 American Journal of Plant Sciences, 2011, 2, 609-618 doi:10.4236/ajps.2011.24072 Published Online October 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS 609 Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis Wen-Hu Cai1, Katsuyoshi Matsunami1, Hideaki Otsuka1, Yoshio Takeda2 1Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima, Japan; 2Faculty of Pharmacy, Yasuda Women’s Uni- versity, Hiroshima, Japan. Email: hotsuka@hiroshima-u.ac.jp Received July 8th, 2011; revised August 31st, 2011; accepted September 9th, 2011. ABSTRACT From the 1-BuOH-soluble fraction of a MeOH extract of the leaves of Symplocos cochinchinensis var. philippinensis, 12 compounds were isolated. Spectroscopic analyses of compounds 1 - 3 established their structures to be megastig- mane glycosides, named symplocosionosides A-C. The absolute structure of 1 was determined by the modified Mosher’s method. Compound 4 was found to be a neolignan glucoside and named symplocosneolignan. The structures of com- pounds 5 and 6, named symplocosins A and B, were elucidated to be the saponins of hederagenin sugar esters. The structures of the remaining known compounds (7 - 12) were identified by comparison of spectroscopic data with those reported in the literature. Keywords: Symplocos Cochinchinensis var. Philippinensis, Symplocaceae, Megastigmane Glycoside, Neolignan Glucoside, Triterpene Glycosyl Ester, Modified Mosher’s Method 1. Introduction Genus Symplocos comprises about 300 species, which are mainly found in tropical, except for Africa, and subtropical areas, with a small number of species in the temperate zone. Symplocos cochinchinensis (Loureiro) Spencer Le Marchant Moore var. philippinensis (Brand) Nootboom (Symplocaceae) is an evergreen tall tree, which is distributed in the Amami and Okinawa Islands, Taiwan, Southern China and Indochina. It grows up to about 15 m in height and bears white flowers in spikes [1]. So far as we know, no chemical investigation has been performed on this plant. Even for its elementary species, S. cochinchinensis (Loureiro) Spencer Le Mar- chant Moore, only few pharmacological works have been performed on its extract [2-4]. Thus, we were promted to invetigate the chemical constituents in the title plant. From the 1-BuOH-soluble fraction of a MeOH extract of the leaves of S. cochinch inensis var. philippinens is , three new megastigmane glycosides, named symplocosiono- sides A-C (1-3), a new neolignan glucoside, named sym- plocosneolignan A (4), and two new triterpene glycosyl esters, named symplocosins A and B (5,6), together with six known compounds, dendranthemoside A (7) [5], 4,5- dihydroblumenol (8) [6], alangionoside B (9) [7], ampe- lopsisionoside (10) [8], citroside A (11) [9], and niga- ichigoside F1 (12) [10] were isolated. This paper deals with structural elucidation of the new compounds. 2. Results and Discussion From the 1-BuOH-soluble fraction of a MeOH extract of the leaves of S. cochinchinensis var. philippinensis, six new compounds (1-6) and six known (7-12) compounds were isolated by means of a combination of various chromatographic technique. Symplocosionoside A (1), 25 D [] –44.0, was isolated as an amorphous powder and its elemental composition was determined to be C24H42O11 by high-resolution (HR)- electrospray ionization (ESI)-mass spectrometry (MS). 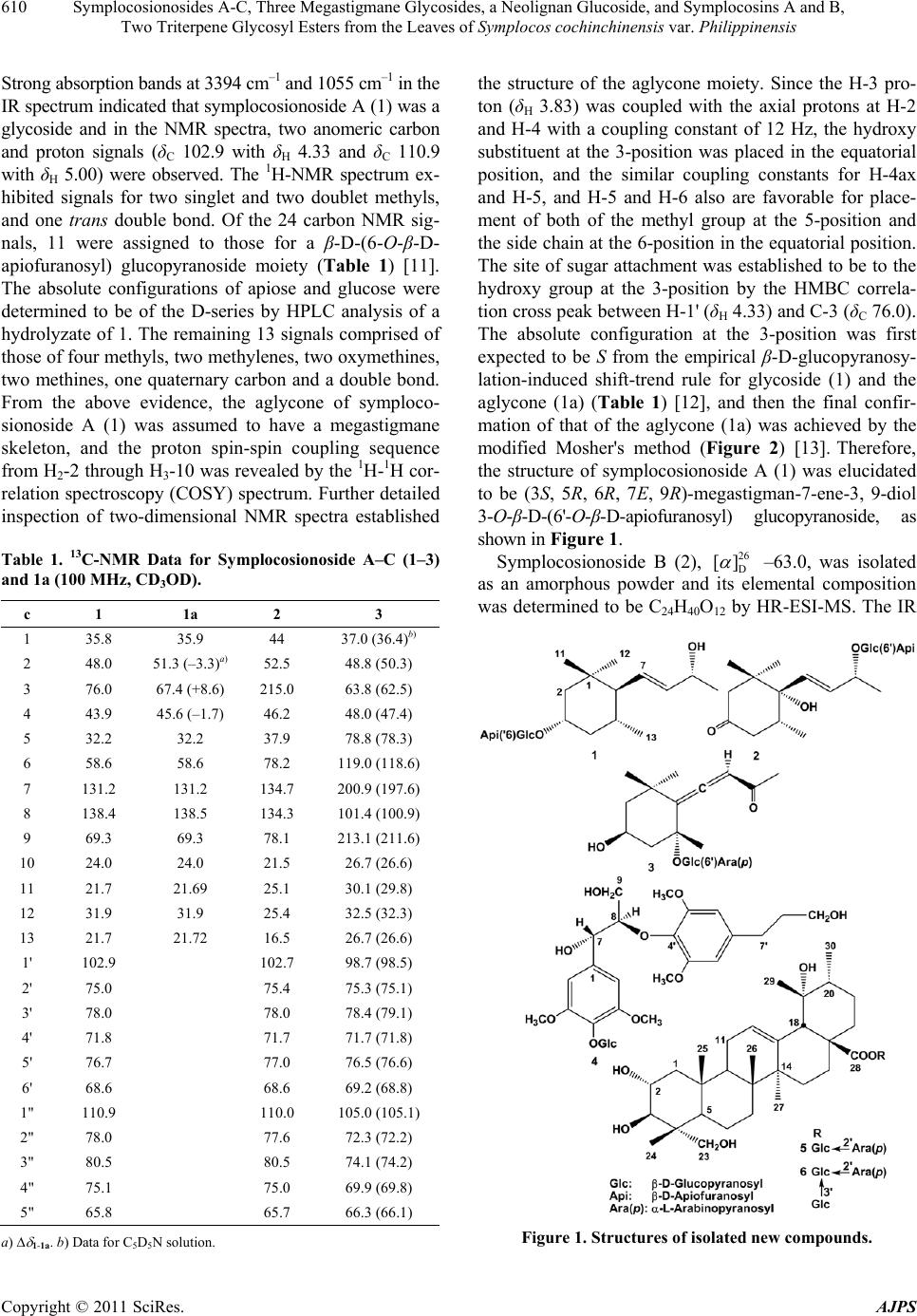 Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 610 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis Strong absorption bands at 3394 cm–1 and 1055 cm–1 in the IR spectrum indicated that symplocosionoside A (1) was a glycoside and in the NMR spectra, two anomeric carbon and proton signals (δC 102.9 with δH 4.33 and δC 110.9 with δH 5.00) were observed. The 1H-NMR spectrum ex- hibited signals for two singlet and two doublet methyls, and one trans double bond. Of the 24 carbon NMR sig- nals, 11 were assigned to those for a β-D-(6-O-β-D- apiofuranosyl) glucopyranoside moiety (Table 1) [11]. The absolute configurations of apiose and glucose were determined to be of the D-series by HPLC analysis of a hydrolyzate of 1. The remaining 13 signals comprised of those of four methyls, two methylenes, two oxymethines, two methines, one quaternary carbon and a double bond. From the above evidence, the aglycone of symploco- sionoside A (1) was assumed to have a megastigmane skeleton, and the proton spin-spin coupling sequence from H2-2 through H3-10 was revealed by the 1H-1H cor- relation spectroscopy (COSY) spectrum. Further detailed inspection of two-dimensional NMR spectra established Table 1. 13C-NMR Data for Symplocosionoside A–C (1–3) and 1a (100 MHz, CD3OD). c 1 1a 2 3 1 35.8 35.9 44 37.0 (36.4)b) 2 48.0 51.3 (–3.3)a)52.5 48.8 (50.3) 3 76.0 67.4 (+8.6) 215.0 63.8 (62.5) 4 43.9 45.6 (–1.7) 46.2 48.0 (47.4) 5 32.2 32.2 37.9 78.8 (78.3) 6 58.6 58.6 78.2 119.0 (118.6) 7 131.2 131.2 134.7 200.9 (197.6) 8 138.4 138.5 134.3 101.4 (100.9) 9 69.3 69.3 78.1 213.1 (211.6) 10 24.0 24.0 21.5 26.7 (26.6) 11 21.7 21.69 25.1 30.1 (29.8) 12 31.9 31.9 25.4 32.5 (32.3) 13 21.7 21.72 16.5 26.7 (26.6) 1' 102.9 102.7 98.7 (98.5) 2' 75.0 75.4 75.3 (75.1) 3' 78.0 78.0 78.4 (79.1) 4' 71.8 71.7 71.7 (71.8) 5' 76.7 77.0 76.5 (76.6) 6' 68.6 68.6 69.2 (68.8) 1" 110.9 110.0 105.0 (105.1) 2" 78.0 77.6 72.3 (72.2) 3" 80.5 80.5 74.1 (74.2) 4" 75.1 75.0 69.9 (69.8) 5" 65.8 65.7 66.3 (66.1) a) 1-1a. b) Data for C5D5N solution. the structure of the aglycone moiety. Since the H-3 pro- ton (δH 3.83) was coupled with the axial protons at H-2 and H-4 with a coupling constant of 12 Hz, the hydroxy substituent at the 3-position was placed in the equatorial position, and the similar coupling constants for H-4ax and H-5, and H-5 and H-6 also are favorable for place- ment of both of the methyl group at the 5-position and the side chain at the 6-position in the equatorial position. The site of sugar attachment was established to be to the hydroxy group at the 3-position by the HMBC correla- tion cross peak between H-1' (δH 4.33) and C-3 (δC 76.0). The absolute configuration at the 3-position was first expected to be S from the empirical β-D-glucopyranosy- lation-induced shift-trend rule for glycoside (1) and the aglycone (1a) (Table 1) [12], and then the final confir- mation of that of the aglycone (1a) was achieved by the modified Mosher's method (Figure 2) [13]. Therefore, the structure of symplocosionoside A (1) was elucidated to be (3S, 5R, 6R, 7E, 9R)-megastigman-7-ene-3, 9-diol 3-O-β-D-(6'-O-β-D-apiofuranosyl) glucopyranoside, as shown in Figure 1. Symplocosionoside B (2), 26 D [] –63.0, was isolated as an amorphous powder and its elemental composition was determined to be C24H40O12 by HR-ESI-MS. The IR Figure 1. Structures of isolated new compounds. Copyright © 2011 SciRes. AJPS  Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 611 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis Figure 2. Results of modified Mosher’s method for symplo- cosionoside A (1). spectrum exhibited a glycosidic feature (3399 cm–1 and 1056 cm–1) and the presence of a ketonic functional group (1694 cm–1). In the 1H-NMR spectrum, the signals for two singlet methyls, two doublet methyls, one trans double bond and two anomeric protons were observed. The 13C-NMR spectrum exhibited 24 signals, 11 of which were assignable to those of a 9-O-β-D-(6-O- β-D-apiofur-anosyl) glucopyranoside moiety. The abso- lute configurations of apiose and glucose were deter- mined to be of the D-series by HPLC analysis of the hy- drolyzate of 2. The remaining 13 signals comprised those of four methyls, two methylenes, one oxymethine, one methine, one double bond, two quaternary carbons and a ketone functional group. From the above evidence to- gether with the information obtained from two dimen- sional spectra, the structure of symplocosonoside B (2) was determined to be 3-keto-6-hydro- xymegastigmane glycoside, as shown in Figure 1. From the proton-proton coupling constant, J4ax-5 = 14 Hz, the methyl group at the 5-position was placed in an equatorial position, and then the correlation cross peak between H-5 (δH 2.28) and H-7 (δH 5.74) observed on phase-sensitive (PS)-nuclear Overhauser effect spectroscopy (NOESY) allowed place- ment of the side chain also in an equatorial position. Based on the octant rule, the absolute configuration at the 5-position was determined to be R from the positive Cotton effect at 289 nm in the CD spectrum and thus the 6-position must have the S configuration. By comparing the reported data, the absolute configuration at the 9-position was determined to be R, and the linkage of the sugar unit to the hydroxy group at the 9-positon was con- firmed by the HMBC correlation cross peak between H-1' (δH 4.34) and C-9 (δC 78.1). Therefore, the structure of symplocosionoside B (2) was elucidated to be (5R, 6S, 7E, 9R)-megastigman-7-en-9-ol-3-one 9-O-β-D-(6'-O-β- D-apiofuranosyl) glucopyranoside. Symplocosonoside C (3), 25 D [] –57.9, was isolated as an amorphous powder and its elemental composition was determined to be C24H38O12 by HR-ESI-MS. The IR spectrum exhibited a characteristic absorption band for an allenic part (1938 cm–1), and the presence of this func- tional group was supported by the 13C-NMR spectrum (δC 118.6, 197.6 and 100.9 in C5D5N). Other 13C-NMR signals showed good similarity to those of citrosides A and B, isolated from Citrus unshiu [9], except for the presence of signals for a terminal α-arabinopyranose, and the upfield and downfield shifts of the C-5' (δC 76.6 in C5D5N) and C-6' (δC 68.8 in C 5D5N) signals. A signifi- cant cross peak, observed between H-1" (δH 5.05 in C5D5N) and C-6' in the HMBC spectrum, confirmed the sugar linkage. The absolute configurations of arabinose and glucose were determined to be of the L- and D-series, respectively, by HPLC analysis of the hydrolyzate of 3 using the chiral detector. The axis chirality of the allene part was determined to be the same as that of citroside A, tentatively R, as judged on comparison of the 13C-NMR chemical shift of C-7 (δC 197.6 in C5D5N) with those for citrosides A (C-7: δC 197.6) and B (C-7: δC 199.0). Therefore, the structure of symplocosionoside C (3) was elucidated to be citroside A 6'-O-α-L-arabinopyranoside, as shown in Figure 1. Symplocosneolignan (4), 25 D [] –13.5, was isolated as an amorphous powder and its elemental composition was determined to be C28H40O14 by HR-ESI-MS. The IR spectrum showed that symplocosneolignan (4) was a glycosidic compound (3395 cm–1) with aromatic ring (s) (1592 cm–1). A UV absorption band (269 nm) also sup- ported the presence of the aromatic ring (s). In the 1H- NMR spectrum, signals for two singlet aromatic protons (δH 6.53 and 6.75), two methoxy protons (δH 3.79 and 3.84), and an anomeric proton (δH 4.83) were observed (Table 2). Each of the aromatic signals accounted for two protons and that of the methoxy signals six protons. The 13C-NMR spectrum exhibited 22 signals, of which six were assignable to β-glucopyranose. As to the re- maining 16 signals, two methoxy signals were expected to include those of four carbons from their peak heights and each of the four aromatic signals was obviously for two carbons. Thus, the two aromatic rings must be sub- stituted symmetrically and the other signals comprised those of two primary alcohols, two methylenes and two oxymethines. The proton chains from H-7 to H-9 and H- 7' to H-9' were assigned by inspection of the 1H-1H COSY spectrum, and the correlation cross peaks between H-8 (δH 4.18), and C-1 (δC 139.5) and C-4' (δC 134.9) in the HMBC spectrum established the structure of sym- plocosneolignan (4), as shown in Figure 1. Other HMBC correlations shown in Figure 3 also supported the struc- ture. The site of the sugar linkage was determined to be to the hydroxy group at the 4-position, judging from the cross peak between H-1' and C-4 (δC 135.6) in the HMBC spectrum, and the mode of linkage to be β from the coupling constant (J = 8 Hz) of the anomeric proton. Copyright © 2011 SciRes. AJPS 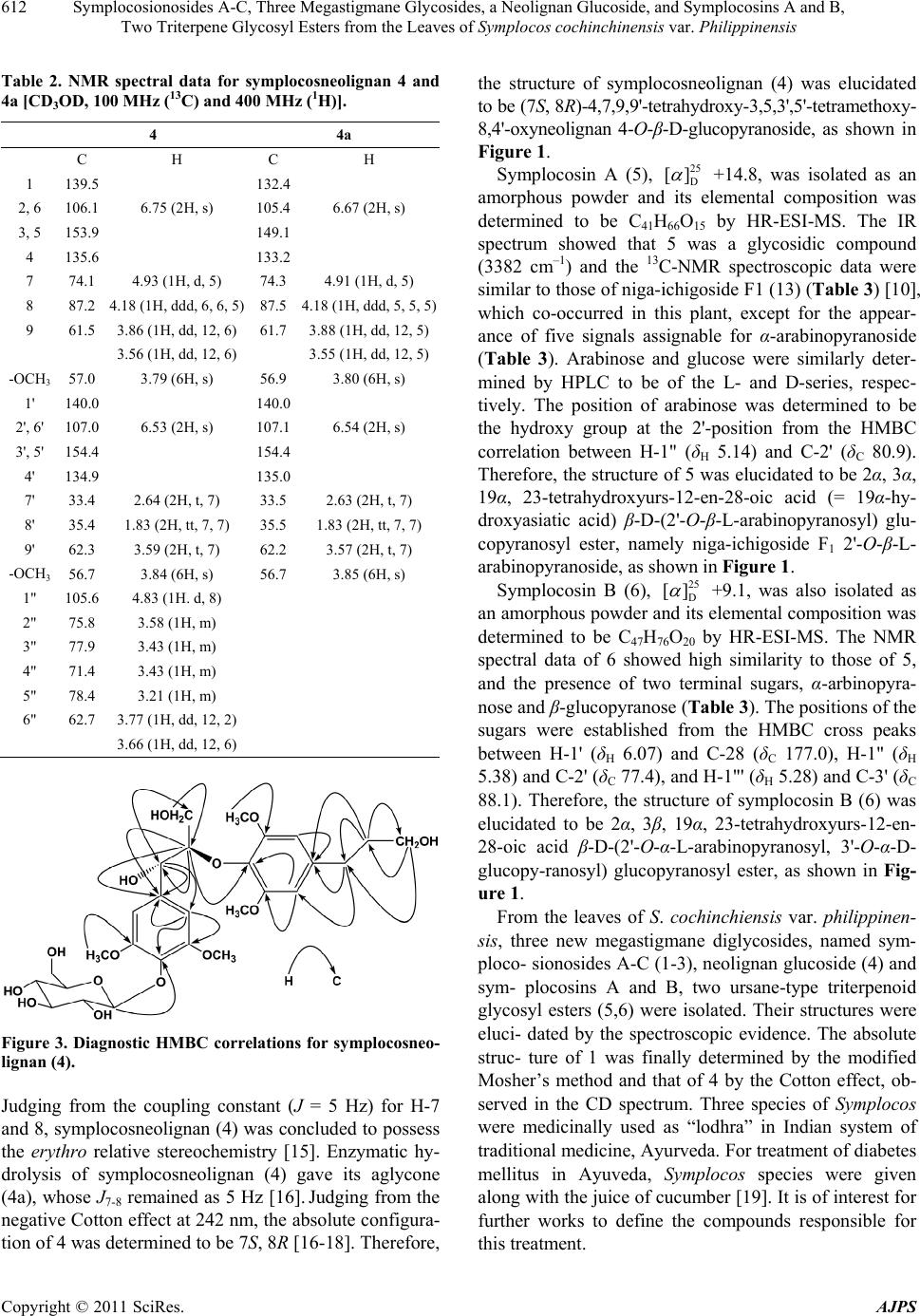 Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 612 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis Table 2. NMR spectral data for symplocosneolignan 4 and 4a [CD3OD, 100 MHz (13C) and 400 MHz (1H)]. 4 4a C H C H 1 139.5 132.4 2, 6 106.1 6.75 (2H, s) 105.4 6.67 (2H, s) 3, 5 153.9 149.1 4 135.6 133.2 7 74.1 4.93 (1H, d, 5) 74.3 4.91 (1H, d, 5) 8 87.2 4.18 (1H, ddd, 6, 6, 5) 87.5 4.18 (1H, ddd, 5, 5, 5) 9 61.5 3.86 (1H, dd, 12, 6)61.7 3.88 (1H, dd, 12, 5) 3.56 (1H, dd, 12, 6) 3.55 (1H, dd, 12, 5) -OCH3 57.0 3.79 (6H, s) 56.9 3.80 (6H, s) 1' 140.0 140.0 2', 6' 107.0 6.53 (2H, s) 107.1 6.54 (2H, s) 3', 5' 154.4 154.4 4' 134.9 135.0 7' 33.4 2.64 (2H, t, 7) 33.5 2.63 (2H, t, 7) 8' 35.4 1.83 (2H, tt, 7, 7) 35.5 1.83 (2H, tt, 7, 7) 9' 62.3 3.59 (2H, t, 7) 62.2 3.57 (2H, t, 7) -OCH3 56.7 3.84 (6H, s) 56.7 3.85 (6H, s) 1" 105.6 4.83 (1H. d, 8) 2" 75.8 3.58 (1H, m) 3" 77.9 3.43 (1H, m) 4" 71.4 3.43 (1H, m) 5" 78.4 3.21 (1H, m) 6" 62.7 3.77 (1H, dd, 12, 2) 3.66 (1H, dd, 12, 6) Figure 3. Diagnostic HMBC correlations for symplocosneo- lignan (4). Judging from the coupling constant (J = 5 Hz) for H-7 and 8, symplocosneolignan (4) was concluded to possess the erythro relative stereochemistry [15]. Enzymatic hy- drolysis of symplocosneolignan (4) gave its aglycone (4a), whose J7-8 remained as 5 Hz [16]. Judging from the negative Cotton effect at 242 nm, the absolute configura- tion of 4 was determined to be 7S, 8R [16-18]. Therefore, the structure of symplocosneolignan (4) was elucidated to be (7S, 8R)-4,7,9,9'-tetrahydroxy-3,5,3',5'-tetramethoxy- 8,4'-oxyneolignan 4-O-β-D-glucopyranoside, as shown in Figure 1. Symplocosin A (5), 25 D [] +14.8, was isolated as an amorphous powder and its elemental composition was determined to be C41H66O15 by HR-ESI-MS. The IR spectrum showed that 5 was a glycosidic compound (3382 cm–1) and the 13C-NMR spectroscopic data were similar to those of niga-ichigoside F1 (13) (Table 3) [10], which co-occurred in this plant, except for the appear- ance of five signals assignable for α-arabinopyranoside (Table 3). Arabinose and glucose were similarly deter- mined by HPLC to be of the L- and D-series, respec- tively. The position of arabinose was determined to be the hydroxy group at the 2'-position from the HMBC correlation between H-1" (δH 5.14) and C-2' (δC 80.9). Therefore, the structure of 5 was elucidated to be 2α, 3α, 19α, 23-tetrahydroxyurs-12-en-28-oic acid (= 19α-hy- droxyasiatic acid) β-D-(2'-O-β-L-arabinopyranosyl) glu- copyranosyl ester, namely niga-ichigoside F1 2'-O-β-L- arabinopyranoside, as shown in Figure 1. Symplocosin B (6), 25 D [] +9.1, was also isolated as an amorphous powder and its elemental composition was determined to be C47H76O20 by HR-ESI-MS. The NMR spectral data of 6 showed high similarity to those of 5, and the presence of two terminal sugars, α-arbinopyra- nose and β-glucopyranose (Tabl e 3 ). The positions of the sugars were established from the HMBC cross peaks between H-1' (δH 6.07) and C-28 (δC 177.0), H-1" (δH 5.38) and C-2' (δC 77.4), and H-1"' (δH 5.28) and C-3' (δC 88.1). Therefore, the structure of symplocosin B (6) was elucidated to be 2α, 3β, 19α, 23-tetrahydroxyurs-12-en- 28-oic acid β-D-(2'-O-α-L-arabinopyranosyl, 3'-O-α-D- glucopy-ranosyl) glucopyranosyl ester, as shown in Fig- ure 1. From the leaves of S. cochinchiensis var. philippinen- sis, three new megastigmane diglycosides, named sym- ploco- sionosides A-C (1-3), neolignan glucoside (4) and sym- plocosins A and B, two ursane-type triterpenoid glycosyl esters (5,6) were isolated. Their structures were eluci- dated by the spectroscopic evidence. The absolute struc- ture of 1 was finally determined by the modified Mosher’s method and that of 4 by the Cotton effect, ob- served in the CD spectrum. Three species of Symplocos were medicinally used as “lodhra” in Indian system of traditional medicine, Ayurveda. For treatment of diabetes mellitus in Ayuveda, Symplocos species were given along with the juice of cucumber [19]. It is of interest for further works to define the compounds responsible for this treatment. Copyright © 2011 SciRes. AJPS 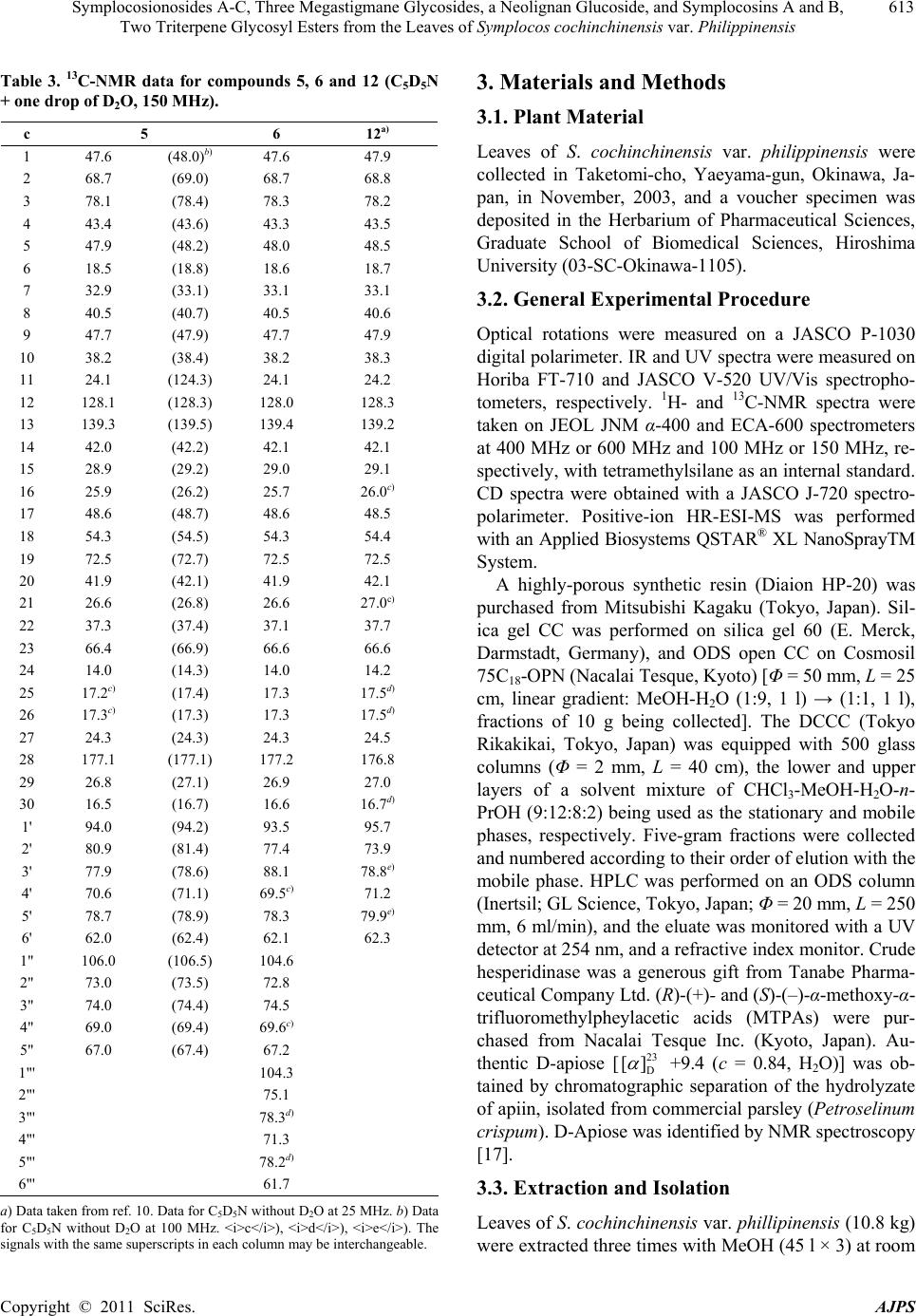 Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 613 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis Table 3. 13C-NMR data for compounds 5, 6 and 12 (C5D5N + one drop of D2O, 150 MHz). c 5 6 12a) 1 47.6 (48.0)b) 47.6 47.9 2 68.7 (69.0) 68.7 68.8 3 78.1 (78.4) 78.3 78.2 4 43.4 (43.6) 43.3 43.5 5 47.9 (48.2) 48.0 48.5 6 18.5 (18.8) 18.6 18.7 7 32.9 (33.1) 33.1 33.1 8 40.5 (40.7) 40.5 40.6 9 47.7 (47.9) 47.7 47.9 10 38.2 (38.4) 38.2 38.3 11 24.1 (124.3) 24.1 24.2 12 128.1 (128.3) 128.0 128.3 13 139.3 (139.5) 139.4 139.2 14 42.0 (42.2) 42.1 42.1 15 28.9 (29.2) 29.0 29.1 16 25.9 (26.2) 25.7 26.0c) 17 48.6 (48.7) 48.6 48.5 18 54.3 (54.5) 54.3 54.4 19 72.5 (72.7) 72.5 72.5 20 41.9 (42.1) 41.9 42.1 21 26.6 (26.8) 26.6 27.0c) 22 37.3 (37.4) 37.1 37.7 23 66.4 (66.9) 66.6 66.6 24 14.0 (14.3) 14.0 14.2 25 17.2c) (17.4) 17.3 17.5d) 26 17.3c) (17.3) 17.3 17.5d) 27 24.3 (24.3) 24.3 24.5 28 177.1 (177.1) 177.2 176.8 29 26.8 (27.1) 26.9 27.0 30 16.5 (16.7) 16.6 16.7d) 1' 94.0 (94.2) 93.5 95.7 2' 80.9 (81.4) 77.4 73.9 3' 77.9 (78.6) 88.1 78.8e) 4' 70.6 (71.1) 69.5c) 71.2 5' 78.7 (78.9) 78.3 79.9e) 6' 62.0 (62.4) 62.1 62.3 1" 106.0 (106.5) 104.6 2" 73.0 (73.5) 72.8 3" 74.0 (74.4) 74.5 4" 69.0 (69.4) 69.6c) 5" 67.0 (67.4) 67.2 1"' 104.3 2"' 75.1 3"' 78.3d) 4"' 71.3 5"' 78.2d) 6"' 61.7 a) Data taken from ref. 10. Data for C5D5N without D2O at 25 MHz. b) Data for C5D5N without D2O at 100 MHz. <i>c</i>), <i>d</i>), <i>e</i>). The signals with the same superscripts in each column may be interchangeable. 3. Materials and Methods 3.1. Plant Material Leaves of S. cochinchinensis var. philippinensis were collected in Taketomi-cho, Yaeyama-gun, Okinawa, Ja- pan, in November, 2003, and a voucher specimen was deposited in the Herbarium of Pharmaceutical Sciences, Graduate School of Biomedical Sciences, Hiroshima University (03-SC-Okinawa-1105). 3.2. General Experimental Procedure Optical rotations were measured on a JASCO P-1030 digital polarimeter. IR and UV spectra were measured on Horiba FT-710 and JASCO V-520 UV/Vis spectropho- tometers, respectively. 1H- and 13C-NMR spectra were taken on JEOL JNM α-400 and ECA-600 spectrometers at 400 MHz or 600 MHz and 100 MHz or 150 MHz, re- spectively, with tetramethylsilane as an internal standard. CD spectra were obtained with a JASCO J-720 spectro- polarimeter. Positive-ion HR-ESI-MS was performed with an Applied Biosystems QSTAR® XL NanoSprayTM System. A highly-porous synthetic resin (Diaion HP-20) was purchased from Mitsubishi Kagaku (Tokyo, Japan). Sil- ica gel CC was performed on silica gel 60 (E. Merck, Darmstadt, Germany), and ODS open CC on Cosmosil 75C18-OPN (Nacalai Tesque, Kyoto) [Φ = 50 mm, L = 25 cm, linear gradient: MeOH-H2O (1:9, 1 l) → (1:1, 1 l), fractions of 10 g being collected]. The DCCC (Tokyo Rikakikai, Tokyo, Japan) was equipped with 500 glass columns (Φ = 2 mm, L = 40 cm), the lower and upper layers of a solvent mixture of CHCl3-MeOH-H2O-n- PrOH (9:12:8:2) being used as the stationary and mobile phases, respectively. Five-gram fractions were collected and numbered according to their order of elution with the mobile phase. HPLC was performed on an ODS column (Inertsil; GL Science, Tokyo, Japan; Φ = 20 mm, L = 250 mm, 6 ml/min), and the eluate was monitored with a UV detector at 254 nm, and a refractive index monitor. Crude hesperidinase was a generous gift from Tanabe Pharma- ceutical Company Ltd. (R)-(+)- and (S)-(–)-α-methoxy-α- trifluoromethylpheylacetic acids (MTPAs) were pur- chased from Nacalai Tesque Inc. (Kyoto, Japan). Au- thentic D-apiose [23 D [] +9.4 (c = 0.84, H2O)] was ob- tained by chromatographic separation of the hydrolyzate of apiin, isolated from commercial parsley (Petroselinum crispum). D-Apiose was identified by NMR spectroscopy [17]. 3.3. Extraction and Isolation Leaves of S. cochinchinensis var. phillipinensis (10.8 kg) were extracted three times with MeOH (45 l × 3) at room Copyright © 2011 SciRes. AJPS  Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 614 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis temperature for one week and then concentrated to 6 l in vacuo. The concentrated extract was washed with n- hexane (6 l, 150 g) and then the MeOH layer was con- centrated to a gummy mass. The latter was suspended in water (6 l) and then extracted with EtOAc (6 l) to give 107 g of an EtOAc-soluble fraction. The aqueous layer was extracted with 1-BuOH (6 l) to give a 1-BuOH- soluble fraction (247 g), and the remaining water-layer was concentrated to furnish 494 g of a water-soluble fraction. The 1-BuOH-soluble fraction (119 g) was sub- jected to Diaion HP-20 CC (Φ = 50 mm, L = 50 cm), us- ing H2O-MeOH (4:1, 4 1), (3:2, 4 1), (2:3, 4 1), and (1:4, 4 l), and MeOH (3 l), 500 ml fractions being collected. The residue (12.5 g in fractions 11 - 18) of the 20% - 40% MeOH eluate obtained on HP-20 CC was subjected to silica gel (500 g) CC with increasing amounts of MeOH in CHCl3 [CHCl3 (3 l), and CHCl3-MeOH (99:1, 3 l), (97:3, 3 l), (19:1, 3 l), (37:3, 3 l), (9:1, 3 l), (7:1, 3 l), (17:3, 3 l), (33:7, 3 l), (4:1, 3 l), (3:1, 3 l) and (7:3, 3 l)], and CHCl3-MeOH-H2O (70: 30: 4, 3 l), 500 ml fractions being collected. The residue (2.52 g) in fractions 39 - 49 was separated by ODS open CC and then the residue (996 mg) in fractions 56 - 81 was subjected to DCCC. The residue (130 mg) in fractions 75 - 88 was purified by HPLC (MeOH:H2O, 1:3) to give 85.7 mg of 11 from the peak at 10 min. The residue (2.18 g) in fractions 50 - 63, obtained on silica gel CC, was separated by ODS open CC to give three subfractions (1.02 g in fractions 42 - 67, 347 mg in fractions 68 - 87 and 138 mg in fractions 110 - 140). The first subfraction was separated again by DCCC to give a residue (877 mg) in fractions 39 - 60, which was then purified by HPLC (MeOH:H2O, 1:4) to afford 6.2 mg of 9 and 11.2 mg of 3 from the peaks at 35 min and 45 min, respectively. The second subfraction was separated by DCCC to give a residue (105 mg) in frac- tions 34 - 42, which was purified by HPLC (MeOH:H2O, 2:5) to yield 23.9 mg of 2 from the peak at 14 min. The last subfraction was separated by DCCC to give a residue (88.1 mg) in fractions 16 - 32, which was purified by HPLC (MeOH:H2O, 9:20) to give 12.3 mg of 1 from the peak at 22 min. The residue (19.7 g in fractions 19 - 25) of the 40% - 60% MeOH eluate obtained on HP-20 CC was subjected to silica gel (500 g) CC with increasing amounts of MeOH in CHCl3 [CHCl3 (3 l), and CHCl3-MeOH (99:1, 3 l), (97:3, 3 l), (19:1, 3 l), (37:3, 3 l), (9:1, 3 l), (7:1, 3 l), (17:3, 3 l), (33:7, 3 l), (4:1, 3 l), (3:1, 3 l) and (7:3, 3 l)], and CHCl3-MeOH-H2O (70:30:4, 3 l), 500 ml fractions being collected. The residue (221 mg) in fractions 8 - 14 was purified by HPLC (MeOH:H2O, 3:10), to give 2.0 mg of 8 from the peak at 44 min. The residue (115 mg) in fractions 16 - 25 was purified by HPLC (MeOH:H2O, 3:10) to give 4.8 mg of 7. The residue (2.94 g) in frac- tions 17 - 43 was separated by ODS open CC to give two subfractions (332 mg in fractions 61 - 63 and 541 mg in fractions 84 - 99). The former was separated by DCCC and then the residue (16.8 mg) in fractions 47 - 54 was purified by HPLC (MeOH:H2O, 1:4) to give 2.8 mg of 10 from the peak at 16 min. The latter was separated by DCCC and then the residue (194 mg) in fractions 67 - 84 was purified by HPLC (MeOH:H2O, 7:20) to afford 11.9 mg of 4 from the peak at 13 min. The residue (2.92 g) in fractions 44 - 60, obtained on silica gel CC, was sub- jected to ODS open CC to give a residue (495 mg) in fractions 130 - 170. This residue was separated by DCCC to give two subfactions (136 mg in fractions 16 - 21 and 189 mg in fractions 22 - 41). The former was purified by HPLC (MeOH:H2O, 2:3) to give 20.0 mg of 12 from the peak at 6 min. The latter was also purified by HPLC (MeOH:H2O, 9:20) to give 23.0 mg of 5 at 10 min. The residue (2.37 g) in fractions 61 - 72, obtained on silica gel CC, was subjected to ODS open CC to give a residue (492 mg) in fractions 154 - 172, which was then sepa- rated by DCCC to give 82.9 mg of 6 in fractions 29 - 42. 3.4. Symplocosionoside A (1) Amorphous powder, 25 D [] –44.0 (c = 0.82, MeOH); IR νmax (film): 3394, 2962, 2927, 2877, 1650, 1055, 1014 cm–1; 1H-NMR (CD3OD, 400 MHz) δ: 5.45 (1H, dd, J = 15, 6 Hz, H-8), 5.28 (1H, dd, J = 15, 10 Hz, H-7), 5.00 (1H, d, J = 2 Hz, H-1"), 4.33 (1H, d, J = 8 Hz, H-1'), 4.21 (1H, qd, J = 6, 6 Hz, H-9), 3.96 (1H, J = 10Hz, H-4"a), 3.94 (1H, dd, J = 12, 2 Hz, H-6'a), 3.87 (1H, d, J = 2 Hz, H-2"), 3.83 (1H, dddd, J = 12, 12, 4, 4 Hz, H-3), 3.78 (1H, d, J = 10 Hz, H-4"b), 3.59 (1H, dd, J = 12, 6 Hz, H- 6'b), 3.54 (2H, s, H2-5"), 3.39 (1H, ddd, J = 9, 6, 2 Hz, H-5'), 3.34 (1H, dd, J = 9, 9 Hz, H-3'), 3.25 (1H, dd, J = 9, 9 Hz, H-4'), 3.11 (1H, dd, J = 9, 8 Hz, H-2'), 2.12 (1H, dddd, J = 12, 4, 4, 2 Hz, H-4eq), 1.83 (1H, ddd, J = 12, 4, 2 Hz, H-2eq), 1.52 (1H, m, H-5), 1.30 (1H, dd, J = 10, 10 Hz, H-6), 1.21 (3H, d, J = 6 Hz, H3-10), 1.16 (1H, dd, J = 12, 12 Hz, H-2ax), 1.10 (1H, ddd, J = 12, 12, 12 Hz, H- 4ax), 0.91 (3H, s, H3-11), 0.87 (3H, s, H3-12), 0.83 (3H, d, J = 7 Hz, H3-13); 13C-NMR (CD3OD, 100 MHz): Table 1; HR-ESI-MS (positive-ion mode) m/z: 529.2620 [M + Na]+ (Calcd for C24H42O11Na: 529.2619). 3.5. Symplocosionoside B (2) Amorphous powder, [α]D 26 –63.0 (c = 1.59, MeOH); IR νmax (film): 3399, 2969, 2931, 2880, 1694, 1651, 1150, 1056 cm–1; 1H-NMR (CD3OD, 400 MHz) δ: 5.89 (1H, dd, J = 16, 6 Hz, H-8), 5.74 (1H, d, J = 16 Hz, H-7), 4.98 (1H, d, J = 2 Hz, H-1"), 4.43 (1H, qd, J = 6, 6 Hz, H-9), 4.34 (1H, d, J = 8 Hz, H-1'), 3.97 (1H, d, J = 10 Hz, H- Copyright © 2011 SciRes. AJPS  Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 615 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis 4"a), 3.94 (1H, dd, J = 12, 2 Hz, H-6'a), 3.91 (1H, d, J = 2 Hz, H-2"), 3.75 (1H, d, J = 10 Hz, H-4"b), 3.58 (1H, dd, J = 12, 6 Hz, H-6'b), 3.57 (2H, s, H2-5"), 3.34 (1H, m, H- 5'), 3.32 (1H, m, H-3'), 3.29 (1H, dd, J = 9, 9, H-4'), 3.18 (1H, dd, J = 9, 8 Hz, H-2'), 2.87 (1H, d, J = 14 Hz, H- 2ax), 2.44 (1H, dd, J = 14, 14 Hz, H-4ax), 2.28 (1H, dqd, J = 14, 7, 4 Hz, H-5), 2.12 (1H, ddd, J = 14, 4, 2 Hz, H- 4eq), 1.82 (1H, dd, J = 14, 2 Hz, H-2eq), 1.32 (3H, d, J = 6 Hz, H3-10), 0.99 (3H, s, H3-11), 0.93 (3H, s, H3-12), 0.90 (3H, d, J = 7 Hz, H3-13); 13C-NMR (CD3OD, 100 MHz): Table 1; CD (MeOH) nm (ε): 289 (+0.89), 246 (+0.63) (c = 2.51 × 10–5 M); HR-ESI-MS (positive-ion mode) m/z: 543.2404 [M + Na]+ (Calcd for C24H40O12Na: 543.2411). 3.6. Symplocosionoside C (3) Amorphous powder, [δ]D 25 – 57.9 (c = 0.61, MeOH); IR max (film): 3399, 3366, 2958, 2927, 1938, 1669, 1515, 1366, 1243, 1069, 1042 cm–1; UV λmax (MeOH) nm (log ): 321 (4.53), 283 (3.91), 220 (4.04); 1H-NMR (CD3OD, 400 MHz) δ: 5.96 (1H, s, H-8), 4.52 (1H, d, J = 8 Hz, H- 1'), 4.31 (1H, dddd, J = 12, 12, 4, 4 Hz, H-3), 4.27 (1H, d, J = 7 Hz, H-1"), 4.00 (1H, dd, J = 11, 2 Hz, H-6'a), 3.85 (1H, dd, J = 12, 4 Hz, H-5"a), 3.81 (1H, m, H-4"), 3.69 (1H, dd, J = 11, 5 Hz, H-6'b), 3.64 (1H, dd, J = 8, 7 Hz, H-2"), 3.54 (1H, m, H-3"), 3.53 (1H, dd, J = 12, 3 Hz, H- 5"b), 3.37 (1H, m, H-5'), 3.35 (1H, dd, J = 9, 9 Hz, H-4'), 3.33 (1H, dd, J = 9, 9 Hz, H-3'), 3.14 (1H, dd, J = 9, 8 Hz, H-2'), 2.48 (1H, ddd, J = 12, 4, 2 Hz, H-4eq), 2.19 (3H, s, H3-10), 1.91 (1H, ddd, J = 12, 4, 2 Hz, H-2eq), 1.47 (3H, s, H3-13), 1.37 (3H, s, H3-11), 1.36 (1H, dd, J = 12, 12 Hz, H-4ax), 1.34 (1H, dd, J = 12, 12 Hz, H-2ax), 1.16 (3H, s, H3-12); 13C-NMR (CD3OD and C5D5N, 100 MHz): Table 1; CD (MeOH) nm (ε): 319 (–0.47), 263 (–2.87), 207 (–23.1) (c = 3.41 × 10–5 M). HR-ESI-MS (positive-ion mode) m/z: 541.2257 [M + Na]+ (Calcd for C24H38O12Na: 541.2255). 3.7. Symplocosneolignan (4) Amorphous powder, 25 D [] –13.5 (c = 0.79, MeOH); IR νmax (film): 3395, 2939, 1592, 1419, 1328, 1229, 1123, 1061 cm–1; UV λmax (MeOH) nm (log ε): 269 (3.59), 213 (4.31); 1H-NMR (CD3OD, 400 MHz): Table 2; 13C- NMR (CD3OD, 100 MHz): Table 2; CD (MeOH) nm (ε): 304 (+1.71), 242 (–4.67) (c = 4.00 × 10–5 M); HR- ESI-MS (positive-ion mode) m/z: 623.2294 [M + Na]+ (Calcd for C28H40O14Na: 623.2310). 3.8. Symplocoside A (5) Amorphous powder, [α]D 22 +14.8 (c = 0.54, MeOH); IR νmax (film): 3382, 2930, 2882, 1730, 1635, 1140, 1074, 1036 cm–1; 1H-NMR (C5D5N + one drop of D2O, 600 MHz) δ: 6.07 (1H, d, J = 8 Hz, H-1'), 5.50 (1H, dd, J = 4, 4 Hz, H-12), 5.14 (1H, d, J = 7 Hz, H-1"), 4.44 (1H, dd, J = 9, 7 Hz, H-2"), 4.40 (2H, dd, J = 14, 2 Hz, H-6'a), 4.28 (1H, dd, J = 9, 9 Hz, H-3'), 4.27 (1H, m, H-6'b and 5"a), 4.26 (2H, m, H-2' and 4"), 4.19 (2H, m, H-2 and 4'), 4.14 (1H, dd, J = 9, 3 Hz, H-3"), 4.10 (1H, d, J = 10 Hz, H-3), 4.05 (1H, d, J = 11 Hz, H-23a), 3.94 (1H, ddd, J = 9, 5, 2 Hz, H-5'), 3.71 (1H, dd, J = 13, 2 Hz, H-5"b), 3.58 (1H, d, J = 11 Hz, H-23b), 2.99 (1H, ddd, J = 13, 13, 5 Hz, H- 16a), 2.86 (1H, br s, H-18), 2.24 (1H, dd, J = 12, 4 Hz, H-1a), 2.30 (1H, ddd, J = 14, 13, 5 Hz, H-15a), 2.07 (2H, m, H2-11), 2.02 (1H, m, H-16b), 1.97 (3H, m, H-21a and H2-22), 1.96 (1H, m, H-9), 1.78 (1H, m, H-7a), 1.70 (1H, br d, J = 12 Hz, H-5), 1.60 (2H, m, H-6a and 7b), 1.57 (3H, s, H3-27), 1.43 (1H, m, H-20), 1.38 (3H, s, H3-29), 1.34 (1H, m, H-6b), 1.32 (1H, m, H-15b), 1.24 (1H, m, H-21b), 1.30 (1H, m, H-1b), 1.09 (3H, s, H3-26), 1.06 (3H, d, J = 7 Hz, H3-30), 1.05 (3H, s, H3-25), 0.96 (3H, s, H3-24); 13C-NMR (C5D5N, 100 MHz): Table 3 ; HR-ESI- MS (positive-ion mode) m/z: 821.4277 [M + Na]+ (Calcd for C41H66O15Na: 821.4293). 3.9. Symplocoside B (6) Amorphous powder, [α]D 22 +9.1 (c = 1.22, MeOH); IR νmax (film): 3395, 2931, 2883, 1730, 1647, 1156, 1076, 1035cm–1; 1H-NMR (C5D5N + one drop of D2O, 600 MHz) δ: 6.07 (1H, d, J = 8 Hz, H-1'), 5.49 (1H, dd, J = 4, 4 Hz, H-12), 5.38 (1H, d, J = 8 Hz, H-1"), 5.28 (1H, d, J = 8 Hz, H-1"'), 4.48 (1H, dd, J = 12, 2 Hz, H-6'a), 4.35 (1H, dd, J = 9, 8 Hz, H-2'), 4.32 (1H, dd, J = 12, 2 Hz, H- 6"'a), 4.31 (1H, m, H-2"), 4.28 (1H, dd, J = 9, 9 Hz, H-3'), 4.20 (2H, m, H-5"a and H-6"'b), 4.18 (1H, m, H-2), 4.16 (3H, m, H-4', 4" and 3"'), 4.15 (1H, m, H-6'b), 4.10 (1H, dd, J = 9, 3 Hz, H-3"), 4.09 (1H, d, J = 9 Hz, H-3), 4.04 (1H, dd, J = 9, 9 Hz, H-4"'), 4.03 (1H, d, J = 11 Hz, H- 23a), 3.98 (1H, dd, J = 9, 8 Hz, H-2"'), 3.94 (1H, ddd, J = 9, 6, 2 Hz, H-5"'), 3.89 (1H, ddd, J = 9, 5, 2 Hz, H-5'), 3.64 (1H, br d, J = 12 Hz, H-5"), 3.54 (1H, d, J = 11 Hz, H-23b), 2.99 (1H, ddd, J = 13, 13, 5 Hz, H-16a), 2.87 (1H, br s, H-18), 2.27 (1H, m, H-15a), 2.24 (1H, dd, J = 12, 4 Hz, H-1a), 2.16 (1H, br d, J = 13 Hz, H-16b), 2.07 (2H, m, H2-11), 1.98 (1H, m, H-21a), 1.97 (2H, m, H2- 22), 1.96 (1H, m, H-9), 1.77 (1H, ddd, J = 13, 13, 4 Hz, H-7a), 1.67 (1H, br d, J = 12 Hz, H-5), 1.65 (1H, ddd, J = 13, 2, 2 Hz, H-7b), 1.57 (3H, s, H3-27), 1.56 (1H, m, H-6a), 1.48 (1H, m, H-15b), 1.46 (1H, m, H-20), 1.37 (3H, s, H3-29), 1.31 (1H, m, H-6b), 1.29 (1H, m, H-1b), 1.26 (1H, m, H-21b), 1.12 (3H, s, H3-26), 1.06 (3H, d, J = 7 Hz, H3-30), 1.04 (3H, s, H3-25), 0.95 (3H, s, H3-24); 13C-NMR (C5D5N, 100 MHz): Table 3; HR-ESI-MS (positive-ion mode) m/z: 983.4823 [M + Na]+ (Calcd for C47H76O20Na: 983.4822). Copyright © 2011 SciRes. AJPS  Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 616 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis 3.10. Enzymatic Hydrolysis of Symplocosionoside A (1) Symplocosionoside A (1) (6.6 mg) in 2 ml of H2O was hydrolyzed with emulsin (14.2 mg) and crude hesperidi- nase (6.0 mg) for 15 h at 37˚C. The reaction mixture was evaporated to dryness, and then the methanolic solu- tion was absorbed on silica gel and subjected to silica gel CC (30 g, Φ = 18 mm, L = 21 cm) with CHCl3 (100 ml) and CHCl3-MeOH (19:1, 100 ml, 9:1, 100 ml, 17:3, 100 ml and 7:3, 300 ml), 12 ml fractions being collected. An aglycone (1a) (1.8 mg, 65%) was recovered in fractions 19 - 23. Aglycone (megastigman-7-ene-3,9-diol) (1a): Colorless syrup, 29 D [] –20.0 (c = 0.12, MeOH); 1H-NMR (CD3OD, 400 MHz) δ: 5.45 (1H, dd, J = 15, 6 Hz, H-8), 5.29 (1H, dd, J = 15, 10, H-7), 4.21 (1H, qd, J = 6, 6 Hz, H-9), 3.71 (1H, dddd, J = 12, 12, 4, 4 Hz, H-3), 1.96 (1H, dddd, J = 12, 12, 4, 2 Hz, H-4eq), 1.70 (1H, ddd, J = 12, 4, 2 Hz, H-2eq), 1.52 (1H, ddq, J = 12, 10, 7 Hz, H-5), 1.30 (1H, dd, J = 10 , 10 Hz, H-6), 1.21 (3H, d, J = 6 Hz, H3-10), 1.10 (1H, dd, J = 12, 12 Hz, H-2ax), 0.89 (1H, ddd, J = 12, 12, 12 Hz, H-4ax), 0.88 (3H, s, H3-12), 0.86 (3H, s, H3-11), 0.82 (3H, d, J = 7 Hz, H3-13); 13C-NMR (CD3OD, 100 MHz): Table 1; HR-ESI-MS (positive-ion mode) m/z: 235.1661 [M + Na]+ (Calcd for C13H24O2Na: 235.1668). 3.11. Preparation of (R)- and (S)-MPTA Diesters (1b and 1c) from 1a A solution of 1a (0.7 mg) in 1 ml of dehydrated CH2Cl2 was reacted with (R)-MTPA (42 mg) in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydro- chloride (EDC) (31 mg) and N,N-dimethyl-4-amino- pyridine (4-DMAP) (31 mg), and then the mixture was occasionally stirred at 25˚C for 30 min. After the addi- tion of 1 ml of CH2Cl2, the solution was washed with H2O (1 ml), 5% HCl (1 ml), NaHCO3-saturated H2O, and then brine (1 ml), successively. The organic layer was dried over Na2SO4 and then evaporated under reduced pressure. The residue was purified by preparative TLC [silica gel (0.25 mm thickness), being applied for 18 cm, developed with CHCl3-(CH3)2CO (19:1) for 9 cm, and then eluted with CHCl3-MeOH (9:1)] to furnish an ester, 1b (1.5 mg, 71%). Through a similar procedure, 1c (1.1 mg, 61%) was prepared from 1a (0.6 mg) using (S)- MTPA (53 mg), EDC (41 mg), and 4-DMAP (24 mg). (3S, 5R, 6R, 7E, 9R)-Megastigman-7-ene-3,9-diol 3,9- O-(R)-MTPA diester (1b) An amorphous powder; 1H-NMR (CDCl3, 400 MHz) δ: 7.47 - 7.43 (4H, m, aro- matic protons), 7.35 - 7.30 (6H, m, aromatic protons), 5.56 (1H, qd, J = 6, 6 Hz, H-9), 5.43 (1H, m, H-8), 5.42 (1H, m, H-7), 5.15 (1H, dddd, J = 12, 12, 4, 4 Hz, H-3), 3.48 (3H, br s, -OCH3), 3.47 (3H, br s, -OCH3), 2.13 (1H, dddd, J = 12, 4, 4, 2 Hz, H-4eq), 1.76 (1H, ddd, J = 12, 4, 2 Hz, H-2eq), 1.68 (1H, m, H-5), 1.43 (1H, dd, J = 10, 10 Hz, H-6), 1.36 (3H, d, J = 6 Hz, H3-10), 1.16 (1H, dd, J = 12, 12 Hz, H-2ax), 0.99 (1H, ddd, J = 12, 12, 12 Hz, H-4ax), 0.88 (3H, d, J = 6 Hz, H3-13), 0.84 (3H, s, H3-11), 0.82 (3H, s, H3-12); HR-ESI-TOF-MS (posi- tive-ion mode) m/z: 667.2472 [M+Na]+ (Calcd for C33H38O6F6Na, 667.2464). (3S, 5R, 6R, 7E, 9R)- Megas- tigman-7-ene-3,9-diol 3,9-O-(S)-MTPA diester (1c) An amorphous powder; 1H-NMR (CDCl3, 400 MHz) δ: 7.47 - 7.43 (4H, m, aromatic protons), 7.35 - 7.30 (6H, m, aromatic protons), 5.56 (1H, qd, J = 6, 6 Hz, H-9), 5.39 (1H, m, H-8), 5.38 (1H, m, H-7), 5.14 (1H, dddd, J = 12, 12, 4, 4 Hz, H-3), 3.48 (3H, br s, -OCH3), 3.46 (3H, br s, -OCH3), 2.04 (1H, dddd, J = 12, 4, 4, 2 Hz, H-4eq), 1.82 (1H, ddd, J = 12, 4, 2 Hz, H-2eq), 1.60 (1H, m, H-5), 1.41 (3H, d, J = 6 Hz, H3-10), 1.37 (1H, dd, J = 10, 10 Hz, H-6), 1.19 (1H, dd, J = 12, 12 Hz, H-2ax), 0.88 (1H, ddd, J = 12, 12, 12 H-4ax), 0.87 (3H, s, H3-11), 0.82 (3H, s, H3-12), 0.77 (3H, d, J = 6 Hz, H3-13); HR-ESI-MS (positive-ion mode) m/z: 667.2471 [M+Na]+ (Calcd for C33H38O6F6Na, 667.2464). 3.12. Enzymatic Hydrolysis of Symplocosneolignan (4) Symplocosneolignan (4) (4.7 mg) in 2 ml of H2O was hydrolyzed with emulsin (12 mg) and crude hesperidi- nase (6.0 mg) for 15 h at 37˚C. The reaction mixture was evaporated to dryness, and then the methanolic solution was absorbed on silica gel and subjected to silica gel CC (30 g, Φ = 18 mm, L = 21 cm) with CHCl3 (100 ml) and CHCl3-MeOH (19:1, 100 ml, 9:1, 100 ml, 17:3, 100 ml and 7:3, 300 ml), 12 ml fractions being collected. An aglycone (1a) (0.9 mg, 26%) was recovered in fractions 23 - 27. Aglycone (4a): Colorless syrup, 25 D [] –21.0 (c = 0.06, MeOH); 1H-NMR (CD3OD, 400 MHz): Table 2; 13C-NMR (CD3OD, 100 MHz): Table 2; HR-ESI-MS (positive-ion mode) m/z: 461.1786 [M+Na]+ (Calcd for C22H30O9Na, 461.1788). 3.13. Sugar Analysis About 350 μg each of 1 and 2 was hydrolyzed with 1N HCl (0.2 ml) at 90˚C for 2 h. The reaction mixtures were washed with an equal amount of EtOAc and then passed through Amberlite MB-3. The pass-through fractions were evaporated to dryness to give residues. The residues were dissolved in 0.1 ml of dry pyridine and then 0.5 mg of L-cysteine methyl ester was added. To these mixtures, 1.4 mg of o-tolylthioisocyanate in 70 μl of pyridine was added, followed by standing at 60˚C for 1 h. The reaction Copyright © 2011 SciRes. AJPS  Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, 617 Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis mixtures were analyzed by HPLC [ODS: Cosmosil 5C18ARII (4.6 mm × 250 mm), CH3CN-50 mM H3PO4 (1:3), 0.8 ml/min, UV detector at 250 nm] to give peaks for thiocarbamoylthiazolidine derivatives of D-glucose and D-apiose at 18.0 min and 31.0 min, respectively [21]. The peaks were identified by co-chromatography with thiocarbamoyl-thiazolidine derivatives of authentic D- glucose and D-apiose. About 500 μg of each compound, except for 1 and 2, was hydrolyzed with 1N HCl (0.1 ml) at 90˚C for 2 h. The reaction mixtures were partitioned with an equal amount of EtOAc (0.1 ml), and the water layers were analyzed with a chiral detector (JASCO OR-2090plus) on an amino column [Asahipak NH2P-50 4E, CH3CN- H2O (3:1), 1 ml/min]. A hydrolyzate of 4 gave a peak for D-glucose at 7.9 min, and ones of 3, 5 and 6 gave peaks for L-arabinose and D-glucose at 6.1 min and 13.7 min, respectively, with positive optical rotation signs. The peaks were identified by co-chromatography with au- thentic samples. 4. Acknowledgements The authors are grateful for access to the superconduct- ing NMR instrument (JEOL JNM α-400) at the Analyti- cal Center of Molecular Medicine of the Hiroshima Uni- versity Faculty of Medicine and an Applied Biosystem QSTAR XL system ESI (Nano Spray)-MS at the Analy- sis Center of Life Science of the Graduate School of Biomedical Sciences, Hiroshima University. The authors are also grateful for the use of the NMR instrument (JEOL ECA-600) at the Natural Science Center for Basic Research and Development, Hiroshima University. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. Nos. 22590006 and 23590130), the Japan Society for the Promotion of Science, and the Ministry of Health, Labour and Welfare. Thanks are also due to the Research Foundation for Pharmaceutical Sci- ences and the Takeda Science Foundation for the finan- cial support. REFERENCES [1] S. Hatusima, “Flora of the Ryukyus. Added and Cor- rected,” The Biological Society of Okinawa, Naha, 1975, p. 476. [2] C. Sunil and S. Ignacimuthu, “In vivo and in vitro Anti- oxidant Activity of Symplocos cochinchinensis S. Moore Leaves Containing Phenolic Compounds,” Food and Chemical Toxicology, Vol. 49, No. 7, 2011, pp. 1604- 1609. doi:10.1016/j.fct.2011.04.010 [3] C. Sunil, S. Ignacimuthu and P. Agastian, “Antidiabetic Effect of Symplocos cochinchinensis (Lour.) S. Moore in Type 2 Diabetic Rats,” Journal of Ethnopharmacology, Vol. 134, No. 2, 2011, pp. 298-304. doi:10.1016/j.jep.2010.12.018 [4] M. R. Khan, M. Kihara and A. D. Ornoloso, “Antimicro- bial Activity of Symplocos cochinensis,” Fitoterapia, Vol. 72, No. 7, 2001, pp. 825-828. doi:10.1016/S0367-326X(01)00314-8 [5] H. Otsuka, Ya. Takeda, K. Yamasaki and Yo. Takeda, “Structural Eelucidation of Dendranthemosides A and B: Two New β-Ionone Glucoside from Dendranthema shi- wogiku,” Planta Medica, Vol. 58, No. 4, 1992, pp. 373- 375. doi:10.1055/s-2006-961489 [6] A. G. González, J. A. Guillermo, A. G. Ravelo, I. A. Jimenez and M. P. Gupta, “4,5-Dihydroblumenol A, a New Nor-Isoprenoid from Perrottetia multiflora,” Jour- nal of Natural Products, Vol. 57, No. 3, 1994, pp. 400-402. doi:10.1021/np50105a013 [7] H. Otsuka, K. Kamada, C. Ogimi, E. Hirata, A. Takushi and Y. Takeda, “Alangionosides A and B, Ionol Gly- cosides from Leaves of Alangium premnifolium,” Phyto- chemistry, Vol. 35, No. 5, 1994, pp. 1331-1334. doi:10.1016/S0031-9422(00)94848-9 [8] A. Inada, Y. Nakamura, M. Konishi, H. Murata, F. Kita- mura, H. Toya and T. Nakanishi, “A New Ionone Gluco- side and a New Phenylpropanoid Rhamnoside from Stems of Ampelopsis brevipedunculata (Maxim.) Trautv.,” Chemical and Pharmaceutical Bulletin, Vol. 39, No. 9, 1991, pp. 2437-2439. [9] K. Umehara, I. Hattori, T. Miyase, A .Ueno, S. Hara and C. Kageyama, “Studies on the Constituents of Leaves of Citrus unshiu Marcov.,” Chemical and Pharmaceutical Bulletin, Vol. 36, No. 12, 1988, pp. 5004-5008. [10] T. Seto, T. Tanaka, O. Tanaka and N. Naruhashi, “β-Gluco-syl Esters of 19α-Hydroxyursolic Acid Deriva- tives in Leaves of Rubus Species,” Phytochemistry, Vol. 23, No. 12, 1984, pp. 2829-2834. doi:10.1016/0031-9422(84)83023-X [11] T. Morikawa, L. -B. Wang, K. Ninomiya, S. Nakamura, H. Matsuda, O. Muraoka, L. -J. Wu and M. Yoshikawa, “Medicinal Flowers. XXX. Eight New Glycosides, Ever- lastosides F-M, from the Flowers of Helichrysum arenarium,” Chemical and Pharmaceutical Bulletin, Vol. 57, No. 8, 2009, pp. 853-859. doi:10.1248/cpb.57.853 [12] R. Kasai, M. Suzuno, J. Asakawa and O. Tanaka, “Car- bon-13 Chemical Shifts of Isoprenoid-β-D-glucopy- rano-sides and -β-D-mannopyranosides. Stereochemical Influences of Aglycone Alcohols,” Tetrahedron Letter, Vol. 18, No. 2, 1977, pp. 175-178. doi:10.1016/S0040-4039(01)92581-X [13] I. Ohtani, T. Kusumi, Y. Kashman and H. Kakisawa, “High-Field FT NMR Application of Mosher’s Method. The Absolute Configurations of Marine Terpenoids,” Journal of American Chemical Society, Vol. 113, No. 11, 1991, pp. 4092-4096. doi:10.1021/ja00011a006 [14] K. Matsunami, H. Otsuka and Y. Takeda, “Structural Revisions of Blumenol C Glucoside and Byzantionoside Copyright © 2011 SciRes. AJPS  Symplocosionosides A-C, Three Megastigmane Glycosides, a Neolignan Glucoside, and Symplocosins A and B, Two Triterpene Glycosyl Esters from the Leaves of Symplocos cochinchinensis var. Philippinensis Copyright © 2011 SciRes. AJPS 618 B,” Chemical and Pharmaceutical Bulletin, Vol. 58, No. 3, 2010, pp. 438-441. doi:10.1248/cpb.58.438 [15] A. C. H. Braga, S. Zacchino, H. Badano, M. G. Sierra and E. A. Rúveda, “13C NMR Spectral and Conformational Analysis of 8-O-4′ Neolignans,” Phytochemistry, Vol. 23, No. 9, 1984, pp. 2025-2028. [16] C. Huo, H. Liang, Y. Zhao, B. Wang and Q. Zhang, “Neo-Lignan Glycosides from Symplocos caudata,” Phy- tochemistry, Vol. 69, No. 3, 2008, pp. 788-795. doi:10.1016/j.phytochem.2007.08.022 [17] A. Arnoldi and L. Merlini, “Asymmetric Synthesis of 3- Methyl-2-phenyl-1,4-Benzodioxanes. Absolute Configu- ration of the Neolignans Eusiderin and Eusiderin C and D,” Journal of Chemical Society Perkin Transaction I, No. 6, 1985, pp. 2555-2557. doi:10.1039/p19850002555 [18] K. Kónya, T. Kurtán, A. Kiss-Szikszai, L. Juhász and S. Antus, “A General CD-Method for the Configurational Assignment of Erythro-8,4'-Oxyneolign-8'-Enes,” Arki- voc, Vol. 8, 2004, pp. 72-78. [19] C. Sunil, S. Ignacimuthu and P. Agastian, “Antidiabetic Effect of Symplocos cochinchinensis (Lour.) S. Moore. in Type 2 Diabetic Rats,” Journal of Ethnopharmacology, Vol. 134, No. 2, 2011, pp. 298-304. doi:10.1016/j.jep.2010.12.018 [20] T. Ishii and M. Yanagisawa, “Synthesis, Separation and NMR Spectral Analysis of Methyl Apiofuranosides,” Carbohydrate Research, Vol. 313, No. 3-4, 1998, pp. 189-192. doi:10.1016/S0008-6215(98)00262-6 [21] T. Tanaka, T. Nakashima, T. Ueda, K. Tomii and I. Kouno, “Facile Discrimination of Aldose Enantiomers by Reversed-Phase HPLC,” Chemical and Pharmaceutical Bulletin, Vol. 55, No. 6, 2007, pp. 899-901. doi:10.1248/cpb.55.899
|