 American Journal of Anal yt ical Chemistry, 2011, 2, 820-831 doi:10.4236/ajac.2011.27094 Published Online November 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Solid-Contact Perchlorate Sensor with Nanomolar Detection Limit Based on Cobalt Phthalocyanine Ionophores Covalently Attached to Polyacrylamide Mohammad Nooredeen Abbas1*, Abdel Lattief A. Radwan1, Philippe Bühlmann2, Mahmud A. Abd El Ghaffar3 1Analytic al Laboratory , Department of Applied Organic Chemistry, National Research Centre, Cairo, Egypt 2Department of Chemistry, University of Minnesota, Saint Pau l, United St a t es 3Department of Polymers and Pigments, National Research Centre, Cairo, Egypt E-mail: *dr.nooreldin@yahoo.com Received July 29, 2011; revised September 3, 2011; accepted Septembe r 15, 2011 Abstract Novel solid-contact perchlorate sensors based on cobalt phthalocyanine-C-monocarboxylic acid (I), and cobalt phthalocyanine-C,C,C,C-tetracarboxylic acid (II) as free ionophores and covalently attached to polyacryla- mide (PAA)—ionophores III and IV, respectively were prepared. The all solid-state sensors were constructed by the application of a thin film of a polymer cocktail containing a phthalocyanine ionophore and cetyl- trimethylammonium bromide (CTMAB) as a lipophilic cationic additive onto a gold electrode precoated with the conducting polymer poly (3,4-ethylenedioxythiophene) (PEDOT) as an ion and electron transducer. The sensor with 10.3% of ionophore (III) covalently attached to plasticizer-free poly (butyl methacrylate-co-do- decyl methacrylate) (PBDA) exhibited a good selectivity for perchlorate and discriminated many ions, in- cluding F–, Cl–, Br–, I–, SCN–, 3 – O, S2– and 2 4 SO . The covalent attachment of the ionophore to the polymer resulted in a near-Nernstian anionic slope of –62.3 mV/decade whereas a super-Nernstian slope of –79.9 mV/ decade was obtained for the free ionophore. The sensor covered a linear concentration range of 5 × 10–9 - 1 × 10–2 mol·L–1 with a lower detection limit (LDL) of 1 × 10–9 mol·L–1 and gave a stable response over a pH range of 4 - 10.5. The all-solid state sensors were utilized for the selective flow injection potentiometric de- termination of perchlorate in natural water and human urine samples in the nanomolar concentration range. Keywords: Ion-Selective Electrode, Solid Contact, Covalent Ionophore Attachment, Perchlorate, Flow-Injection Analysis, Urine 1. Introduction Perchlorate () is an environmental contaminant 4 whose occurrence is most clearly linked with its use as an oxidizer in rocket propellants, fireworks, matches, and highway safety flares [1]. Through accidental releases and improper disposal of materials containing its salts, perchlorate has entered the soil, surface water, and g- roundwater, and its solubility, mobility, and persistence characteristics have allowed it to contaminate drinking water. Perchlorate presents an environmental health risk to humans as it interferes with iodine uptake by the thy- roid gland and is associated with the disruption of its function [2,3]. These reasons have stimulated research towards the accurate determination of perchlorate ions in different samples, such as urine and natural water [4-7]. Recently, chemical sensors have been used in many fie- lds of applications, including clinical diagnosis, biome- dical analysis and monitoring of environmentally hazar- dous materials [8-10]. The life span of these sensors and their lower limit of detection are among their most im- portant characteristics. Therefore, increasing efforts are being directed towards the optimum design and operating conditions of long-living sensors. In particular, covalent binding of ionophores to polymeric sensing membranes provides long-term stability by preventing the ionophore from crystallizing, evaporating [11,12] and leaching into sample solutions. In addition, it can improve the sensor – ClO  821 M. N. ABBAS ET AL. selectivities and detection limits [13,14]. At the same time, all-solid-state ion-selective electrodes (ISEs) based on polymeric membranes doped with electrically neutral or charged ionophores (carriers) have attracted consid- erable interest since the invention of the so-called coat- ed-wire electrode (CWE) more than 30 years ago [15]. Their further development led to ion-selective electrodes with a solid internal contact (SCISEs) [16,17] character- ized by a well-defined ion-to-electron transduction proc- ess between the ionically conducting ion-selective mem- brane and the electronically conducting substrate. The notable progress in sensor technology is manifested in the wide application of ion sensors in environmental analysis [18]. However, while there a considerable num- ber of perchlorate ISEs were reported in the literature, most of them are not sensitive and selective enough to permit accurate measurements of the low levels of per- chlorate usually encountered in real samples [19] and only have a limited lifetime. Therefore, the development of durable perchlorate sensor with improved sensitivity and selectivity is still an urgent need. In this contribution, we report long-living highly selec- tive and sensitive membrane electrodes for the determina- tion of subnanomolar amounts of perchlorate in tap water, ground water and urine. We have utilized the combined use of covalent ionophore attachment and solid internal con- tacts for the preparation of these perchlorate selective elec- trodes. The construction and evaluation of perchlorate SCISEs based on cobalt phthalocyanine (Co-Pc) covalently attached to a polyacrylamide polymer backbone is de- scribed. As the ion-to-electron transducer, the conducting polymer poly (ethylenedioxy-thiophene) (PEDOT) was used. It was coated with either ionophore-doped plasticized poly (vinyl chloride) (PVC) or ionophore-doped plasti- cizer-free polymethacrylate membranes. At last, it is wor- thy to note that we have integrated the excellent potenti- ometric performance of the developed sensors with the agreed advantages of the flow injection technique with the high sample throughput and low sample volume. 2. Experimental 2.1. Reagents and Materials All chemicals used were of analytical reagent grade unless stated otherwise, and doubly distilled water was used throughout. o-Nitrophenyl octyl ether (o-NPOE), bis (2-ethylhexyl) phthalate (DOP), dibutyl sebacate (DBS), dodecyl methacrylate and tetrahydrofuran (THF) were purchased from Sigma-Aldrich. Gold wires and conducting polymer dispersion in water composed of 0.5% poly (3,4-ethylene-dioxythiophene) with 0.8% poly (styrene sulfonate) (PEDOT/PSS) as dopant were pur- chased from Sigma-Aldrich. Sodium tetraphenylborate was obtained from Riedel de Haen, and oleic acid (OA) and high relative molecular weight PVC from Fluka. Tetrahydrofuran (THF), methyl methacrylate, n-butyl methacrylate and the sodium and potassium salts of all anions were purchased from Merck. Cetylpyridinium chloride (CPC) was purchased from ACROS, and dibutyl phthalate (DBP) and cetyltrimethylammonium bromide (CTMAB) from BDH. A stock solution of 0.1 mol·L–1 potassium perchlorate was prepared in water, and 8 working standards in the range from 10−2 mol·L–1 to 10−9 mol·L–1 (each differing from the next more concentrated standard by a factor of 10) were freshly prepared by stepwise dilution. Phos- phate buffer (0.1 mol·L−1) of pH 7.2 was used to adjust the pH of all sample solutions. 2.2. Instrumentation All pH measurements were made at 25 ± 1˚C using a pH/Ion meter Model 692 (Metrohm). A PC-based EMF- 16 high-resolution data logger (Lawson) was used to re- cord the output signals. A combination pH glass elec- trode Model Metrohm 6.0202.100 was used for all pH measurements, and a Metrohm double junction Ag/AgCl reference electrode Model 6.0726.100 containing 3 mol·L−1 KCl solution in the outer compartment and 3 mol·L−1 KCl solution saturated with AgCl in the inner compartment was used for all other potentiometric measurements. Standard solutions were freshly prepared with doubly distilled water. 2.3. Synthesis of Ionophores The ionophores cobalt phthalocyanine-C-monocarboxylic acid (I) and cobalt phthalocyanine-C,C,C,C-tetracarbox ylic acid (II) and the adducts of phthalocyanine-C-mono carboxylic acid-PAA (III) and cobalt phthalocyanine- C,C,C,C-tetracarboxylic acid-PAA (IV), were synthesized according to the Figure 1 and characterized by elemental analysis and UV-Vis and IR spectroscopy. In brief, con- densation of trimellitic anhydride in the presence of urea, ammonium molybdate and cobalt acetate was used to form the tetraformamido-phthalocyanine cobalt. By hy- drolyzing the product in alkaline medium, ionophore II was obtained. The synthesis of ionophore I was carried out according to a procedure described by Chen et al. [20]. By heating a mixture of trimellitic anhydride and phthalic anhydride at a ratio of 1:7 to 190˚C in the pre- sence of urea, cobalt acetate, and ammonium molybdate, a mixture of cobalt 2-formamido-phthalocyanine and cobalt phthalocyanine was obtained. The cobalt forma- mido-phthalocyanine thus prepared was hydrolyzed under Copyright © 2011 SciRes. AJAC 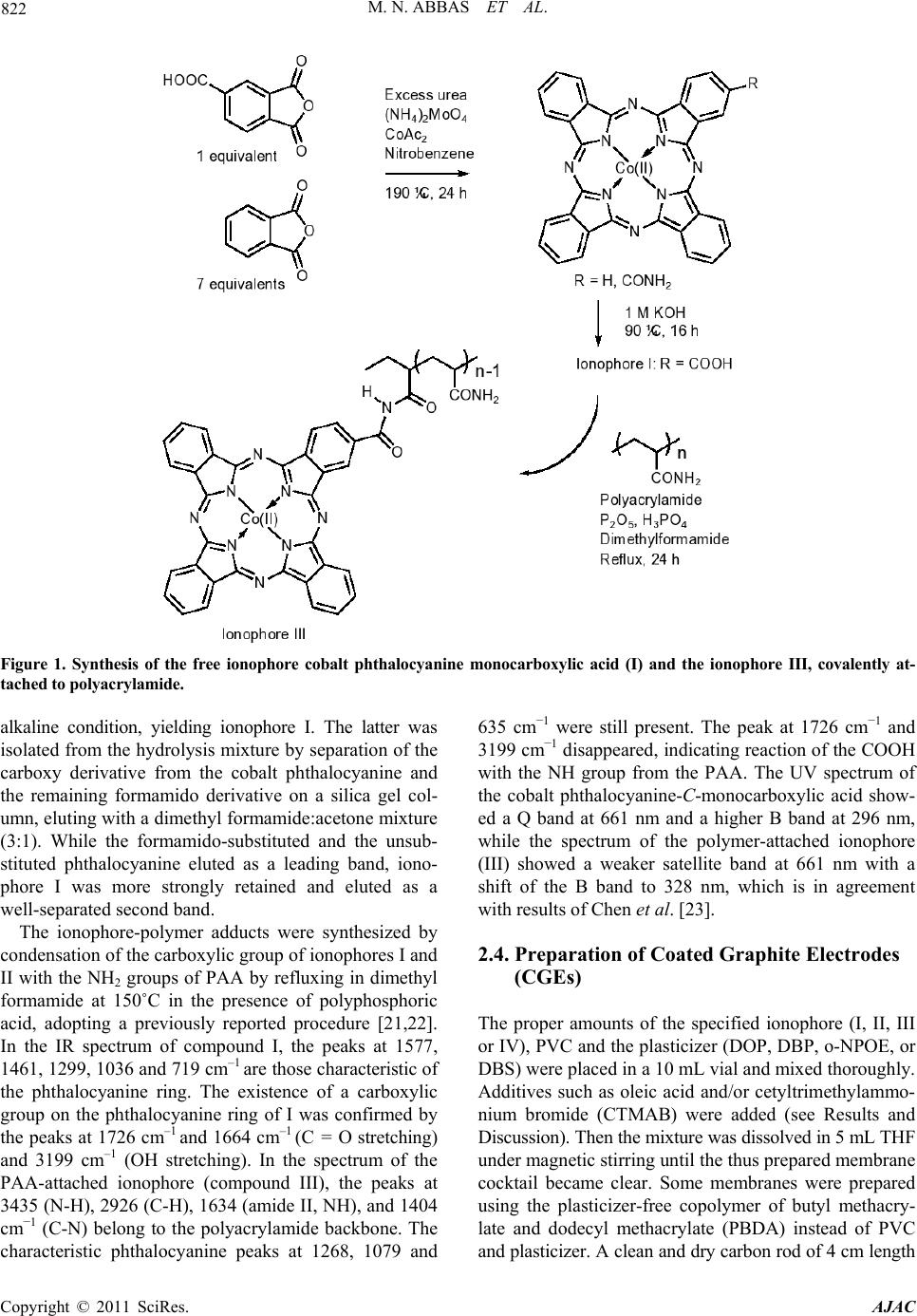 M. N. ABBAS ET AL. Copyright © 2011 SciRes. AJAC 822 Figure 1. Synthesis of the free ionophore cobalt phthalocyanine monocarboxylic acid (I) and the ionophore III, covalently at- tached to polyacrylamide. alkaline condition, yielding ionophore I. The latter was isolated from the hydrolysis mixture by separation of the carboxy derivative from the cobalt phthalocyanine and the remaining formamido derivative on a silica gel col- umn, eluting with a dimethyl formamide:acetone mixture (3:1). While the formamido-substituted and the unsub- stituted phthalocyanine eluted as a leading band, iono- phore I was more strongly retained and eluted as a well-separated second band. The ionophore-polymer adducts were synthesized by condensation of the carboxylic group of ionophores I and II with the NH2 groups of PAA by refluxing in dimethyl formamide at 150˚C in the presence of polyphosphoric acid, adopting a previously reported procedure [21,22]. In the IR spectrum of compound I, the peaks at 1577, 1461, 1299, 1036 and 719 cm–1 are those characteristic of the phthalocyanine ring. The existence of a carboxylic group on the phthalocyanine ring of I was confirmed by the peaks at 1726 cm–1 and 1664 cm–1 (C = O stretching) and 3199 cm–1 (OH stretching). In the spectrum of the PAA-attached ionophore (compound III), the peaks at 3435 (N-H), 2926 (C-H), 1634 (amide II, NH), and 1404 cm–1 (C-N) belong to the polyacrylamide backbone. The characteristic phthalocyanine peaks at 1268, 1079 and 635 cm–1 were still present. The peak at 1726 cm–1 and 3199 cm–1 disappeared, indicating reaction of the COOH with the NH group from the PAA. The UV spectrum of the cobalt phthalocyanine-C-monocarboxylic acid show- ed a Q band at 661 nm and a higher B band at 296 nm, while the spectrum of the polymer-attached ionophore (III) showed a weaker satellite band at 661 nm with a shift of the B band to 328 nm, which is in agreement with results of Chen et al. [23]. 2.4. Preparation of Coated Graphite Electrodes (CGEs) The proper amounts of the specified ionophore (I, II, III or IV), PVC and the plasticizer (DOP, DBP, o-NPOE, or DBS) were placed in a 10 mL vial and mixed thoroughly. Additives such as oleic acid and/or cetyltrimethylammo- nium bromide (CTMAB) were added (see Results and Discussion). Then the mixture was dissolved in 5 mL THF under magnetic stirring until the thus prepared membrane cocktail became clear. Some membranes were prepared using the plasticizer-free copolymer of butyl methacry- late and dodecyl methacrylate (PBDA) instead of PVC and plasticizer. A clean and dry carbon rod of 4 cm length  823 M. N. ABBAS ET AL. and 2 mm diameter was dipped to about 1 cm depth into the membrane cocktail for 2 s, and then lifted out of the solution to evaporate the THF, leaving the polymeric membrane layer coating the carbon rod. That operation was repeated for 12 - 17 times to give a proper mem- brane thickness. The rod was fitted into a plastic body and connected with the membrane-free end to the pH/ion meter using a copper wire. 2.5. Preparation of Solid-Contact Electrodes (SCEs) with Gold Contacts Gold wire (1 cm length and 0.1 mm diameter) was care- fully washed with 1 mol·L–1 H2SO4, water and acetone, dried, and attached to a silver wire using conducting sil- ver-epoxy glue (silver epoxy E10-101, Alfa). The con- ducting polymer films were cast from an aqueous disper- sion of PEDOT/PSS containing of FeCl3 onto Au wire. The thus obtained polymer films possess low water solu- bility and allowed for stabilized standard electrode po- tential [24]. Finally, the membrane cocktail was applied onto the dry PEDOT/PSS polymer layer deposited onto the gold electrode. The electrodes were conditioned in a 1 × 10–2 mol·L–1 perchlorate solution for at least 48 h before their first use and kept in such a solution over- night when not in use. Figure 2 shows a schematic drawing of the all-solid contact sensor. 2.6. ISE Calibration The ISEs were calibrated by immersion, along with an Ag/AgCl/Cl– reference electrode, in a 50-mL beaker containing 9.0 mL phosphate buffer solution (0.1 mol·L−1) of pH 7.2. Then aliquots of a standard perchlorate solu- tion were added successively under continuous stirring to obtain solutions with a perchlorate concentration ranging from 1 × 10-10 to 1 × 10–1 mol·L–1, and the potential was recorded after stabilization to ±0.5 mV within approxi- mately 1 min. A calibration graph was then constructed by plotting the recorded potentials as a function of the logarithm of the perchlorate concentration. Figure 2. Schematic of the solid contact electrode (SCE). 2.7. Sensor Selectivity Potentiometric selectivity coefficients , ot AB were deter- mined according to IUPAC guidelines using the separate solutions method (SSM) [25,26]. Different interfering anions of a concentration of 1 × 10−3 mol·L−1 in phos- phate buffer (pH 7.2, 0.1 mol·L−1) were utilized, and se- lectivity coefficients were obtained using Equation (1). ,1log log pot BA A AB A B EE zc KSz where S is the slope of the calibration curve, cA the con- centration of perchlorate, and zA and zB are the charges of perchlorate and the interfering anion, respectively. 2.8. Flow Injection Analysis Test solutions were injected using an injection valve Model 5060 (Rheodyne). The carrier buffer solution was propelled by means of a four channel peristaltic pump model MCP (Ismatec) through Tygon tubing R- 3603 of 0.25 mm i.d. The all-solid state perchlorate selective electrodes along with Ag/AgCl/Cl− double jun- ction reference electrodes were used to detect perchlo- rate in a home-built micro-flow cell of 250 uL volume. Figure 3 shows a schematic diagram for the flow injec- tion set-up. 3. Results and Discussions 3.1. Effect of Ionophores Type, Plasticizer, and Ionic Sites on Response Slope and Detection Limit Phthalocyanines and metal phthalocyanines are well-kno- wn, readily available pigments that have good chemical, acid-base, and thermal stabilities. They represent one of the most studied classes of organic functional materials [26-28]. In particular, they have been successfully used as ionophores for potentiometric sensing, which takes advantage of their selective coordination chemistry and structural diversity [29,30]. The type of phthalocyanine ring, the nature of their peripheral substituents, and the choice of the central metal control the axial ligation of different anions to these compounds [31,32]. We at- tached the phthalocyanine ionophores I and II covalently to polyacrylamide (PAA) through the condensation reac- tion between the carboxyl groups of the ionophores I and II and the NH2 groups of PAA, giving ionophores III and IV, respectively. The four compounds were investigated as anion sensing ionophores, and preliminary results showed a strong response towards perchlorate. Copyright © 2011 SciRes. AJAC 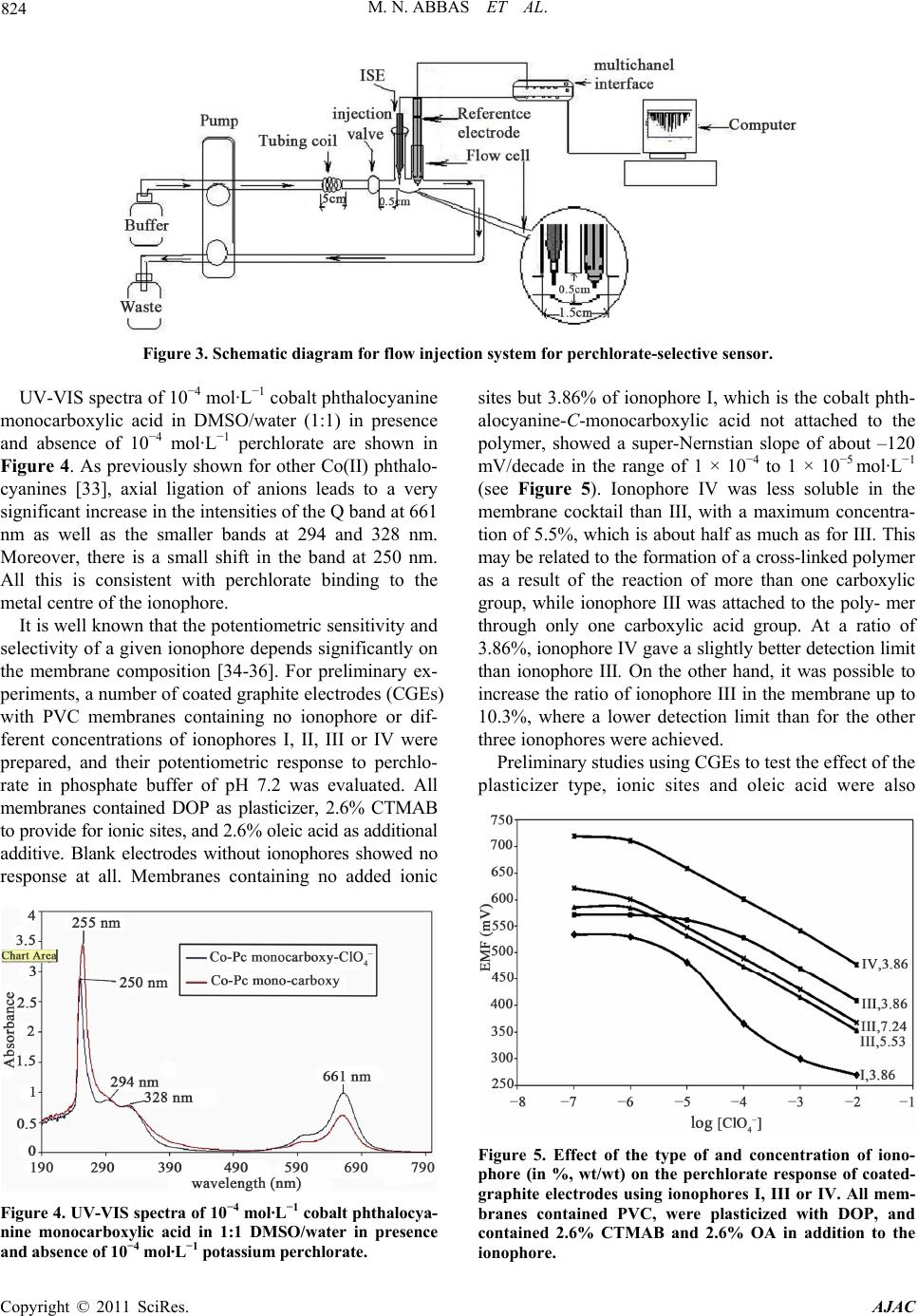 M. N. ABBAS ET AL. Copyright © 2011 SciRes. AJAC 824 Figure 3. Schematic diagram for flow injection system for perchlorate-selective sensor. UV-VIS spectra of 10−4 mol·L−1 cobalt phthalocyanine monocarboxylic acid in DMSO/water (1:1) in presence and absence of 10−4 mol·L−1 perchlorate are shown in Figure 4. As previously shown for other Co(II) phthalo- cyanines [33], axial ligation of anions leads to a very significant increase in the intensities of the Q band at 661 nm as well as the smaller bands at 294 and 328 nm. Moreover, there is a small shift in the band at 250 nm. All this is consistent with perchlorate binding to the metal centre of the ionophore. sites but 3.86% of ionophore I, which is the cobalt phth- alocyanine-C-monocarboxylic acid not attached to the polymer, showed a super-Nernstian slope of about –120 mV/decade in the range of 1 × 10−4 to 1 × 10−5 mol ·L−1 (see Figure 5). Ionophore IV was less soluble in the membrane cocktail than III, with a maximum concentra- tion of 5.5%, which is about half as much as for III. This may be related to the formation of a cross-linked polymer as a result of the reaction of more than one carboxylic group, while ionophore III was attached to the poly- mer through only one carboxylic acid group. At a ratio of 3.86%, ionophore IV gave a slightly better detection limit than ionophore III. On the other hand, it was possible to increase the ratio of ionophore III in the membrane up to 10.3%, where a lower detection limit than for the other three ionophores were achieved. It is well known that the potentiometric sensitivity and selectivity of a given ionophore depends significantly on the membrane composition [34-36]. For preliminary ex- periments, a number of coated graphite electrodes (CGEs) with PVC membranes containing no ionophore or dif- ferent concentrations of ionophores I, II, III or IV were prepared, and their potentiometric response to perchlo- rate in phosphate buffer of pH 7.2 was evaluated. All membranes contained DOP as plasticizer, 2.6% CTMAB to provide for ionic sites, and 2.6% oleic acid as additional additive. Blank electrodes without ionophores showed no response at all. Membranes containing no added ionic Preliminary studies using CGEs to test the effect of the plasticizer type, ionic sites and oleic acid were also Figure 5. Effect of the type of and concentration of iono- phore (in %, wt/wt) on the perchlorate response of coated- graphite electrodes using ionophores I, III or IV. All mem- branes contained PVC, were plasticized with DOP, and contained 2.6% CTMAB and 2.6% OA in addition to the ionophore. Figure 4. UV-VIS spectra of 10−4 mol·L−1 cobalt phthalocya- nine monocarboxylic acid in 1:1 DMSO/water in presence and absence of 10−4 mol·L−1 potassium perchlorate. 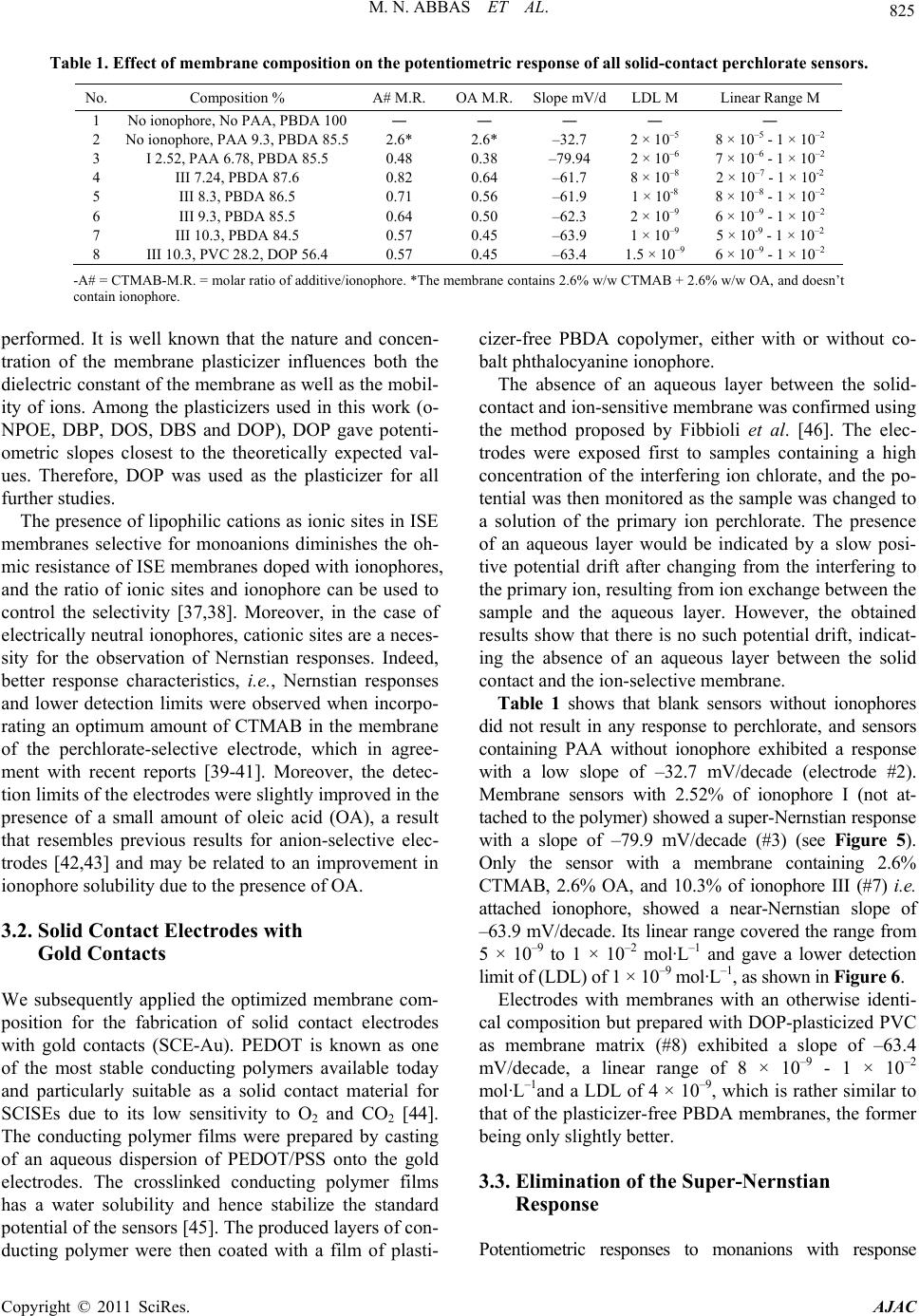 825 M. N. ABBAS ET AL. Table 1. Effect of membrane composition on the potentiometric response of all solid-contact perchlorate sensors. No. Composition % A# M.R. OA M.R.Slope mV/dLDL M Linear Range M 1 No ionophore, No PAA, PBDA 100 ― ― ― ― ― 2 No ionophore, PAA 9.3, PBDA 85.5 2.6* 2.6* –32.7 2 × 10–5 8 × 10–5 - 1 × 10–2 3 I 2.52, PAA 6.78, PBDA 85.5 0.48 0.38 –79.94 2 × 10–6 7 × 10–6 - 1 × 10–2 4 III 7.24, PBDA 87.6 0.82 0.64 –61.7 8 × 10–8 2 × 10–7 - 1 × 10-2 5 III 8.3, PBDA 86.5 0.71 0.56 –61.9 1 × 10-8 8 × 10–8 - 1 × 10–2 6 III 9.3, PBDA 85.5 0.64 0.50 –62.3 2 × 10–9 6 × 10–9 - 1 × 10–2 7 III 10.3, PBDA 84.5 0.57 0.45 –63.9 1 × 10–9 5 × 10-9 - 1 × 10–2 8 III 10.3, PVC 28.2, DOP 56.4 0.57 0.45 –63.4 1.5 × 10–9 6 × 10–9 - 1 × 10–2 -A# = CTMAB-M.R. = molar ratio of additive/ionophore. *The membrane contains 2.6% w/w CTMAB + 2.6% w/w OA, and doesn’t contain ionophore. performed. It is well known that the nature and concen- tration of the membrane plasticizer influences both the dielectric constant of the membrane as well as the mobil- ity of ions. Among the plasticizers used in this work (o- NPOE, DBP, DOS, DBS and DOP), DOP gave potenti- ometric slopes closest to the theoretically expected val- ues. Therefore, DOP was used as the plasticizer for all further studies. The presence of lipophilic cations as ionic sites in ISE membranes selective for monoanions diminishes the oh- mic resistance of ISE membranes doped with ionophores, and the ratio of ionic sites and ionophore can be used to control the selectivity [37,38]. Moreover, in the case of electrically neutral ionophores, cationic sites are a neces- sity for the observation of Nernstian responses. Indeed, better response characteristics, i.e., Nernstian responses and lower detection limits were observed when incorpo- rating an optimum amount of CTMAB in the membrane of the perchlorate-selective electrode, which in agree- ment with recent reports [39-41]. Moreover, the detec- tion limits of the electrodes were slightly improved in the presence of a small amount of oleic acid (OA), a result that resembles previous results for anion-selective elec- trodes [42,43] and may be related to an improvement in ionophore solubility due to the presence of OA. 3.2. Solid Contact Electrodes with Gold Contacts We subsequently applied the optimized membrane com- position for the fabrication of solid contact electrodes with gold contacts (SCE-Au). PEDOT is known as one of the most stable conducting polymers available today and particularly suitable as a solid contact material for SCISEs due to its low sensitivity to O2 and CO2 [44]. The conducting polymer films were prepared by casting of an aqueous dispersion of PEDOT/PSS onto the gold electrodes. The crosslinked conducting polymer films has a water solubility and hence stabilize the standard potential of the sensors [45]. The produced layers of con- ducting polymer were then coated with a film of plasti- cizer-free PBDA copolymer, either with or without co- balt phthalocyanine ionophore. The absence of an aqueous layer between the solid- contact and ion-sensitive membrane was confirmed using the method proposed by Fibbioli et al. [46]. The elec- trodes were exposed first to samples containing a high concentration of the interfering ion chlorate, and the po- tential was then monitored as the sample was changed to a solution of the primary ion perchlorate. The presence of an aqueous layer would be indicated by a slow posi- tive potential drift after changing from the interfering to the primary ion, resulting from ion exchange between the sample and the aqueous layer. However, the obtained results show that there is no such potential drift, indicat- ing the absence of an aqueous layer between the solid contact and the ion-selective membrane. Table 1 shows that blank sensors without ionophores did not result in any response to perchlorate, and sensors containing PAA without ionophore exhibited a response with a low slope of –32.7 mV/decade (electrode #2). Membrane sensors with 2.52% of ionophore I (not at- tached to the polymer) showed a super-Nernstian response with a slope of –79.9 mV/decade (#3) (see Figure 5). Only the sensor with a membrane containing 2.6% CTMAB, 2.6% OA, and 10.3% of ionophore III (#7) i.e. attached ionophore, showed a near-Nernstian slope of –63.9 mV/decade. Its linear range covered the range from 5 × 10–9 to 1 × 10–2 mol·L–1 and gave a lower detection limit of (LDL) of 1 × 10–9 mol·L–1, as shown in Figure 6. Electrodes with membranes with an otherwise identi- cal composition but prepared with DOP-plasticized PVC as membrane matrix (#8) exhibited a slope of –63.4 mV/decade, a linear range of 8 × 10–9 - 1 × 10–2 mol·L–1and a LDL of 4 × 10–9, which is rather similar to that of the plasticizer-free PBDA membranes, the former being only slightly better. 3.3. Elimination of the Super-Nernstian Response Potentiometric responses to monanions with response Copyright © 2011 SciRes. AJAC 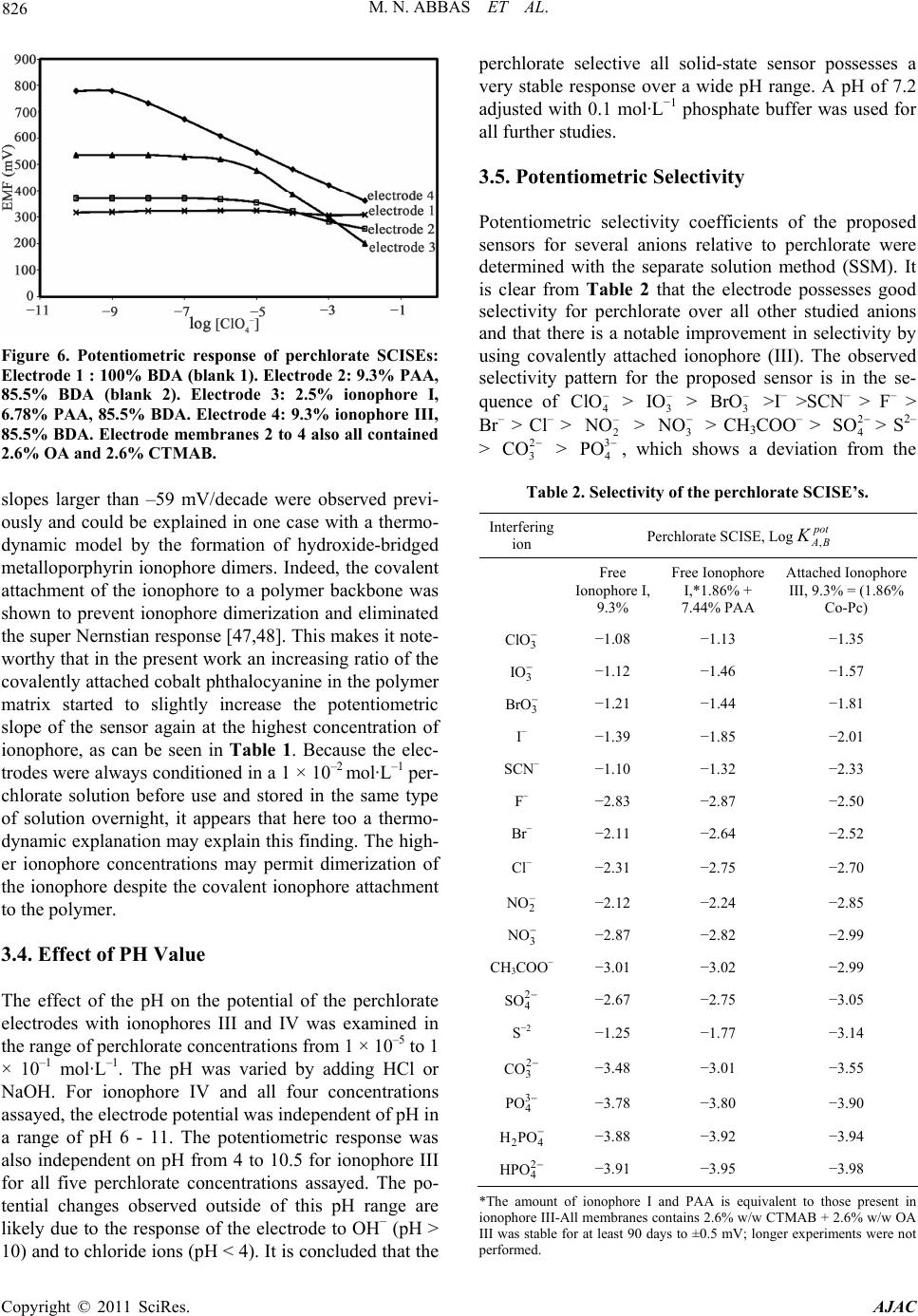 M. N. ABBAS ET AL. 826 Figure 6. Potentiometric response of perchlorate SCISEs: Electrode 1 : 100% BDA (blank 1). Electrode 2: 9.3% PAA, 85.5% BDA (blank 2). Electrode 3: 2.5% ionophore I, 6.78% PAA, 85.5% BDA. Electrode 4: 9.3% ionophore III, 85.5% BDA. Electrode membranes 2 to 4 also all contained 2.6% OA and 2.6% CTMAB. slopes larger than –59 mV/decade were observed previ- ously and could be explained in one case with a thermo- dynamic model by the formation of hydroxide-bridged metalloporphyrin ionophore dimers. Indeed, the covalent attachment of the ionophore to a polymer backbone was shown to prevent ionophore dimerization and eliminated the super Nernstian response [47,48]. This makes it note- worthy that in the present work an increasing ratio of the covalently attached cobalt phthalocyanine in the polymer matrix started to slightly increase the potentiometric slope of the sensor again at the highest concentration of ionophore, as can be seen in Table 1. Because the elec- trodes were always conditioned in a 1 × 10–2 mol·L–1 per- chlorate solution before use and stored in the same type of solution overnight, it appears that here too a thermo- dynamic explanation may explain this finding. The high- er ionophore concentrations may permit dimerization of the ionophore despite the covalent ionophore attachment to the polymer. 3.4. Effect of PH Value The effect of the pH on the potential of the perchlorate electrodes with ionophores III and IV was examined in the range of perchlorate concentrations from 1 × 10–5 to 1 × 10–1 mol·L–1. The pH was varied by adding HCl or NaOH. For ionophore IV and all four concentrations assayed, the electrode potential was independent of pH in a range of pH 6 - 11. The potentiometric response was also independent on pH from 4 to 10.5 for ionophore III for all five perchlorate concentrations assayed. The po- tential changes observed outside of this pH range are likely due to the response of the electrode to OH− (pH > 10) and to chloride ions (pH < 4). It is concluded that the perchlorate selective all solid-state sensor possesses a very stable response over a wide pH range. A pH of 7.2 adjusted with 0.1 mol·L−1 phosphate buffer was used for all further studies. 3.5. Potentiometric Selectivity Potentiometric selectivity coefficients of the proposed sensors for several anions relative to perchlorate were determined with the separate solution method (SSM). It is clear from Table 2 that the electrode possesses good selectivity for perchlorate over all other studied anions and that there is a notable improvement in selectivity by using covalently attached ionophore (III). The observed selectivity pattern for the proposed sensor is in the se- quence of > > >I– >SCN– > F– > Br– > Cl– > – 2 > 3 – 4 ClO NO – 3 IO NO – 3 BrO > CH3COO– > 2 4 SO > S2– 2 3 > CO >3 4 PO , which shows a deviation from the Table 2. Selectivity of the perchlorate SCISE’s. Interfering ion Perchlorate SCISE, Log, ot AB Free Ionophore I, 9.3% Free Ionophore I,*1.86% + 7.44% PAA Attached Ionophore III, 9.3% = (1.86% Co-Pc) 3 ClO −1.08 −1.13 −1.35 3 IO −1.12 −1.46 −1.57 3 BrO −1.21 −1.44 −1.81 I− −1.39 −1.85 −2.01 SCN− −1.10 −1.32 −2.33 F− −2.83 −2.87 −2.50 Br− −2.11 −2.64 −2.52 Cl− −2.31 −2.75 −2.70 – 2 NO −2.12 −2.24 −2.85 3 NO −2.87 −2.82 −2.99 CH3COO−−3.01 −3.02 −2.99 2 4 SO −2.67 −2.75 −3.05 S−2 −1.25 −1.77 −3.14 2 3 CO −3.48 −3.01 −3.55 3 4 PO −3.78 −3.80 −3.90 24 HPO −3.88 −3.92 −3.94 2 4 HPO −3.91 −3.95 −3.98 *The amount of ionophore I and PAA is equivalent to those present in ionophore III-All membranes contains 2.6% w/w CTMAB + 2.6% w/w OA III was stable for at least 90 days to ±0.5 mV; longer experiments were not performed. Copyright © 2011 SciRes. AJAC 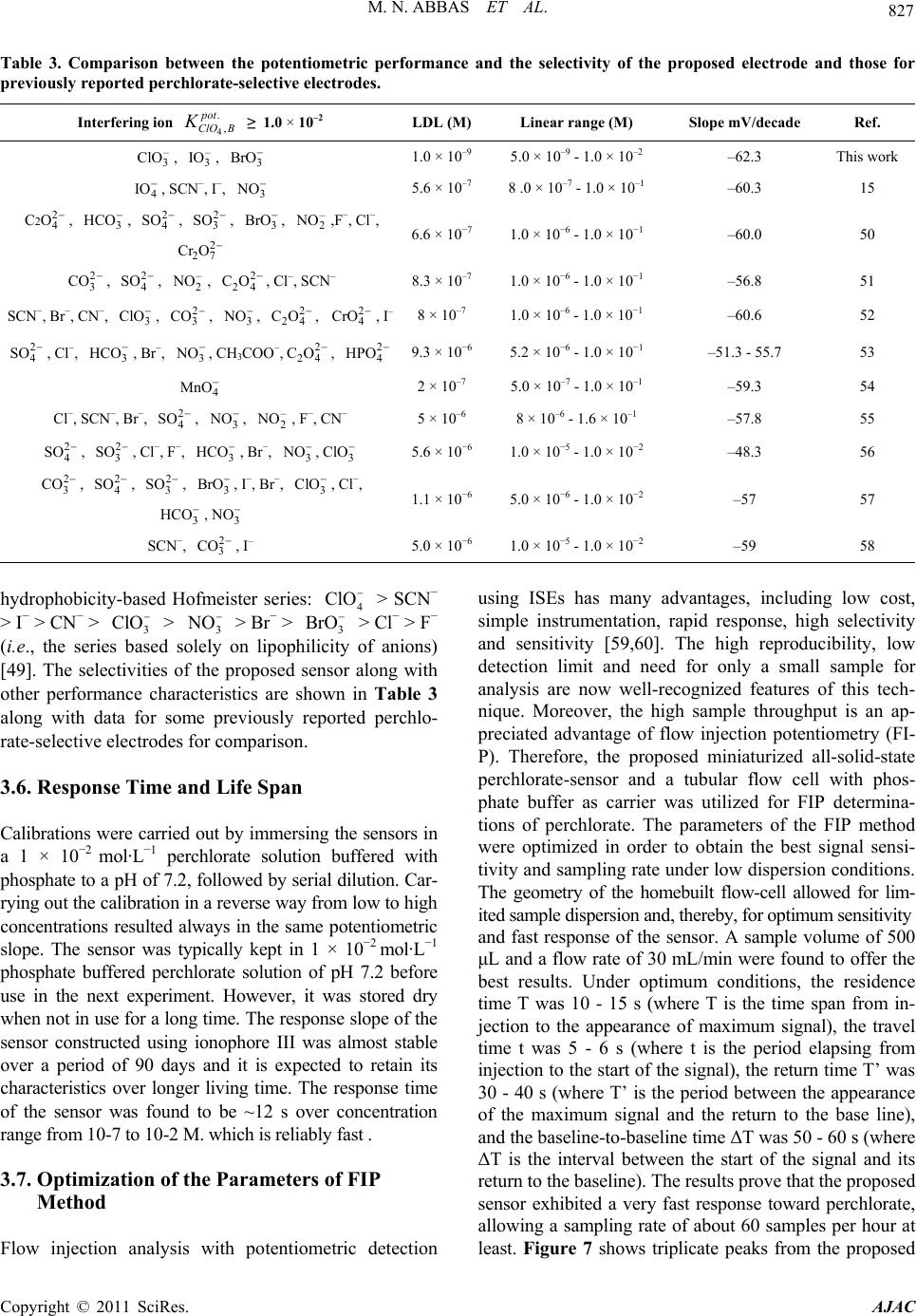 M. N. ABBAS ET AL. Copyright © 2011 SciRes. AJAC 827 Table 3. Comparison between the potentiometric performance and the selectivity of the proposed electrode and those for previously reported perchlorate-selective electrodes. Interfering ion ≥ 1.0 × 10–2 Ref. Slope mV/decade Linear range (M) LDL (M) 4 . , pot ClO B K This work –62.3 5.0 × 10–9 - 1.0 × 10–2 1.0 × 10–9 – 3 ClO, , – 3 IO 3 BrO 15 –60.3 8 .0 × 10–7 - 1.0 × 10–1 5.6 × 10–7 4 IO, SCN−, I−, 3 NO 50 –60.0 1.0 × 10−6 - 1.0 × 10−1 6.6 × 10−7 2 24 CO, , , , , ,F−, Cl−, 3 HCO 2 4 SO 2 3 SO 2 27 Cr O 3 BrO – 2 NO 51 –56.8 1.0 × 10−6 - 1.0 × 10−1 8.3 × 10–7 2 3 CO , , , , Cl−, SCN− 2 4 SO – 2 NO 2 24 CO 52 –60.6 1.0 × 10–6 - 1.0 × 10−1 8 × 10–7 SCN−, Br−, CN−, , , , , 3 ClO2 3 CO 3 NO 2 24 CO2 4 CrO , I– 53 –51.3 - 55.7 5.2 × 10−6 - 1.0 × 10−1 9.3 × 10−6 2 4 SO , Cl−, , Br−, , CH3COO−,, 3 HCO 3 NO 2 24 CO2 4 HPO 54 –59.3 5.0 × 10–7 - 1.0 × 10–1 2 × 10–7 4 MnO 55 –57.8 8 × 10–6 - 1.6 × 10–1 5 × 10–6 Cl−, SCN−, Br−, , 2 4 SO 3 NO , , F−, CN− – 2 NO 56 –48.3 1.0 × 10−5 - 1.0 × 10−2 5.6 × 10−6 2 4 SO , , Cl−, F−, 2 3 SO 3 HCO , Br−, , 3 NO 3 ClO 57 –57 5.0 × 10−6 - 1.0 × 10−2 1.1 × 10−6 2 3 CO , , , , I−, Br−, , Cl−, , 2 4 SO 2 3 SO HCO 3 BrO 3 3 NO 3 ClO 58 –59 1.0 × 10−5 - 1.0 × 10−2 5.0 × 10−6 SCN−, 2 3 CO , I– hydrophobicity-based Hofmeister series: 4 > SCN− > I− > CN− > 3 > 3 > Br− > 3 > Cl− > F− (i.e., the series based solely on lipophilicity of anions) [49]. The selectivities of the proposed sensor along with other performance characteristics are shown in Table 3 along with data for some previously reported perchlo- rate-selective electrodes for comparison. ClO O ClONOBr 3.6. Response Time and Life Span Calibrations were carried out by immersing the sensors in a 1 × 10−2 mol·L−1 perchlorate solution buffered with phosphate to a pH of 7.2, followed by serial dilution. Car- rying out the calibration in a reverse way from low to high concentrations resulted always in the same potentiometric slope. The sensor was typically kept in 1 × 10−2 mol· L−1 phosphate buffered perchlorate solution of pH 7.2 before use in the next experiment. However, it was stored dry when not in use for a long time. The response slope of the sensor constructed using ionophore III was almost stable over a period of 90 days and it is expected to retain its characteristics over longer living time. The response time of the sensor was found to be ~12 s over concentration range from 10-7 to 10-2 M. which is reliably fast . 3.7. Optimization of the Parameters of FIP Method Flow injection analysis with potentiometric detection using ISEs has many advantages, including low cost, simple instrumentation, rapid response, high selectivity and sensitivity [59,60]. The high reproducibility, low detection limit and need for only a small sample for analysis are now well-recognized features of this tech- nique. Moreover, the high sample throughput is an ap- preciated advantage of flow injection potentiometry (FI- P). Therefore, the proposed miniaturized all-solid-state perchlorate-sensor and a tubular flow cell with phos- phate buffer as carrier was utilized for FIP determina- tions of perchlorate. The parameters of the FIP method were optimized in order to obtain the best signal sensi- tivity and sampling rate under low dispersion conditions. The geometry of the homebuilt flow-cell allowed for lim- ited sample dispersion and, thereby, for optimum sensitivity and fast response of the sensor. A sample volume of 500 μL and a flow rate of 30 mL/min were found to offer the best results. Under optimum conditions, the residence time T was 10 - 15 s (where T is the time span from in- jection to the appearance of maximum signal), the travel time t was 5 - 6 s (where t is the period elapsing from injection to the start of the signal), the return time T’ was 30 - 40 s (where T’ is the period between the appearance of the maximum signal and the return to the base line), and the baseline-to-baseline time ΔT was 50 - 60 s (where ΔT is the interval between the start of the signal and its return to the baseline). The results prove that the proposed sensor exhibited a very fast response toward perchlorate, allowing a sampling rate of about 60 samples per hour at least. Figure 7 shows triplicate peaks from the proposed 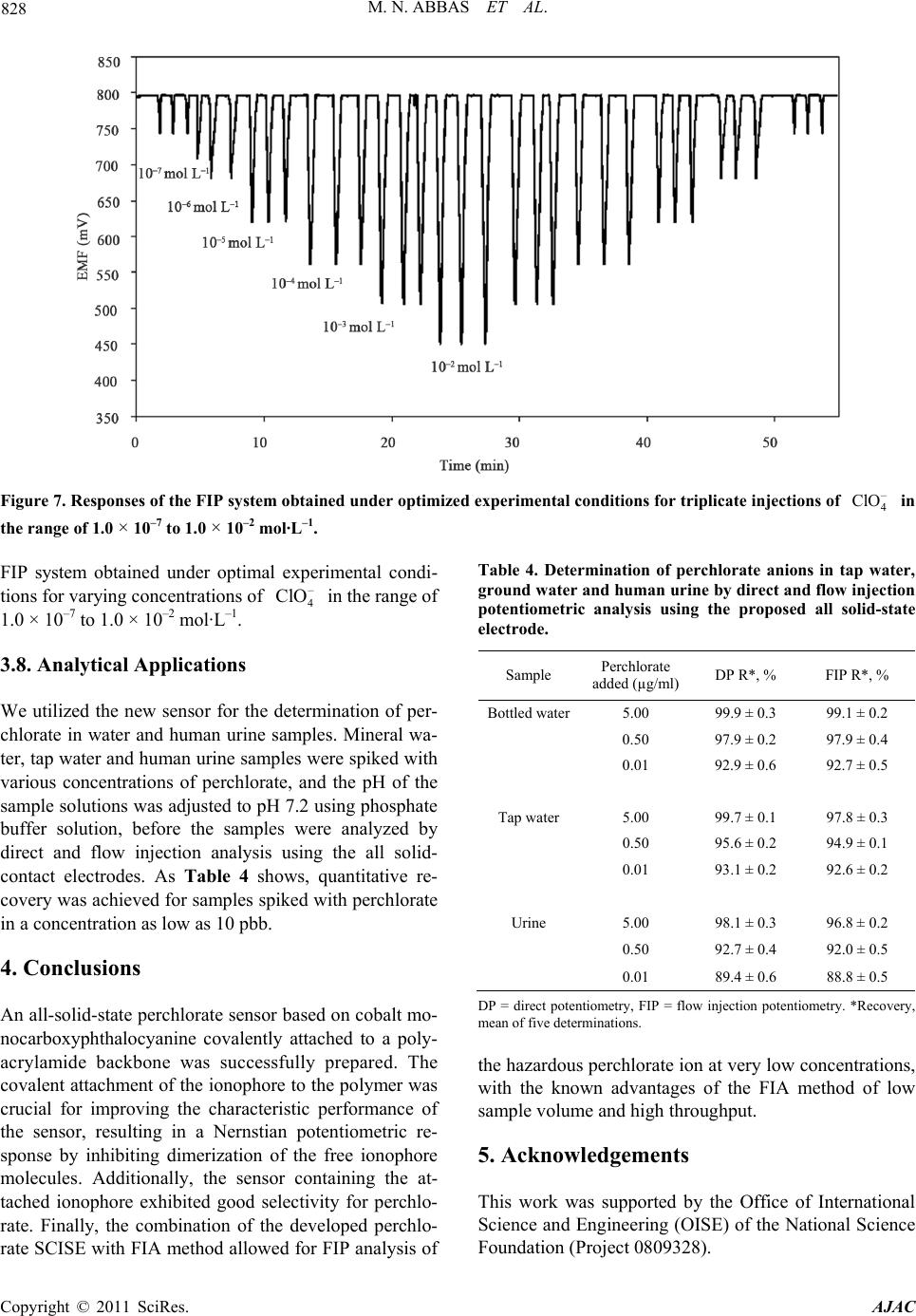 M. N. ABBAS ET AL. 828 Figure 7. Responses of the FIP system obtained under optimized experimental conditions for triplicate injections of in the range of 1.0 × 10–7 to 1.0 × 10–2 mol·L–1. – 4 ClO FIP system obtained under optimal experimental condi- tions for varying concentrations of in the range of 1.0 × 10–7 to 1.0 × 10–2 mol·L–1. – 4 ClO 3.8. Analytical Applications We utilized the new sensor for the determination of per- chlorate in water and human urine samples. Mineral wa- ter, tap water and human urine samples were spiked with various concentrations of perchlorate, and the pH of the sample solutions was adjusted to pH 7.2 using phosphate buffer solution, before the samples were analyzed by direct and flow injection analysis using the all solid- contact electrodes. As Table 4 shows, quantitative re- covery was achieved for samples spiked with perchlorate in a concentration as low as 10 pbb. 4. Conclusions An all-solid-state perchlorate sensor based on cobalt mo- nocarboxyphthalocyanine covalently attached to a poly- acrylamide backbone was successfully prepared. The covalent attachment of the ionophore to the polymer was crucial for improving the characteristic performance of the sensor, resulting in a Nernstian potentiometric re- sponse by inhibiting dimerization of the free ionophore molecules. Additionally, the sensor containing the at- tached ionophore exhibited good selectivity for perchlo- rate. Finally, the combination of the developed perchlo- rate SCISE with FIA method allowed for FIP analysis of Table 4. Determination of perchlorate anions in tap water, ground water and human urine by direct and flow injection potentiometric analysis using the proposed all solid-state electrode. Sample Perchlorate added (µg/ml)DP R*, % FIP R*, % Bottled water5.00 99.9 ± 0.3 99.1 ± 0.2 0.50 97.9 ± 0.2 97.9 ± 0.4 0.01 92.9 ± 0.6 92.7 ± 0.5 Tap water 5.00 99.7 ± 0.1 97.8 ± 0.3 0.50 95.6 ± 0.2 94.9 ± 0.1 0.01 93.1 ± 0.2 92.6 ± 0.2 Urine 5.00 98.1 ± 0.3 96.8 ± 0.2 0.50 92.7 ± 0.4 92.0 ± 0.5 0.01 89.4 ± 0.6 88.8 ± 0.5 DP = direct potentiometry, FIP = flow injection potentiometry. *Recovery, mean of five determinations. the hazardous perchlorate ion at very low concentrations, with the known advantages of the FIA method of low sample volume and high throughput. 5. Acknowledgements This work was supported by the Office of International Science and Engineering (OISE) of the National Science Foundation (Project 0809328). Copyright © 2011 SciRes. AJAC  829 M. N. ABBAS ET AL. 6. References [1] P. E. Jackson, S. Gokhale, T. Streib, J. S. Rohrer and C. A. Pohl, “Improved Method for the Determination of Trace Perchlorate in Ground and Drinking Waters by Ion Chromatography,” Journal of Chromatography A, Vol. 888, No. 1-2, 2000, pp. 151-158. doi:10.1016/S0021-9673(00)00557-4 [2] K. O. Yu, L. Narayanan, D. R. Mattie, R. J. Godfrey, P. N. Todd, T. R. Sterner, D. A. Mahle, M. N. Lumpkin and J. W. Fisher, “The Pharmacokinetics of Perchlorate and Its Effect on the Hypothalamus-Pituitary-Thyroid Axis in the Male Rat,” Toxicology and Applied Pharmacology, Vol. 182, No. 2, 2002, pp. 148-159. doi:10.1006/taap.2002.9432 [3] R. A. Clewell, E. A. Merrill, K. O. Yu, D. A. Mahle, T. R. Sterner, D. R. Mattie, P. J. Robinson, J. W. Fisher and J. M. Gearhart, “Predicting Neonatal Perchlorate Dose and Inhi- bition of Iodide Uptake in the Rat during Lactation Using Physiologically-Based Pharmacokinetic Modeling,” Toxi- cological Sciences, Vol. 74, No. 2, 2003, pp. 416-436. doi:10.1093/toxsci/kfg147 [4] B. Rezaei, S. Meghdadi and S. Bagherpour, “Perchlorate- Selective Polymeric Membrane Electrode Based on Bis (Dibenzoylmethanato) Cobalt(II) Complex as a Neutral Carrier,” Journal of Hazardous Materials, Vol. 161, No. 2-3, 2009, pp. 641-648. doi:10.1016/j.jhazmat.2008.04.005 [5] A. Soleymanpour, B. Garaili and S. M. Nabavizadeh, “Perchlorate Selective Membrane Electrodes Based on a Platinum Complex,” Monatshefte fur Chemie, Vol. 139, No. 12, 2008, pp. 1439-1445. doi:10.1007/s00706-008-0947-8 [6] M. R. Ganjali, P. Norouzi, F. Faridbod, M. Yousefi, L. Naji and M. Salavati-Niasari, “Perchlorate-Selective Me- mbrane Sensors Based on Two Nickel-Hexaaza-macro- cycle Complexes,” Sensors and Actuators B: Chemical, Vol. 120, No. 2, 2007, pp. 494-499. doi:10.1016/j.snb.2006.03.002 [7] M. Arvand, A. Pourhabib, R. Shemshadi and M. Giahi, “The Potentiometric Behavior of Polymer-Supported Me- tallophthalocyanines Used as Anion-Selective Electro- des,” Analytical and Bioanalytical Chemistry, Vol. 387, No. 3, 2007, pp. 1033-1039. doi:10.1007/s00216-006-0988-y [8] E. Pretsch, “The New Wave of Ion-Selective Electrodes,” Trends in Analytical Chemistry, Vol. 26, No. 1, 2007, pp. 46-51. doi:10.1016/j.trac.2006.10.006 [9] E. Bakker and E. Pretsch, “Potentiometric Sensors for Trace-Level Analysis,” Trends in Analytical Chemistry, Vol. 24, No. 3, 2005, pp. 199-207. doi:10.1016/j.trac.2005.01.003 [10] A. Michalska and K. Maksymiuk, “All-Plastic, Dispo- sable, Low Detection Limit Ion-Selective Electrodes,” Analytica Chimica Acta, Vol. 523, No. 1, 2004, pp. 97- 105. doi:10.1016/j.aca.2004.07.020 [11] S. Daunert and L. G. Bachas, “Ion-Selective Electrodes Using an Ionophore Covalently Attached to Carboxylated poly (Vinyl Chloride),” Analytical Chemistry, Vol. 62, No. 14, 1990, pp. 1428-1431. doi:10.1021/ac00213a016 [12] R. Bereczki, R. E. Gyurcsányi, B. Ágai and K. Tóth, “Sy- nthesis and Characterization of Covalently Immobilized Bis-Crown Ether Based Potassium Ionophore,” Analyst, Vol. 130, No. 1, 2005, pp. 63-70. doi:10.1039/b410410b [13] Y. Qin, S. Peper, A. Radu, A. Ceresa and E. Bakker, “Plasticizer-Free Polymer Containing a Covalently Im- mobilized Ca2+-Selective Ionophore for Potentiometric and Optical Sensors,” Analytical Chemistry, Vol. 75, No. 13, 2003, pp. 3038-3045. doi:10.1021/ac0263059 [14] M. Püntener, T. Vigassy, E. Baier, A. Ceresa and E. Pre- tsch, “Improving the Lower Detection Limit of Potenti- ometric Sensors by Covalently Binding the Ionophore to a Polymer Backbone,” Analytica Chimica Acta, Vol. 503, No. 2, 2004, pp. 187-194. doi:10.1016/j.aca.2003.10.030 [15] R. W. Cattrall and H. Freiser, “Coated Wire Ion Selective Electrodes,” Analytical Chemistry, Vol. 43, No. 13, 1971, pp. 1905-1906. doi:10.1021/ac60307a032 [16] B. P. Nikolskii and E. A. Materova, “Solid Contact in Membrane Ion-Selective Electrodes,” Ion-Selective Elec- trode Reviews, Vol. 7, No. 1, 1985, pp. 3-39. [17] E. Lindner and R. E. Gyurcsányi, “Quality Control Crite- ria for Solid-Contact, Solvent Polymeric Membrane Ion-Selective Electrodes,” Journal of Solid State Elec- trochemistry, Vol. 13, No. 1, 2009, pp. 51-68. doi:10.1007/s10008-008-0608-1 [18] R. De Marco, G. Clarke and B. Pejcic, “Ion-Selective Electrode Potentiometry in Environmental Analysis,” El- ectroanalysis, Vol. 19, No. 19-20, 2007, pp. 1987-2001. doi:10.1002/elan.200703916 [19] Interim Drinking Water Health Advisory for Perchlorate, “Health and Ecological Criteria Division,” Office of Sci- ence and Technology, Office of Water U.S. EPA, Wash- ington D.C., December 2008. [20] J. Chen, N. Chen, J. Huang, J. Wang and M. Huang, “De- rivatizable Phthalocyanine with Single Carboxyl Group: Synthesis and Purification,” Inorganic Chemistry Com- munications, Vol. 9, No. 3, 2006, pp. 313-315. doi:10.1016/j.inoche.2005.12.002 [21] M. A. Abd El-Ghaffar, N. R. El-Halawany and H. A. Essawy, “Phthalocyanine/Laponite Nanocomposites as Multifunction Additives for Stabilization of Polymeric Materials,” Journal of Applied Polymer Science, Vol. 108, No. 5, 2008, pp. 3225-3232. doi:10.1002/app.27558 [22] H. A. Essawy, N. A. Abd El-Wahab and M. A. Abd El-Ghaffar, “PVC-Laponite Nanocomposites: Enhanced Resistance to UV Radiation,” Polymer Degradation and Stability, Vol. 93, No. 8, 2008, pp. 1472-1478. doi:10.1016/j.polymdegradstab.2008.05.015 [23] W. Chen, B. Zhao, Y. Pan, Y. Yao, S. Lu, S. Chen and L. Du, “Preparation of a Thermosensitive Cobalt Phthalo- Copyright © 2011 SciRes. AJAC  M. N. ABBAS ET AL. 830 cyanine/N-Isopropylacrylamide Copolymer and Its Cata- lytic Activity on Thiol,” Journal of Colloid and Interface Science, Vol. 300, No. 2, 2006, pp. 626-632. doi:10.1016/j.jcis.2006.04.008 [24] M. Vázquez, P. Danielsson, J. Bobacka, A. Lewenstam and A. Ivaska, “Solution-Cast Films of Poly(3,4-Ethy- lene-Dioxythiophene) as Ion-to-Electron Transducers in All-Solid-State Ion-Selective Electrodes,” Sensors and Actuators B: Chemical, Vol. 97, No. 2-3, 2004, pp. 182- 189. doi:10.1016/j.snb.2003.08.010 [25] E. Lindner and Y. Umezawa, “Performance Evaluation Criteria for Preparation and Measurement of Macro- and Microfabricated Ion-Selective Electrodes (IUPAC Tech- nical Report),” Pure and Applied Chemistry, Vol. 80, No. 1, 2008, pp. 85-104. doi:10.1351/pac200880010085 [26] Y. umezawa, P. Bühlmann, K. Umezawa, K. Tohda and S. Amemiya, “Potentiometric Selectivity Coefficients of Ion-Selective Electrodes Part I. Inorganic Cations (Tech- nical Report),” Pure and Applied Chemistry, Vol. 72, No. 10, 2000, pp. 1851-2082. doi:10.1351/pac200072101851 [27] N. B. McKeown, “Phthalocyanine Materials: Synthesis, Structure and Function,” Cambridge University Press, Cambridge, 1998. [28] N. B. McKeown, “Out of the Blue,” Chemistry and In- dustry (London), No. 3, 1999, pp. 92-98. [29] W. Xu, R. Yuan, Y. Chai, T. Zhang, W. Liang and X. Wu, “Fabrication of an Iodide-Selective Electrode Based on Phthalocyaninatotitanium(IV) Oxide and the Selective Determination of Iodide in Actual Samples,” Analytical and Bioanalytical Chemistry, Vol. 392, 2008, pp. 297- 303. doi:10.1007/s00216-008-2243-1 [30] J. Liu, Y. Masuda and E. Sekido, “Response Properties of an Ion-Selective Polymeric Membrane Phosphate Elec- Trode Prepared with Cobalt Phthalocyanine and Charac- terization of the Electrode Process,” Journal of Electro- analytical Chemistry, Vol. 291, No. 1-2, 1990, pp. 67-79. doi:10.1016/0022-0728(90)87178-M [31] J. Li, X. Wu, R. Yuan, H. Lin and R. Yu, “Cobalt Phthalocyanine Derivatives as Neutral Carriers for Nitr- ite-Sensitive Poly (Vinyl Chloride) Membrane Electro- des,” Analyst, Vol. 119, 1994, pp. 1363-1366. doi:10.1039/an9941901363 [32] S. Shahrokhian, M. Amini, S. Kolagar and S. Tanges- taninejad, “Coated-Graphite Electrode Based on Poly (V- inyl Chloride)-Aluminum Phthalocyanine Membrane for Determination of Salicylate,” Microchemical Journal, Vol. 63, No. 2, 1999, pp. 302-310. doi:10.1006/mchj.1999.1794 [33] T. Nakamura, C. Hayashi and T. Ogawara, “Potentiomet- ric Response Properties of Sensor Membranes Based on Cobalt Phthalocyanine Conjugate-Polymer in Nonaque- ous Solutions,” Bulletin of the Chemical Society of Japan, Vol. 69, No. 6, 1996, pp. 1555-1559. doi:10.1246/bcsj.69.1555 [34] V. V. Egorov and A. A. Bolotin, “Ion-Selective Elec- trodes for Determination of Organic Ammonium Ions: Ways for Selectivity Control,” Talanta, Vol. 70, No. 5, 2006, pp. 1107-1116. doi:10.1016/j.talanta.2006.02.025 [35] E. Bakker, “Generalized Selectivity Description for Poly- Meric Ion-Selective Electrodes Based on the Phase Bound-Ary Potential Model,” Journal of Electroanalyti- cal Chemistry, Vol. 639, No. 1-2, 2010, pp. 1-7. doi:10.1016/j.jelechem.2009.09.031 [36] U. Schaller, E. Bakker and U. Spichiger, “Nitrite-Selec tive Microelectrodes,” Talanta, Vol. 41, No. 6, 1994, pp. 1001-1005. doi:10.1016/0039-9140(94)E0048-V [37] P. M. Gehrig, W. E. Morf, M. Welti, E. Pretsch and W. Simon, “Catalysis of Ion Transfer by Tetraphenylborates in Neutral Carrier-Based Ion-Selective Electrodes,” Hel- vetica Chimica Acta, Vol. 73, No. 1, 1990, pp. 203-212. doi:10.1002/hlca.19900730124 [38] E. Bakker, E. Malinowska, R. D. Schiller and M. E. Meyerhoff, “Anion-Selective Membrane Electrodes Ba- sed on Metalloporphyrins: The Influence of Lipophilic Anionic and Cationic Sites on Potentiometric Selectiv- ity,” Talanta, Vol. 41, No. 6, 1994, pp. 881-890. doi:10.1016/0039-9140(94)E0041-O [39] V. K. Gupta, R. N. Goyal and R. A. Sharma, “Anion Recognition Using Newly Synthesized Hydrogen Bond- ing Disubstituted Phenylhydrazone-Based Receptors: Po- ly (Vi-Nyl Chloride)-Based Sensor for Acetate,” Talanta, Vol. 76, No. 4, 2008, pp. 859-864. doi:10.1016/j.talanta.2008.04.046 [40] W. Zhou, Y. Chai, R, Yuan, X. Wu and J. Guo, “Poten- tiometric Iodide Selectivity of Polymer-Membrane Sen- sors Based on Co(II) Triazole Derivative,” Electroana- lysis, Vol. 20, No. 13, 2008, pp. 1434-1439. doi:10.1002/elan.200704197 [41] M. A. Zanjanchi, M. Arvand, M. Akbari, K. Tabatabaeian and G. Zaraei, “Perchlorate-Selective Polymeric Mem- Brane Electrode Based on a cobaloxime as a Suitable Carrier,” Sensors and Actuators B: Chemical, Vol. 113, No. 1, 2006, pp. 304-309. doi:10.1016/j.snb.2005.03.003 [42] S. Sadeghi, A. Gafarzade, M. A. Naseri and H. Shargi, “Triiodide-Selective Polymeric Membrane Electrodes Based on Schiff Base Complexes of Cu (II) and Fe (III),” Sensors and Actuators B: Chemical, Vol. 98, No. 2-3, 2004, pp. 174-179. doi:10.1016/j.snb.2003.10.005 [43] M. Shamsipur, M. Yousefi, M. R. Ganjali, T. Poursaberi and M. Faal-Rastgar, “Highly Selective Sulfate PVC-Me- mbrane Electrode Based on 2,5-Diphenyl-1,2,4,5-Tetra- aza-bicyclo[2.2.1 Heptane as a Neutral Carrier,” Sensors and Actuators B: Chemical, Vol. 82, No. 1, 2002, pp. 105 -110. doi:10.1016/S0925-4005(01)00997-2 [44] M. Vázquez, J. Bobacka, A. Ivaska and A. Lewenstam, “Influence of Oxygen and Carbon Dioxide on the Elec- trochemical Stability of Poly(3,4-Ethylene Dioxy Thio- phene) Used as Ion-to-Electron Transducer in All-Solid- State Ion-Selective Electrodes,” Sensors and Actuators B: Chemical, Vol. 82, No. 1, 2002, pp. 7-13. Copyright © 2011 SciRes. AJAC  M. N. ABBAS ET AL. Copyright © 2011 SciRes. AJAC 831 [45] M. Vázquez, P. Danielsson, J. Bobacka, A. Lewenstam and A. Ivaska, “Solution-Cast Films of Poly(3,4-Ethyle- ne-dioxythiophene) as Ion-to-Electron Transducers in All-Solid-State Ion-Selective Electrodes,” Sensors and Actuators B: Chemical, Vol. 97, No. 2-3, 2004, pp. 182- 189. doi:10.1016/j.snb.2003.08.010 [46] M. Fibbioli, W. E. Morf, M. Badertscher, N. F. de Rooij and E. pretsch, “Potential Drifts of Solid-Contacted Ion- Selective Electrodes Due to Zero-Current Ion Fluxes through the Sensor Membrane,” Electroanalysis, Vol. 12, No. 16, 2000, pp. 1286-1292. doi:10.1002/1521-4109(200011)12:16<1286::AID-ELAN 1286>3.0.CO;2-Q [47] E. Steinle, S. Amemiya, P. Buhlmann and M. Meyerhoff, “Origin of Non-Nernstian Anion Response Slopes of Metalloporphyrin-Based Liquid/Polymer Membrane El- ectrodes,” Analytical Chemistry, Vol. 72, No. 23, 2000, pp. 5766-5773. doi:10.1021/ac000643x [48] J. Casabó, L. Escriche, C. Pérez-Jiménez, J. A. Munoz, F. Teixidor, J. Bausells and A. Errachid, “Application of a New Phosphadithiamacrocycle to 4 ClO-Selective CHE- MFET and Ion-Selective Electrode Devices,” Analytica Chimica Acta, Vol. 320, No. 1, 1996, pp. 63-68. doi:10.1016/0003-2670(95)00526-9 [49] Y. Marcus, “Thermodynamic Functions of Transfer of Single Ions from Water to Nonaqueous and Mixed Solvents: Part I―Gibbs Free Energies of Transfer to Nonaqueous Solvents,” Pure and Applied Chemistry, Vol. 55, No. 6, 1983, pp. 977-1021. doi:10.1351/pac198355060977 [50] B. Rezaei, S. Meghdadi and V. Nafisi, “Fast Response and Selective Perchlorate Polymeric Membrane Electrode Based on Bis(Dibenzoylmethanato) Nickel(II) Complex as a Neutral Carrier,” Sensors and Actuators B: Chemi cal, Vol. 121, No. 2, 2007, pp. 600-605. doi:10.1016/j.snb.2006.04.093 [51] M. A. Zanjanchi, M. Arvand, M. Akbari, K. Tabatabaeian and G. Zaraei, “Perchlorate-Selective Polymeric Mem- brane Electrode Based on a Cobaloxime as a Suitable Carrier,” Sensors and Actuators B: Chemical, Vol. 113, No. 1, 2006, pp. 304-309. doi:10.1016/j.snb.2006.04.093 [52] M. M. Ardakani, M. Jalayer, H. Naeimi and H. R. Zare, “Perchlorate-Selective Membrane Electrode Based on a New Complex of Uranil,” Analytical and Bioanalytical Chemistry, Vol. 381, No. 6, 2005, pp. 1186-1192. doi:10.1007/s00216-004-3011-5 [53] J. Lizondo-Sabater, M. J. Segui, J. M. Lloris, R. Martínez- Mañez, T. Pardo, F. Sancenón and J. Soto, “New Membrane Perchlorate-Selective Electrodes Containing Polyazacycloa- lkanes as Carriers,” Sensors and Actuators B: Chemical, Vol. 101, No. 1-2, 2004, pp. 20- 27. doi:10.1016/j.snb.2004.02.018 [54] M. R. Ganjali, M. Yousefi, T. Poursaberi, L. Naji, M. Salavati-Niasari and M. Shamsipur, “Highly Selective and Sensitive Perchlorate Sensors Based on Some Re- cently Synthesized Ni(II)-Hexaazacyclotetradecane Com- plexes,” Electroanalysis, Vol. 15, No. 18, 2003, pp. 1476 -1480. doi:10.1002/elan.200302679 [55] M. Shamsipur, A. Soleymanpour, M. Akhond, H. Sharghi and A. R. Hasaninejad, “Perchlorate Selective Membrane Electrodes Based on a Phosphorus(V)-Tetraphenylpor- phyrin Complex,” Sensors and Actuators B: Chemical, Vol. 89, No. 1-2, 2003, pp. 9-14. doi:10.1016 /S09 25-400 5(02) 00401 -X [56] C. Sanchez-Pedreño, J. A. Ortuño and J. Hernández, “Flow Injection Potentiometry of Primary and Interfering Ion with a Gold Complex Ion-Exchange Membrane,” Ta- lanta, Vol. 55, No. 1, 2001, pp. 201-207. doi:10.1016 /S00 39-914 0(01) 00418 -0 [57] C. Sanchez-Pedreño, J. A. Ortuño and J. Hernández, “Perchlorate-Selective Polymeric Membrane Electrode Based on a Gold (I) Complex: Application to Water and Urine Analysis,” Analytica Chimica Acta, Vol. 415, No. 1-2, 2000, pp. 159-164. doi:10.1016 /S00 03-267 0(00) 00872 -2 [58] T. A. Bendikov and T. C. Harmon, “Long-Lived Solid State Perchlorate Ion Selective Sensor Based on Doped Poly(3,4-Ethylenedioxythiophene) (PEDOT) Films,” An- alytica Chimica Acta, Vol. 551, No. 1-2, 2005, pp. 30-36. doi:10.1016/j.aca.20 05.07 .004 [59] J. Ruzicka and E. H. Hansen, “Flow Injection Analysis,” 2nd Edition, Wiley, New York, 1988. [60] P. W. Alexander, T. Dimitrakopoulos and D. B. Hibbert, “A Six Sensor Array of Coated-Wire Electrodes for Use in a Portable Flow Injection Analyzer,” Electroanalysis, Vol. 10, No. 10, 1998, pp. 707-712. doi:10.1002/(SICI)1521-4109(199808)10:10<707::AID-E LAN707>3.0.CO;2-V
|