C.-W. CHENG ET AL.
297
in–1) D
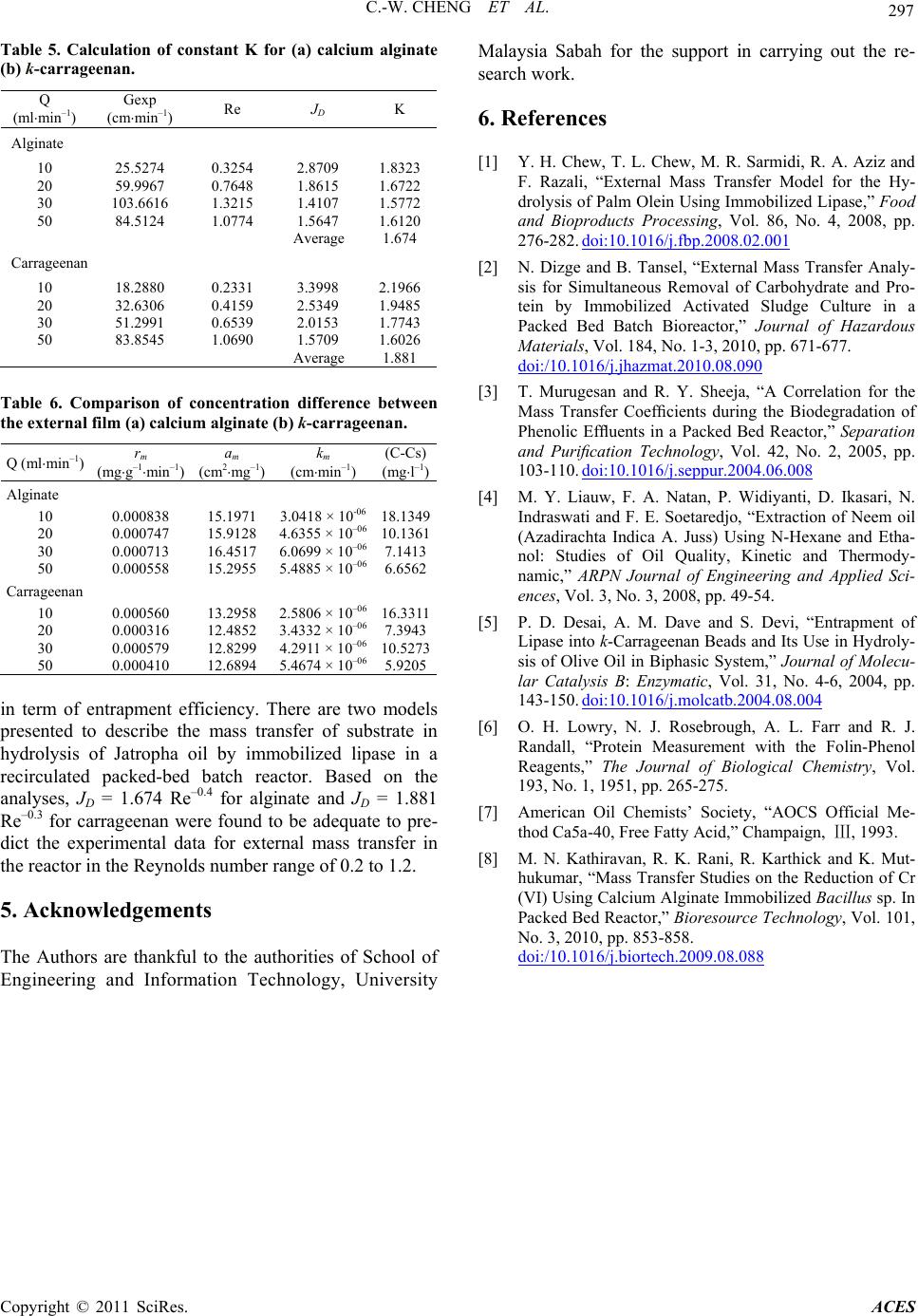
Table 5. Calculation of constant K for (a) calcium alginate
(b) k-carrageenan.
Q
(mlmin–1)
Gexp
(cmmRe J K
Alginate
10 25.5274 0.2.8709 1.83
59.9967 0.7648 1.8615 1.6
Carrageenan
10 18.2880 0.2331
32.6306 0.4159 2.5349 1.9485
3254 32
20 722
30 103.6616 1.3215 1.4107 1.5772
50 84.5124 1.0774 1.5647 1.6120
Average 1.674
3.3998 2.1966
20
30 51.2991 0.6539 2.0153 1.7743
50 83.8545 1.0690 1.5709 1.6026
Average 1.881
Table 6. Comparison of concentrationce
e external film (a) calcium alginate (b) k-carrageenan
)
differenbetween
. th
Q (mlmin–1) rm
(mgg–1min –1)
am
(cm2mg–1)
km
(cmmin–1)
(C-Cs)
(mgl–1
Alginate
10 0.000838 15.1971 3.
0.000747 15.9128 4.6355 × 10610.
–06
Carrageenan
0.000316 12.4852 3.4332 × 10–06 7.3943
–06
0418 × 10-06
–0
18.1349
20 1361
30 0.000713 16.4517 6.0699 × 10
–0
7.1413
50 0.000558 15.2955 5.4885 × 1066.6562
10 0.000560 13.2958 2.5806 × 10–06 16.3311
20
30 0.000579 12.8299 4.2911 × 10
–0
10.5273
50 0.000410 12.6894 5.4674 × 1065.9205
in teof enteffiT s
presented to describe the mss transfer of substrate in
the authorities of School of
ngineering and Information Technology, University
alaysia Sabah for the support in carrying out the re-
. References
] Y. H. Chew, T. L. Chew, M. R. Sarmidi, R. A. Aziz and
rm rapment ciency. here are two model
a
hydrolysis of Jatropha oil by immobilized lipase in a
recirculated packed-bed batch reactor. Based on the
analyses, JD = 1.674 Re–0.4 for alginate and JD = 1.881
Re–0.3 for carrageenan were found to be adequate to pre-
dict the experimental data for external mass transfer in
the reactor in the Reynolds number range of 0.2 to 1.2.
5. Acknowledgements
The Authors are thankful to
E
M
search work.
6
[1
F. Razali, “External Mass Transfer Model for the Hy-
drolysis of Palm Olein Using Immobilized Lipase,” Food
and Bioproducts Processing, Vol. 86, No. 4, 2008, pp.
276-282. doi:10.1016/j.fbp.2008.02.001
[2] N. Dizge and B. Tansel, “External Mass Transfer Analy-
sis for Simultaneous Removal of Carbohydrate and Pro-
tein by Immobilized Activated Sludge Culture in a
Packed Bed Batch Bioreactor,” Journal of Hazardous
Materials, Vol. 184, No. 1-3, 2010, pp. 671-677.
doi:/10.1016/j.jhazmat.2010.08.090
[3] T. Murugesan and R. Y. Sheeja, “A Correlation for the
Mass Transfer Coefficients during the Biodegradation of
Phenolic Effluents in a Packed Bed Reactor,” Separation
and Purification Technology, Vol. 42, No. 2, 2005, pp.
103-110. doi:10.1016/j.seppur.2004.06.008
[4] M. Y. Liauw, F. A. Natan, P. Widiyanti, D. Ikasari, N.
vi, “Entrapment of
Indraswati and F. E. Soetaredjo, “Extraction of Neem oil
(Azadirachta Indica A. Juss) Using N-Hexane and Etha-
nol: Studies of Oil Quality, Kinetic and Thermody-
namic,” ARPN Journal of Engineering and Applied Sci-
ences, Vol. 3, No. 3, 2008, pp. 49-54.
[5] P. D. Desai, A. M. Dave and S. De
Lipase into k-Carrageenan Beads and Its Use in Hydroly-
sis of Olive Oil in Biphasic System,” Journal of Molecu-
lar Catalysis B: Enzymatic, Vol. 31, No. 4-6, 2004, pp.
143-150. doi:10.1016/j.molcatb.2004.08.004
[6] O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J.
ty, “AOCS Official Me-
t-
9.08.088
Randall, “Protein Measurement with the Folin-Phenol
Reagents,” The Journal of Biological Chemistry, Vol.
193, No. 1, 1951, pp. 265-275.
[7] American Oil Chemists’ Socie
thod Ca5a-40, Free Fatty Acid,” Champaign, Ⅲ, 1993.
[8] M. N. Kathiravan, R. K. Rani, R. Karthick and K. Mu
hukumar, “Mass Transfer Studies on the Reduction of Cr
(VI) Using Calcium Alginate Immobilized Bacillus sp. In
Packed Bed Reactor,” Bioresource Technology, Vol. 101,
No. 3, 2010, pp. 853-858.
doi:/10.1016/j.biortech.200
Copyright © 2011 SciRes. ACES