 Journal of Environmental Protection, 2011, 2, 1218-1226 doi:10.4236/jep.2011.29140 Published Online November 2011 (http://www.scirp.org/journal/jep) Copyright © 2011 SciRes. JEP Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, Musa Estuary (Persian Gulf) Alireza Safahieh, Fazel Abdolahpur Monikh*, Ahmad Savari, Abdolmajid Doraghi Department of Marine Biology, Faculty of Marine Science, Marine Science and Technology University, Khorramshahr, Iran. Email: *Fazel_abdolahpur2@yahoo.com Received June 21st, 2011; revised August 23rd, 2011; accepted September 28th, 2011. ABSTRACT Fish in estuaries, especially those live near petrochemical area, may be exposed to various kinds of contaminants such as heavy metals. This study was to investigate the varia tions of heavy metals in Liza abu from the Musa estuary which receives petrochemical wastes. Fish samples were obtained from five different creeks. The samples were dissected into liver, gill and muscle, acid digested and their heavy metals level were analyzed. The results showed that the concentra- tion of Cd, Co, Cu, Ni and Pb in muscle ranged 0.08-0.44, ND-1.63, 0.89 - 4.28, 0.48 - 2.73 and 0.5 - 2.50 (µg/g) re- spectively. Their concentrations in liver were 0.44 - 2.03, 0.5 - 2.80, 5.05 - 36.22, 0.48 - 4.91 and 0.66 - 5.74 (µg/g) respectively. The concentra tion of these metals in gill was 0.32 - 2.72, 0.29 - 1.10, 4.33 - 6.03, 4.61 - 17.52 and 2.64 - 21.41 (µg/g) respectively. Generally, the level of heavy metals in muscle tissue of Liza abu was lower than the general standard. Keywords: Wastewater, Khor-Jafari, Khor-Ghazale, Liver, Muscle 1. Introduction Fish occupies a particular position in the aquatic con- tamination studies, because it play significant roles in establishing water equality guidelines and is considered as a main part of human diet [1,2]. The nutritional bene- fits of fish might be related to essential proteins, vitamin and two kinds of potential healthful omega-3 polyun- saturated fatty acids (PUFA); docosahexaenoic acid (DHA, is found most abundantly in brain and retina) and eicosapentaenoic acid (EPA, exists profusely in nervous system) [3-5]. Nevertheless, fish is influenced by various types of contaminants such as heavy metals, PAHs and PCBs and subsequently could be changed into a main source of contaminant uptake for human being. Indus- tries, agriculture activities and transportation are poten- tial sources of toxic contaminants in the marine envi- ronment [6-8]. Heavy metals constitute a highlighted group of pollu- tants in aquatic ecosystems because of their accumulative behavior [9,10]. Since they are not biodegradable, they could enter aquatic food chain [11,12] and consequently accumulate in organisms positioned in various trophic levels. This condition may also produce health problem in people who consume contaminated seafood [13]. For example, they may appear in threatening form for chil- dren and women [14]. They cause neurological damages [15], increase the risk of cancer and increase the risk of abortion in pregnant woman [16]. The transferring of heavy metals from mother to fetus through placenta is another kind of threat rises from excess consumption of heavy metals by human [16]. These harmful peculiarities of heavy metals can turn fish consumption into an envi- ronmental mishap. Physicochemical factors of seawater (pH, salinity, temperature and suspended matters) [17], as well as ecological and biological factors (weight, size, age, sex, species and season) determine the accumulation of metals in fish [18]. However, metal toxicity via con- taminated fish is mainly dependent upon metal speciation [19] quantity of consumed fishes and age of consumer [20]. Musa Estuary, one of the largest estuaries in the Per- sian Gulf, is located in the northwest of the Gulf. It has subtropical climate with tow marked seasons including winter and summer. This estuary consists of many bran- ches and creeks, which each creek is locally called Khor. Thus, it provides a suitable habitat for fish, shrimp and 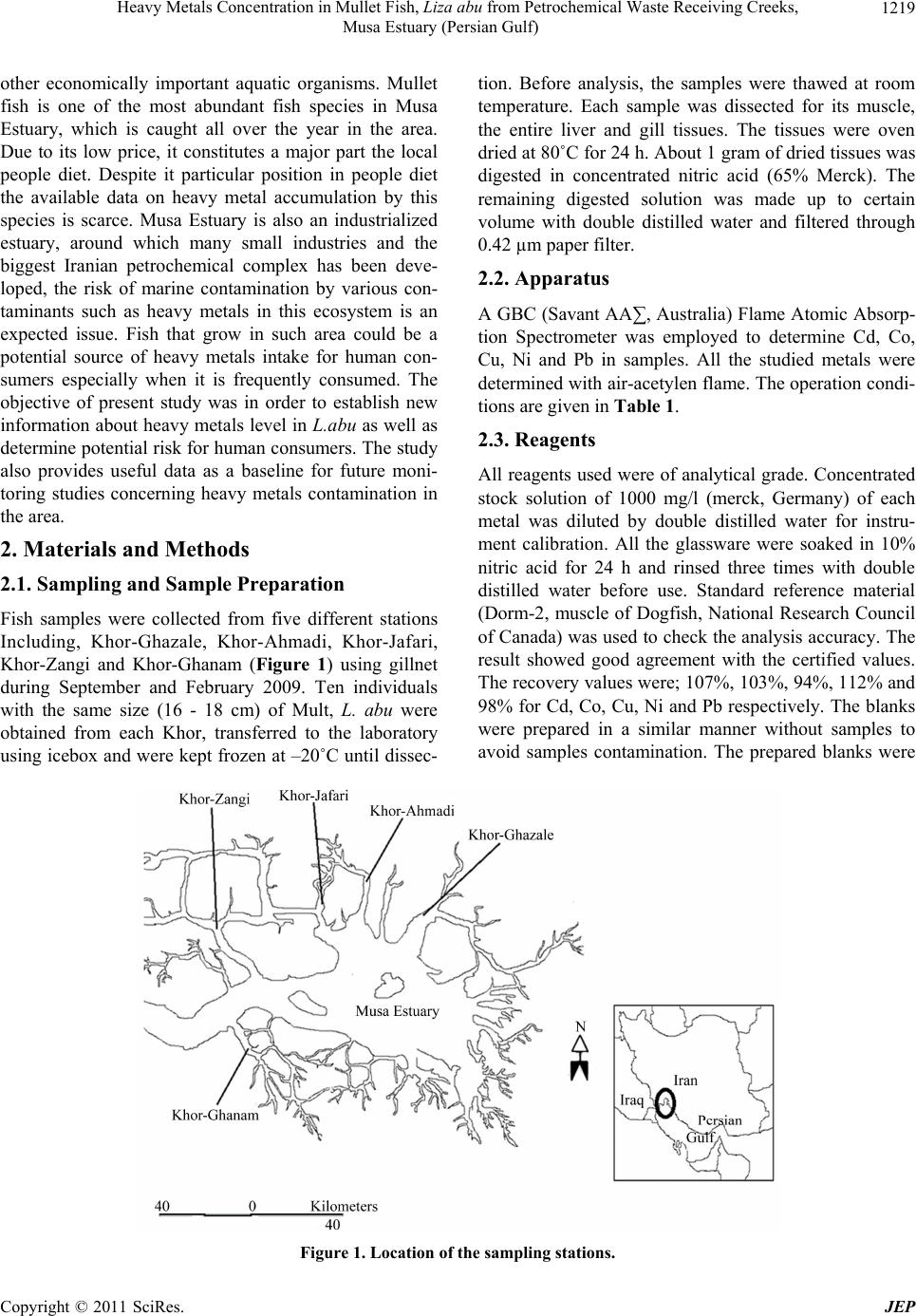 Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, 1219 Musa Estuary (Persian Gulf) other economically important aquatic organisms. Mullet fish is one of the most abundant fish species in Musa Estuary, which is caught all over the year in the area. Due to its low price, it constitutes a major part the local people diet. Despite it particular position in people diet the available data on heavy metal accumulation by this species is scarce. Musa Estuary is also an industrialized estuary, around which many small industries and the biggest Iranian petrochemical complex has been deve- loped, the risk of marine contamination by various con- taminants such as heavy metals in this ecosystem is an expected issue. Fish that grow in such area could be a potential source of heavy metals intake for human con- sumers especially when it is frequently consumed. The objective of present study was in order to establish new information about heavy metals level in L.abu as well as determine potential risk for human consumers. The study also provides useful data as a baseline for future moni- toring studies concerning heavy metals contamination in the area. 2. Materials and Methods 2.1. Sampling and Sample Preparation Fish samples were collected from five different stations Including, Khor-Ghazale, Khor-Ahmadi, Khor-Jafari, Khor-Zangi and Khor-Ghanam (Figure 1) using gillnet during September and February 2009. Ten individuals with the same size (16 - 18 cm) of Mult, L. abu were obtained from each Khor, transferred to the laboratory using icebox and were kept frozen at –20˚C until dissec- tion. Before analysis, the samples were thawed at room temperature. Each sample was dissected for its muscle, the entire liver and gill tissues. The tissues were oven dried at 80˚C for 24 h. About 1 gram of dried tissues was digested in concentrated nitric acid (65% Merck). The remaining digested solution was made up to certain volume with double distilled water and filtered through 0.42 µm paper filter. 2.2. Apparatus A GBC (Savant AA∑, Australia) Flame Atomic Absorp- tion Spectrometer was employed to determine Cd, Co, Cu, Ni and Pb in samples. All the studied metals were determined with air-acetylen flame. The operation condi- tions are given in Table 1. 2.3. Reagents All reagents used were of analytical grade. Concentrated stock solution of 1000 mg/l (merck, Germany) of each metal was diluted by double distilled water for instru- ment calibration. All the glassware were soaked in 10% nitric acid for 24 h and rinsed three times with double distilled water before use. Standard reference material (Dorm-2, muscle of Dogfish, National Research Council of Canada) was used to check the analysis accuracy. The result showed good agreement with the certified values. The recovery values were; 107%, 103%, 94%, 112% and 98% for Cd, Co, Cu, Ni and Pb respectively. The blanks were prepared in a similar manner without samples to avoid samples contamination. The prepared blanks were Figure 1. Location of the sampling stations. Copyright © 2011 SciRes. JEP  Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, 1220 Musa Estuary (Persian Gulf) Table 1. The operation condition of the AAS. Cd Co Cu Ni Pb Wavelengh (nm) 228.8 240.7 324.7 232 217 Silt Width (nm) 0.05 0.2 0.5 0.2 1 Lamp Current (MA) 3 6 3 4 5 used to calibrate the instrument. They also routinely were analyzed after every 10 analysis in order to auto zero of the AAS. 2.4. Statistical Analysis All data were tested for normal distribution with Shapiro- wilk normality test. Significant differences between heavy metals concentration in the samples of various stations were determined using One-Way analysis of variance (ANOVA) fallowed by Duncan post hoc test. Seasonal differences in metal concentration were examined using t_test. The level of significance was set at α= 0.05. 3. Results and Discussion The concentration of Cd, Co, Cu, Ni and Pb (mean ± standard error) in the liver tissue of L. abu is shown in Table 2. The maximum concentration of Co (2.80 µg/g), Cu (36.22 µg/g) and Ni (4.91 µg/g) were observed in the liver tissue of fish from Khor-Jafari. The maximum con- centration of Cd (2.03 µg/g) and Pb (5.74 µg/g) were obtained in the fish from Khor-Ghanam and Khor-Zangi respectively. The results of heavy metal analysis in the muscle are presented in Table 3. The concentration of Co in muscle tissue was below the detection limit in summer. The highest concentration of Cd (0.35 µg/g) and Pb (2.54 µg/g) were obtained in the muscle of fish from Khor-Ghanam and Khor-Jafari respectively. The highest concentration of Co (1.63 µg/g), Cu (4.28 µg/g) and Ni (2.73 µg/g) were found in the muscle tissue be- long to fish from Khor-Zangi. The concentration of heavy metals detected in the gill samples has given in Table 4. The highest concentration of Cd (2.72 µg/g) and Co (1.10 µg/g) were noted in Khor-Jafari, while the maximum of Cu (6.03 µg/g ), Ni (17.52 µg/g) and Pb (21.41 µg/g) were detected in the gill tissue belong to fish from Khor-Ahmadi, Khor-Ghazale and Khor-Zangi respectively. 3.1. Inter-Station Distribution of Heavy Metal Khor-Jafari and Khor-Zangi originate from Musa Estu- ary and are stretched along PETZONE (Petrochemical Special Economic Zone) up to Mashahr and Sarbandar cities. These creeks are used as municipal wastes recep- tors. Moreover, they receive copious amount of petroche- mical wastewater along their courses. Thus, the higher level of accumulated metals in fish from Khor-Jafari and Khor-Zangi could be related to anthropogenic activities and effluent discharges into the mentioned Khors. The proximity of Khor-Ghazale to oil terminal turns this area into a waterway for oil tankers. Therefore, the enrich- ment of heavy metals in this creek might reflect oil pol- lution in the area. Khor-Ahmadi is located between Khor-Jafari and Ghazale and consequently a great amount of heavy metal may displace to this creek by movements produced by waves and tidal current [21,22]. 3.2. Differences of Metals Accumulation among Season Heavy metals accumulate in muscle, liver and gills dis- played significant variation among different seasons (p < 0.05). Significant seasonal differences for heavy metal accumulation in the liver, gill and muscle are given in Tables 2, 3 and 4 respectively. There was a clear sea- sonal resemblance between concentration of Cd in the liver, muscle and gill and concentration of Co, Cu and Ni in the gills, except in the few cases. Generally the results showed that the concentration of Co in the liver and gill, Cu in the liver and muscle and Pb in the gills during winter were higher than summer. On the other hand, Ni concentration in the liver and muscle during summer was significantly higher than winter in all Khors. Seasonal variations observed in the metals concentration could be attributed to the differences in local pollution, bioavail- ability of metals (variations among physiochemical fac- tors) and fish metabolism (growth cycle, reproduction and feeding) [23-25]. In addition, some other indirect activities such as energy demand activities, atmospheric deposition and runoff inputting, that could lead to metal contamination, are variable among seasons. The spawn- ing period of L. abu takes place in winter (January and February). During this period, the feeding habits of L. abu might get altered [26]. This alteration in feeding habits could be the reason of observed difference in metal concentrations between the seasons [27]. Mullet fish is known as Phytoplanktivores (diatoms) and detritus feeder [28]. Due to decrease of Phytoplank- ton productivity during winter, the fish tends to leave surface waters and goes down near the sediment and here it mainly feeds on detritus. It is known that sediment is a main sink of contaminants particularly heavy metals en- C opyright © 2011 SciRes. JEP 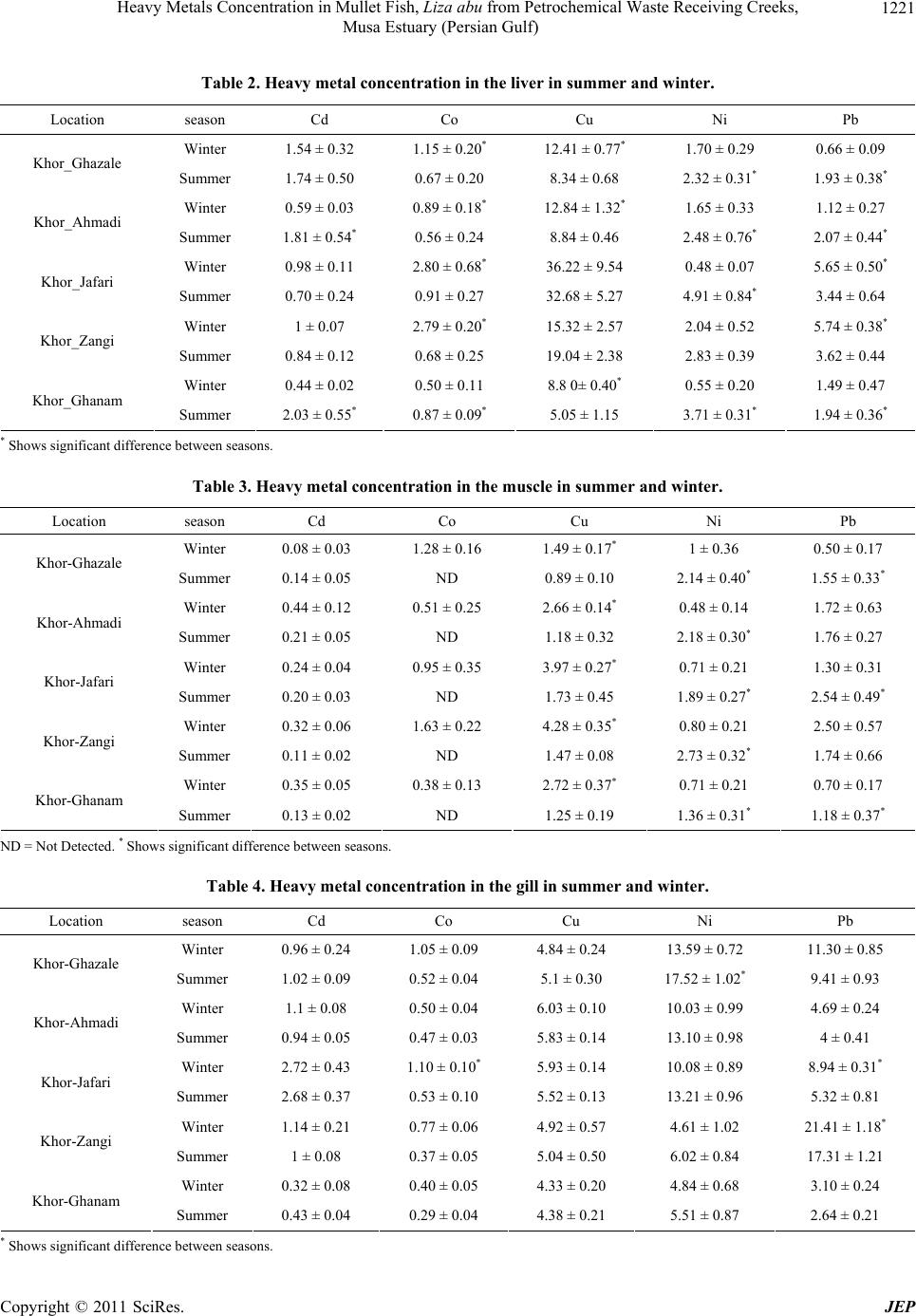 Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, 1221 Musa Estuary (Persian Gulf) Table 2. Heavy metal concentration in the liver in summer and winter. Location season Cd Co Cu Ni Pb Winter 1.54 ± 0.32 1.15 ± 0.20* 12.41 ± 0.77* 1.70 ± 0.29 0.66 ± 0.09 Khor_Ghazale Summer 1.74 ± 0.50 0.67 ± 0.20 8.34 ± 0.68 2.32 ± 0.31* 1.93 ± 0.38* Winter 0.59 ± 0.03 0.89 ± 0.18* 12.84 ± 1.32* 1.65 ± 0.33 1.12 ± 0.27 Khor_Ahmadi Summer 1.81 ± 0.54* 0.56 ± 0.24 8.84 ± 0.46 2.48 ± 0.76* 2.07 ± 0.44* Winter 0.98 ± 0.11 2.80 ± 0.68* 36.22 ± 9.54 0.48 ± 0.07 5.65 ± 0.50* Khor_Jafari Summer 0.70 ± 0.24 0.91 ± 0.27 32.68 ± 5.27 4.91 ± 0.84* 3.44 ± 0.64 Winter 1 ± 0.07 2.79 ± 0.20* 15.32 ± 2.57 2.04 ± 0.52 5.74 ± 0.38* Khor_Zangi Summer 0.84 ± 0.12 0.68 ± 0.25 19.04 ± 2.38 2.83 ± 0.39 3.62 ± 0.44 Winter 0.44 ± 0.02 0.50 ± 0.11 8.8 0± 0.40* 0.55 ± 0.20 1.49 ± 0.47 Khor_Ghanam Summer 2.03 ± 0.55* 0.87 ± 0.09* 5.05 ± 1.15 3.71 ± 0.31* 1.94 ± 0.36* * Shows significant difference between seasons. Table 3. Heavy metal concentration in the muscle in summer and winter. Location season Cd Co Cu Ni Pb Winter 0.08 ± 0.03 1.28 ± 0.16 1.49 ± 0.17* 1 ± 0.36 0.50 ± 0.17 Khor-Ghazale Summer 0.14 ± 0.05 ND 0.89 ± 0.10 2.14 ± 0.40* 1.55 ± 0.33* Winter 0.44 ± 0.12 0.51 ± 0.25 2.66 ± 0.14* 0.48 ± 0.14 1.72 ± 0.63 Khor-Ahmadi Summer 0.21 ± 0.05 ND 1.18 ± 0.32 2.18 ± 0.30* 1.76 ± 0.27 Winter 0.24 ± 0.04 0.95 ± 0.35 3.97 ± 0.27* 0.71 ± 0.21 1.30 ± 0.31 Khor-Jafari Summer 0.20 ± 0.03 ND 1.73 ± 0.45 1.89 ± 0.27* 2.54 ± 0.49* Winter 0.32 ± 0.06 1.63 ± 0.22 4.28 ± 0.35* 0.80 ± 0.21 2.50 ± 0.57 Khor-Zangi Summer 0.11 ± 0.02 ND 1.47 ± 0.08 2.73 ± 0.32* 1.74 ± 0.66 Winter 0.35 ± 0.05 0.38 ± 0.13 2.72 ± 0.37* 0.71 ± 0.21 0.70 ± 0.17 Khor-Ghanam Summer 0.13 ± 0.02 ND 1.25 ± 0.19 1.36 ± 0.31* 1.18 ± 0.37* ND = Not Detected. * Shows significant difference between seasons. Table 4. Heavy metal concentration in the gill in summer and winter. Location season Cd Co Cu Ni Pb Winter 0.96 ± 0.24 1.05 ± 0.09 4.84 ± 0.24 13.59 ± 0.72 11.30 ± 0.85 Khor-Ghazale Summer 1.02 ± 0.09 0.52 ± 0.04 5.1 ± 0.30 17.52 ± 1.02* 9.41 ± 0.93 Winter 1.1 ± 0.08 0.50 ± 0.04 6.03 ± 0.10 10.03 ± 0.99 4.69 ± 0.24 Khor-Ahmadi Summer 0.94 ± 0.05 0.47 ± 0.03 5.83 ± 0.14 13.10 ± 0.98 4 ± 0.41 Winter 2.72 ± 0.43 1.10 ± 0.10* 5.93 ± 0.14 10.08 ± 0.89 8.94 ± 0.31* Khor-Jafari Summer 2.68 ± 0.37 0.53 ± 0.10 5.52 ± 0.13 13.21 ± 0.96 5.32 ± 0.81 Winter 1.14 ± 0.21 0.77 ± 0.06 4.92 ± 0.57 4.61 ± 1.02 21.41 ± 1.18* Khor-Zangi Summer 1 ± 0.08 0.37 ± 0.05 5.04 ± 0.50 6.02 ± 0.84 17.31 ± 1.21 Winter 0.32 ± 0.08 0.40 ± 0.05 4.33 ± 0.20 4.84 ± 0.68 3.10 ± 0.24 Khor-Ghanam Summer 0.43 ± 0.04 0.29 ± 0.04 4.38 ± 0.21 5.51 ± 0.87 2.64 ± 0.21 * Shows significant difference between seasons. Copyright © 2011 SciRes. JEP 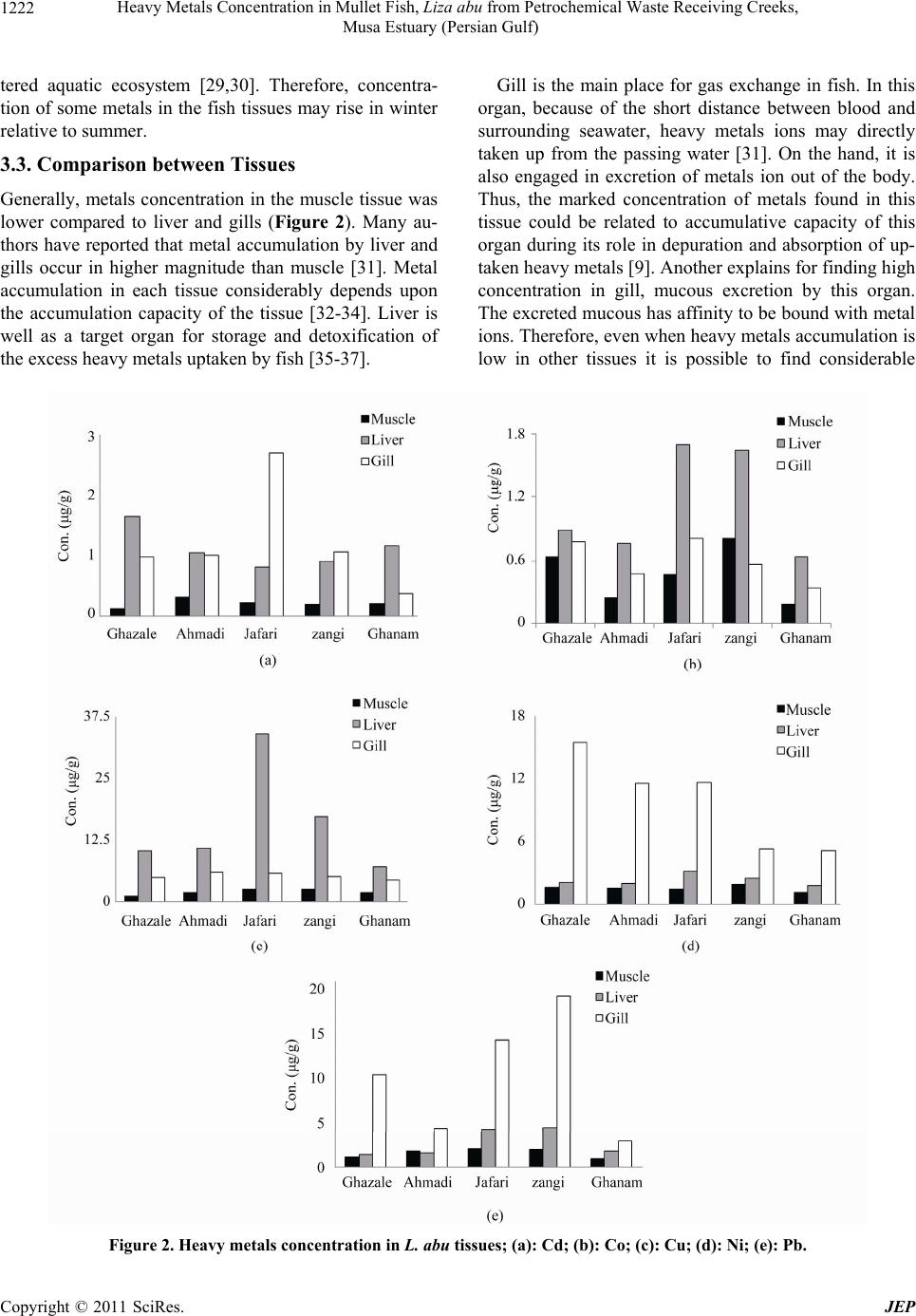 Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, 1222 Musa Estuary (Persian Gulf) tered aquatic ecosystem [29,30]. Therefore, concentra- tion of some metals in the fish tissues may rise in winter relative to summer. 3.3. Comparison between Tissues Generally, metals concentration in the muscle tissue was lower compared to liver and gills (Figure 2). Many au- thors have reported that metal accumulation by liver and gills occur in higher magnitude than muscle [31]. Metal accumulation in each tissue considerably depends upon the accumulation capacity of the tissue [32-34]. Liver is well as a target organ for storage and detoxification of the excess heavy metals uptaken by fish [35-37]. Gill is the main place for gas exchange in fish. In this organ, because of the short distance between blood and surrounding seawater, heavy metals ions may directly taken up from the passing water [31]. On the hand, it is also engaged in excretion of metals ion out of the body. Thus, the marked concentration of metals found in this tissue could be related to accumulative capacity of this organ during its role in depuration and absorption of up- taken heavy metals [9]. Another explains for finding high concentration in gill, mucous excretion by this organ. The excreted mucous has affinity to be bound with metal ions. Therefore, even when heavy metals accumulation is low in other tissues it is possible to find considerable Figure 2. Heavy metals concentration in L. abu tissues; (a): Cd; (b): Co; (c): Cu; (d): Ni; (e): Pb. C opyright © 2011 SciRes. JEP  Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, 1223 Musa Estuary (Persian Gulf) amount of metals in this part [38,39]. 3.4. Comparison with Standard and Previous Study Heavy metals concentration in various tissues of Mullet, L. abu from the northwest Persian Gulf is compared with some previous studies from other locations of the world (Table 5). The concentration of Cd, Cu and Ni in muscle of L. abu from Musa Estuary were higher than Solea Elongate, Psettodes erumeei and Epinephelus coioides from north Persian Gulf [40] and Dicentrarchus labrax, Sparus aurata and Mugil cephalus from Tuzen Lagoon [13]. However, the concentration of Cd and Pb in present study was lower than those given for Liza ramada from Rosario [41]. The concentration of Cd, Co and Cu in the liver of Liza abu was higher than the species that studied by Dural et al., (2007). On the other hand, the level of Cu in the liver of L. abu from Ataturk was higher than L. abu that was caught from Musa Estuary. The maximum concentration of Cu, Ni and Pb in gills during this study were higher than the results that reported by Dural et al., (2007) and similar to the results that reported by Karad- ede et al., (2004) for Cu. The higher concentration of Ni and Co in L. abu from the Persian Gulf may be attributed to the strong oil pollution in the area. Comparison of metals concentration in current study with some available standards (Table 6) showed that Cd concentration in the fish muscle of Khor-Ahmadi, Khor- Table 5. Heavy metal concentration in fish tissues reported in literature (µg/g dw). Tissues Location Species Cd Co Cu Ni Pb Reference Muscle Musa Estuary Liza abu This study Persian Gulf , northern Part Solea elongata 0.072 6.69 2.43 [40] Persian Gulf , northern Part Psettodes erumeei 0.105 1.09 2.09 [40] Persian Gulf northern Part Epinephelus c o io ides 0.111 1.56 2.32 [40] Ataturk Dam Lake Liza abu* ND 1.36 ND [7] Tuzla lagoon Dicentrarchus labrax0.08 0.26 0.40 Tuzla lagoon Sparus aurata 0.10 0.82 2.44 Tuzla lagoon Mugil cephalus 0.11 0.47 0.49 [7] Rosario Liza ramada 0.9 1.6 3.7 [41] Liver Musa Estuary Liza abu This study Ataturk Dam Lake Liza abu* ND 267.45 ND [7] Tuzla lagoon Dicentrarchus labrax0.19 0.69 1.72 Tuzla lagoon Sparus aurata 0.19 2.63 1.87 Tuzla lagoon Mugil cephalus 0.21 4.77 2.12 [13] Gill Musa Estuary Liza abu This study Ataturk Dam Lake Liza abu* ND 6.27 ND [7] Tuzla lagoon Dicentrarchus labrax1.12 1.10 3.71 Tuzla lagoon Sparus aurata 1.01 1.94 3.86 Tuzla lagoon Mugil cephalus 1.27 3.43 4.54 [13] Concentration are in µg/g dry weight, except the cases are denoted with asterisk, which are in µg/g wet weight. ND = Not Detect. Table 6. Heavy metal concentration in guide line. Standard Cd Cu Pb References FAO, (1983) 0.5 ( ppm ) 30 ( ppm ) 0.5 ( ppm ) [42] Turkish Guidelinesa 0.1 ( µg/g ) 20 ( µg/g ) 1 ( µg/g ) [43] Saudi Arabia 0.5 ( µg/g ) 2 ( µg/g ) [44] ECb 0.05 ( µg/g ) 0.5 ( µg/g ) [44] a Turkish Environmental Guidelines (1988). EC = European Communities. Copyright © 2011 SciRes. JEP  Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, 1224 Musa Estuary (Persian Gulf) Zangi and Khor-Ghanam during the summer were below the permissible level of EC (Commission of the Euro- pean Communities, 1997), on the other hand the men- tioned concentration during the winter exceeded the EC limits. In addition, Pb concentration of fish muscle from Khor-Zangi during the winter and in Khor-Jafari during the summer was in agreement with the limits prescribed by both EC an FAO (1983). 4. Conclusions Heavy metals accumulate in different tissues of mullet fish with different magnitudes. Generally, metals accu- mulation in muscle was lower than gills and liver. Lead and nickel mainly accumulated in gills while the main tissue for Cu and Co accumulation was liver. Fish caught from stations closed to the PETZONE and Imam Port was found to containing high level of metals in muscle, liver and gills. The results provide new information on the distribution of heavy metals in liver, gill and edible tissues of Liza abu, and indicate that the levels of some metals exceed the legal limit that designated by some health organization. The major finding of this study de- monstrated that seasons play significant role in metals accumulation. Therefore, in some seasons human might be at risk of metal contamination via fish consumption but in other seasons the level of heavy metal in the fish decreases below the dangerous limits. REFERENCES [1] J. Burger and M. Gochfeld, “Heavy Metals in Commer- cial Fish in New Jersey,” Environmental Research, Vol. 99, No. 3, 2005, pp. 403-412. doi:10.1016/j.envres.2005.02.001 [2] J.L. Domingo, A. Bocio, R. Martı-Cid and J. M. Llobet, “Benefits and Risks of Fish Consumption Part II. RIBEP- EIX, a Computer Program to Optimize the Balance be- tween the Intake of Omega-3 Fatty Acids and Chemical Contaminants,” Toxicology, Vol. 230, No. 2-3, 2007, pp. 227-233. doi:10.1016/j.tox.2006.11.059 [3] N. Shirai, T. Higuchi and H. Suzuki, “Analysis of Lipid Classes and the Fatty Acid Composition of the Salted Fish Roe Food Products, Ikura, Tarako, Tobiko and Ka- zunoko,” Food Chemistry, Vol. 94, No. 1, 2006, pp. 61-67. doi:10.1016/j.foodchem.2004.10.050 [4] N. Sinn and P. R. C. Howe, “Mental Health Benefits of Omega-3 Fatty Acids May Be Mediated by Improve- ments in Cerebral Vascular Function,” Bioscience Hy- potheses, Vol. 1, No. 2, 2008, pp. 103-108. doi:10.1016/j.bihy.2008.02.003 [5] M. I. Gladyshev, N. Sushchik, O. Anishchenko and O. N. Makhutova, “Benefit-Risk Ratio of Food Fish Intake as the Source of Essential Fatty Acids vs. Heavy Metals: A Case Study of Siberian Grayling from the Yenisei River,” Food Chemistry, Vol. 115, No. 2, 2009, pp. 545-550. doi:10.1016/j.foodchem.2008.12.062 [6] N. Tarras-Wahlberg, A. Flachier, S. N. Lane and O. Sangfors, “Environmental Impacts and Metal Exposure of Aquatic Ecosystems in Rivers Contaminated by Small Scale Gold Mining: The Puyango River Basin, Southern Ecuador,” Science of Total Environment, Vol. 278, No. 1-3, 2001, pp. 239-261. [7] H. l. Karadede, S. A. Oymak and E. Unlu, “Heavy Metals in Mullet, Liza abu, and Catfish, Silurus triostegus, from the Ataturk Dam Lake (Euphrates), Turkey,” Environ- ment International, Vol. 30, No. 2, 2004, pp. 183-188. doi:10.1016/S0160-4120(03)00169-7 [8] M. Ozmen, Z. Ayas, A. Gungordu, G. F. Ekmekci and S. Yerli, “Ecotoxicological Assessment of Water Pollution in Sariyar Dam Lake, Turkey,” Ecotoxicology and Envi- ronmental Safety, Vol. 70, No. 1, 2008, pp. 163-173. doi:10.1016/j.ecoenv.2007.05.011 [9] M. H. Al-Yousuf, M. S. El-Shahawi, and S. M. Al-Ghais. “Trace Metals in Liver, Skin and Muscle of Lethrinus lentjan Fish Species in Relation to Body Length and Sex,” The Science of the Total Environment, Vol. 256, No. 2-3, 2000, pp. 87-94. doi:10.1016/S0048-9697(99)00363-0 [10] A. Turkmen, M. Tu¨rkmen, Y. Tepe and I. Akyurt, “Heavy Metals in Three Commercially Valuable Fish Species from Iskenderun Bay, Northern East Mediterra- nean Sea, Turkey,” Food Chemistry, Vol. 91, No. 1, 2005, pp. 167-172. doi:10.1016/j.foodchem.2004.08.008 [11] M. Tuzen, “Determination of Heavy Metals in Fish Sam- ples of the Middle Black Sea (Turkey) by Graphite Fur- nace Atomic Absorption Spectrometry,” Food Chemistry, Vol. 80, No. 1, 2003, pp. 119-123. doi:10.1016/S0308-8146(02)00264-9 [12] A. Altındag and S. Yig, “Assessment of Heavy Metal Concentrations in the Food Web of Lake Beys_Ehir, Tur- key,” Chemosphere, Vol. 60, No. 4, 2005, pp. 552-556. doi:10.1016/j.chemosphere.2005.01.009 [13] M. Dural, M. Ziya Lugal Goksu, and A. A. Ozak. “Inves- tigation of Heavy Metal Levels in Economically Impor- tant Fish Species Captured from the Tuzla Lagoon,” Food Chemistry, Vol. 102, No. 1, 2007, pp. 415-421. doi:10.1016/j.foodchem.2006.03.001 [14] P. Grandjean, “Methylmercury Toxicity and Functional Programming,” Report of Toxicology, Vol. 23, No. 3, 2007, pp. 414-420. doi:10.1016/j.reprotox.2007.03.002 [15] J. G. Dorea, “Persistent, Bioaccumulative and Toxic Sub- stances in Fish: Human Health Considerations,” Science of the Total Environment, Vol. 400, No. 1-3, 2008, pp. 93-114. doi:10.1016/j.scitotenv.2008.06.017 [16] B. L. Gulson, K. R. Mahaffey, C. W. Jameson, K. J. Mizon, M. J. Korsch, M. A. Cameron and J. A. Eisman, “Mobilization of Lead from the Skeleton during the Post- natal Period Is Larger than during Pregnancy,” Journal of Laboratory and Clinical Medicine, Vol. 131, No. 4, 1998, pp. 324-329. doi:10.1016/S0022-2143(98)90182-2 C opyright © 2011 SciRes. JEP  Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, 1225 Musa Estuary (Persian Gulf) [17] M. C. Navarro, C. Perez-Sirvent, M. J. Martınez-Sanchez, J. Vidal and J. Marimon, “Lead, Cadmium and Arsenic Bioavailability in the Abandoned Mine Site of Cabezo Rajao (Murcia, SE Spain),” Chemosphere, Vol. 63, No. 3, 2006, pp. 484-489. doi:10.1016/j.chemosphere.2005.08.017 [18] M. Canli and G. Z. Atli, “The Relationships between Heavy Metal (Cd, Cr, Cu, Fe, Pb, Zn) Levels and the Size of Six Mediterranean Fish Species,” Environmental Pol- lution, Vol. 121, No. 1, 2003, pp. 129-136. doi:10.1016/S0269-7491(02)00194-X [19] M. Tuzen, “Toxic and Essential Trace Elemental Con- tents in Fish Species from the Black Sea, Turkey,” Food and Chemical Toxicology, Vol. 47, No. 8, 2009, pp. 1785-1790. doi:10.1016/j.fct.2009.04.029 [20] X. Wang, T. Sato, B. Xing and S. Tao, “Health Risks of Heavy Metals to the General Public in Tianjin, China via Consumption of Vegetables and Fish,” Science of the Total Environment, Vol. 350, No. 1-3, 2005, pp. 28-37. doi:10.1016/j.scitotenv.2004.09.044 [21] H. Feng, J. K. Cochran and D. J. Hirschberg, “Transport and Sources of Metal Contaminants over the Course of Tidal Cycle in the Turbidity Maximum Zone of the Hud- son River Estuary,” Water Research, Vol. 36, No. 3, 2002, pp. 733-743. doi:10.1016/S0043-1354(01)00268-8 [22] V. K. Mubiana, K. Vercauteren and R. Blust, “The Influ- ence of Body Size, Condition Index and Tidal Exposure on the Variability in Metal Bioaccumulation in Mytilus edulis,” Environmental Pollution, Vol. 144, No. 1, 2006, pp. 272-279. doi:10.1016/j.envpol.2005.12.017 [23] J. Aucoin, R. Blanchard, C. Billiot, C. Partridge, D. Schultz, K. Mandhare, M. J. Beck and J. N. Beck, “Trace Metals in Fish and Sediments from Lake Boeuf, South- eastern Louisiana,” Microchemical Journal, Vol. 62, No. 2, 1999, pp. 299-307. doi:10.1006/mchj.1999.1735 [24] S. Eastwood and P. Couture, “Seasonal Variations in Condition and Liver Metal Concentrations of Yellow Perch (Perca flaescens) from a Metal-Contaminated En- vironment,” Aquatic Toxicology, Vol. 58, No. 1, 2002, pp. 43-56. doi:10.1016/S0166-445X(01)00218-1 [25] D. Mendil, O. D. Uluozlu, E. Hasdemir, M. Tuzen, H. Sari and M. Suic¸mez, “Determination of Trace Metal Levels in Seven Fish Species in Lakes in Tokat, Turkey,” Food Chemistry, Vol. 90, No. 1-2, 2005, pp. 175-179. doi:10.1016/j.foodchem.2004.03.039 [26] T. Ahmad, N. A. Salman and N. A. Hussain, “Effects of Some Experimental Conditions on the Behaviour and Survival of Liza abu (Heckel) from Basrah, Iraq,” Jour- nal of The Faculty of Marine Science, Vol. 3, 1404H, 1983. [27] F. Yılmaz , N. Ozdemir, A. Demirak and A. Levent Tuna, “Heavy Metal Levels in Two Fish Species Leuciscus cephalus and Lepomis gibbosus,” Food Chemistry, Vol. 100, No. 2, 2007, pp. 830-835. doi:10.1016/j.foodchem.2005.09.020 [28] A. R. M. Mohamed, N. A. Hussain, S. S. Al-Noor, F. M. Mutlak, I. M. Al-Sudani, A. M. Mojer, A. J. Toman and M. A. Abdad, “Fish Assemblage of Restored Al-Hawizeh Marsh, Southern Iraq,” Ecohydrological Processes and Sustainable Floodplain Managemen, Vol. 8, No. 2-4, 2008, pp. 375-384. [29] S. Duquesne, L. C. Newton, L. Giusti, S. B. Marriott, H. J. Stark and D. J. Bird, “Evidence for Declining Levels of Heavy-Metals in the Severn Estuary and Bristol Channel, U.K. and Their Spatial Distribution in Sediments,” Envi- ronmental Pollution, Vol. 143, No. 2, 2006, pp. 187-196. doi:10.1016/j.envpol.2005.12.002 [30] E. P. Nobi, E. Dilipan, T. Thangaradjou, K. Sivakumar and L. Kannan, “Geochemical and Geo-Statistical As- sessment of Heavy Metal Concentration in the Sediments of Different Coastal Ecosystems of Andaman Islands, In- dia,” Estuarine, Coastal and Shelf Science, Vol. 87, No. 2, 2010, pp. 253-264. doi:10.1016/j.ecss.2009.12.019 [31] A. Farkas, J. Salanki and A. Specziar, “Age- and Size- Specific Patterns of Heavy Metals in the Organs of Freshwater Fish Abramis brama L. Populating a Low- Contaminated Site,” Water Research, Vol. 37, No. 5, 2003, pp. 959-964. [32] J. Kojadinovic, M. Potier, M. L. Corre, R. P. Cosson and P. Bustamante, “Bioaccumulation of Trace Elements in Pelagic Fish from the Western Indian Ocean,” Environ- mental Pollution, Vol. 146, No. 2, 2007, pp. 548-566. doi:10.1016/j.envpol.2006.07.015 [33] F. Dang and W. X. Wang, “Assessment of Tissue-Spe- cific Accumulation and Effects of Cadmium in a Marine Fish Fed Contaminated Commercially Produced Diet,” Aquatic Toxicology, Vol. 95, No. 3, 2009, pp. 248-255. doi:10.1016/j.aquatox.2009.09.013 [34] J. Kalman, I. Riba, T. A. DelValls and J. Blasco, “Com- parative Toxicity of Cadmium in the Commercial Fish Species Sparus aurata and Solea senegalensis,” Ecotoxi- cology and Environmental Safety, Vol. 73, No. 3, 2010, pp. 306-311. doi:10.1016/j.ecoenv.2009.10.013 [35] A. B. Yılmaz, “Levels of Heavy Metals (Fe, Cu, Ni, Cr, Pb and Zn) in Tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay Turkey,” Environ- mental Research , Vol. 92, No. 3, 2003, pp. 277-281. doi:10.1016/S0013-9351(02)00082-8 [36] L. D. Kraemer, P. G. C. Campbell and L. Hare, “Seasonal Variations in Hepatic Cd and Cu Concentrations and in the Sub-Cellular Distribution of These Metals in Juvenile Yellow Perch (Perca flavescens),” Environmental Pollu- tion, Vol. 142, No. 2, 2006, pp. 313-325. doi:10.1016/j.envpol.2005.10.004 [37] R. Company, H. Felıcia, A. Serafim, A. J. Almeida, M. Biscoito and M. J. Bebianno, “Metal Concentrations and Metallothionein-Like Protein Levels in Deep-Sea Fishes Captured near Hydrothermal Vents in the Mid-Atlantic Ridge off Azores,” Deep-Sea Research I, Vol. 57, No. 7, 2010, pp. 893-908. doi:10.1016/j.dsr.2010.02.005 [38] S. L. R. G. Junior, F. G. Araujo, M. F. Maia and S. B. A. S. Pinto, “Evaluation of Heavy Metals in Fish of the Se- petiba and Ilha Grande Bays, Rio de Janeiro, Brazil,” En- Copyright © 2011 SciRes. JEP  Heavy Metals Concentration in Mullet Fish, Liza abu from Petrochemical Waste Receiving Creeks, Musa Estuary (Persian Gulf) Copyright © 2011 SciRes. JEP 1226 vironmental Research Section A, Vol. 89, 2002, pp. 171-179. doi:10.1006/enrs.2002.4341 [39] J. Usero, C. Izquierdo, J. Morillo and I. Gracia, “Heavy metals in fish (Solea vulgaris, Anguilla anguilla and Liza aurata) from Salt Marshes on the Southern Atlantic Coast of Spain,” Environment International, Vol. 29, No. 7, 2003, pp. 949-956. doi:10.1016/S0160-4120(03)00061-8 [40] N. Pourang, A. Nikouyan and J. H. Dennis, “Trace Ele- ment Concentrations in Fish, Surficial Sediments and Water from Northern Part of the Persian Gulf,” Environ- mental Monitoring and Assessment, Vol. 109, No. 1-3, 2005, pp. 293-316. doi:10.1007/s10661-005-6287-9 [41] S. Franca, C. Vinagre, I. Cacador and H. N. Cabral, “Heavy Metal Concentrations in Sediment, Benthic In- vertebrates and Fish in Three Salt Marsh Areas Subjected to Different Pollution Loads in the Tagus Estuary (Portu- gal),” Marine Pollution Bulletin, Vol. 50, 2005, pp. 993-1018. doi:10.1016/j.marpolbul.2005.06.040 [42] T. V. Sankar, A. A. Zynudheen, R. Anandan and P. G. Viswanathan Nair, “Distribution of Organochlorine Pes- ticides and Heavy Metal Residues in Fish and Shellfish from Calicut Region, Kerala, India,” Chemosphere, Vol. 65, No. 4, 2006, pp. 583-590. doi:10.1016/j.chemosphere.2006.02.038 [43] A. Demirak, F. Yilmaz, L. Tuna and N. Ozdemir, “Heavy Metals in Water, Sediment and Tissues of Leuciscus cephalus from a Stream in Southwestern Turkey,” Chemosphere, Vol. 63, No. 9, 2006, pp. 1451-1458. doi:10.1016/j.chemosphere.2005.09.033 [44] I. Al-Saleh and N. Shinwari, “Preliminary Report on the Levels of Elements in Four Fish Species from the Ara- bian Gulf of Saudi Arabia,” Chemosphere, Vol. 48, No. 7, 2002, pp. 749-755. doi:10.1016/S0045-6535(02)00126-1
|