1210/en.2003-0010 [17] Wehbi, G.J., Zimpelmann, J., Carey, R.M., et al. (2001) Early streptozotocin-diabetes mellitus downregulates rat kidney AT2 receptors. American Journal of Physiology— Renal Physiology, 280, F254-F265. [18] Nakajima, M., Hutchinson, H.G., Fujinaga, M., et al. (1995) The angiotensin II type 2 (AT2) receptor antago- nizes the growth effects of the AT1 receptor: gain-of- function study using gene transfer. Proceedings of the National Academy of Sciences of the USA, 92, 10663- 10667. doi:10.1073/pnas.92.23.10663 [19] Maric, C., Aldred, G.P., Harris, P.J. and Alcorn, D. (1998) Angiotensin II inhibits growth of cultured embryonic renomedullary interstitial cells through the AT2 receptor. Kidney International, 53, 92-99. doi:10.1046/j.1523-1755.1998.00749.x [20] Hussain, T. and Hakam, A.C. (2006) Angiotensin II AT2 receptors inhibit proximal tubular Na+, K+-ATPase activ- ity via a NO/cGMP dependent pathway. Am. J. Physiol. Renal. Physiol., 290, F1430-F1436. doi:10.1152/ajprenal.00218.2005 [21] Hakam, A. C., Siddiqui, A. H. and Hussain, T. (2006) Re- nal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. American Journal of Physiology—Renal Physiology, 290, 503-508. doi:10.1152/ajprenal.00092.2005 [22] Siragy, M.H. (2010) The angiotensin II type 2 receptor and the kidney. Journal of Renin-Angiotensin-Aldoster- one System, 11, 33-36. doi:10.1177/1470320309347786 [23] Miller, J.A. (1999) Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes melli- tus. Journal of the American Society of Nephrology, 10, 1778-1785. [24] Brewster, U.C. and Perazella, M.A. (2004) The renin- angiotensin-aldosterone system and the kidney: effects on kidney disease. American Journal of Medicine, 116, 263-272. doi:10.1016/j.amjmed.2003.09.034 [25] Weir, M.R. (2007) Effects of rennin-angiotensin system inhibition on end-organ protection: Can we do better? Clinical Therapeutics, 29, 1803-1824. doi:10.1016/j.clinthera.2007.09.019 [26] Burns, K.D. (2000) Angiotensin II and its receptors in the diabetic kidney. American Journal of Kidney Diseases, 36, 449-467. [27] Mapanga, R.F. and Musabayane, C.T. (2010) The renal effects of blood glucose-lowering plant-derived extracts in diabetes mellitus-an overview. Renal Failure, 32, 132- 138. doi:10.3109/08860220903367585 [28] Kasuga, S., Ushijima, M., Morihara, N., et al. (1999) Effect of aged garlic extract (AGE) on hyperglycemia induced by immobilization stress in mice. Nippon Yaku- rigaku Zasshi, 114, 191-197. doi:10.1254/fpj.114.191 [29] Hosseini, M., Shafiee, S.M. and Baluchnejad-mojarad, T. (2007) Garlic extract reduces serum angiotensin con- verting enzyme (ACE) activity in nondiabetic and strep- tozotocin-diabetic rats. Pathophysiology, 14, 109-112. doi:10.1016/j.pathophys.2007.07.002 [30] Thomson, M., Al-Amin, Z.M., Al-Qattan, K.K., et al. (2007) Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced dia- betic rats. International Journal of Diabetes and Metabo- lism, 15, 108-115. [31] Al-Qattan, K., Thomson, M. and Ali, M. (2008) Garlic (Allium sativum) and ginger (Zingiber officinale) attenu- ate structural nephropathy progression in streptozoto- cin-induced diabetic rats. Journal of Cancer, 44, 341. [32] Vazquez-Prieto, M.A., González, R.E., Renna, N.F., et al. (2010) Aqueous garlic extracts prevent oxidative stress and vascular remodeling in an experimental model of metabolic syndrome. Journal of Agricultural and Food Chemistry, 58, 6630-6635. doi:10.1021/jf1006819 [33] Liu, C.T., Shen, L.Y. and Li, C.K. (2007) Does garlic have a role as an antidiabetic agent? Molecular Nutrition & Food Research, 51, 1353-1364. doi:10.1002/mnfr.200700082 [34] Kasuga, S., Ushijima, M., Morihara, N., et al. (1999) Effect of aged garlic extract (AGE) on hyperglycemia induced by immobilization stress in mice. Nippon Yaku- rigaku Zasshi, 114, 191-197. doi:10.1254/fpj.114.191 [35] Pedraza-Chaverrí, J., Barrera, D., Maldonado, P.D., et al. (2004) S-allylmercaptocysteine scavenges hydroxyl ra- dical and singlet oxygen in vitro and attenuates gen- tamicin-induced oxidative and nitrosative stress and renal damage in vivo. B.M.C. Clinical Pharmacology, 4, 5-18. [36] Ahmad, M.S. and Ahmed, N. (2006) Antiglycation prop- erties of aged garlic extract: Possible role in prevention of diabetic complication. Journal of Nutrition, 136, 796S- 799S. [37] Mansour, M.H., Al-Qattan, K., Thomson, M. and Ali, M. (2011) Garlic (Allium sativum) down-regulates the ex- pression of angiotensin II AT1 receptor in adrenal and renal tissues of streptozotocin-induced diabetic rats. Sub- mitted to publication. [38] Feng, Y.-H., Zhou, L., Sun, Y. and Douglas, J.G. (2005) Functional diversity of AT2 receptor orthologues in close- ly related species. Kidney International, 67, 1731-1738. doi:10.1111/j.1523-1755.2005.00270.x [39] Al-Qattan, K., Al-Akhawand, S. and Mansour, M.H. (2006) Immuno-histochemical localization of distinct an- giotensin II AT1 receptor isoforms in the kidneys of the Sprague-Dawley rat and the desert rodent Meriones crassus. Anatomia, Histologia, Embryologia, 35, 130- C opyright © 2011 SciRes. ABC  M. H. Mansour et al. / Advances in Biological Chemistry 1 (2011) 93-102 Copyright © 2011 SciRes. 102 ABC 138. doi:10.1111/j.1439-0264.2005.00649.x [40] Drobiova, H., Thomson, M., Al-Qattan, K., et al. (2009) Garlic increases antioxidant levels in diabetic and hyper- tensive rats determined by a modified peroxidase method. eCAM. [41] Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265-275. [42] Laemmli, U.K. (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature, 227, 680-685. doi:10.1038/227680a0 [43] Nouet, S., Amzallag, N., Li, J.M., et al. (2004) Trans- inactivation of receptor tyrosine kinases by novel angio- tensin II AT2 receptor-interacting protein, ATIP. Journal of Biological Chemistry, 279, 28989-28997. doi:10.1074/jbc.M403880200 [44] Belloni, A.S., Andreis, P.G., Macchi, V., et al. (1998) Distribution and functional significance of angiotensin-II AT 1- and AT2-receptor subtypes in the rat adrenal gland. Endocrine Research, 24, 1-15. doi:10.3109/07435809809031865 [45] Servant, G., Dudley, D.T., Escher, E. and Guillimette, G. (1996) Analysis of the role of N-glycosylation in cell- surface expression and binding properties of angiotensin II type-2 receptor of rat pheochromocytoma cells. Bio- chemical Journal, 313, 297-304. [46] Mukoyama, M., Nakajima, M., Horiuchi, M., et al. (1993) Expression cloning of type 2 angiotensin II receptor re- veals a unique class of seven-transmembrane receptors. Journal of Biological Chemistry, 268, 24539-24542. [47] Zhuo, J., MacGregor, D.P. and Mendelsohn, F.A.O. (1996) Comparative distribution of angiotensin II receptor sub- types in mammalian adrenal glands. In: Vinson and An- derson D.C., Eds., Journal of Endocrinology Ltd, Bristol, 53-68. [48] Miyata, N., Park, F., Li, X.F. and Cowley Jr., A.W. (1999) Distribution of angiotensin AT1 and AT2 receptor sub- types in the rat kidney. American Journal of Physiol- ogy—Renal Physiology, 277, F437-F446. [49] Armando, I., Jezova, M., Bregonzio, C., et al. (2004) Angiotensin II AT1 and AT2 receptor types regulate basal and stress-induced adrenomedullary catecholamine pro- duction through transcriptional regulation of tyrosine hy- droxylase. Annals of the New York Academy of Sciences, 1018, 302-309. doi:10.1196/annals.1296.036 [50] Chatelain, D., Montel, V., Dickes-Coopman, A., et al. (2003) Trophic and steroidogenic effects of water depri- vation on the adrenal gland of the adult female rat. Regulatory Peptides, 110, 249-255. doi:10.1016/S0167-0115(02)00217-3 [51] Lehoux, J.-G., Bird, I.M., Brier, N., et al. (1997) Influ- ence of dietary sodium restriction on angiotensin II re- ceptors in rat adrenals. Endocrinology, 138, 5238-5245. doi:10.1210/en.138.12.5238 [52] Wang, D.H., Qiu, J. and Hu, Z. (1998) Differential regu- lation of angiotensin II receptor subtypes in the adrenal gland: Role of aldosterone. Journal of Hypertension, 32, 65-70. [53] Hakam, A.C. and Hussain, T. (2006) Angiotensin II type 2 receptor agonist directly inhibits proximal tubule so- dium pump activity in obese but not in lean zucker rats Journal of Hypertension, 47, 1117-1124. doi:10.1161/01.HYP.0000220112.91724.fc
|