206 Y. BAKHSHAN ET AL.
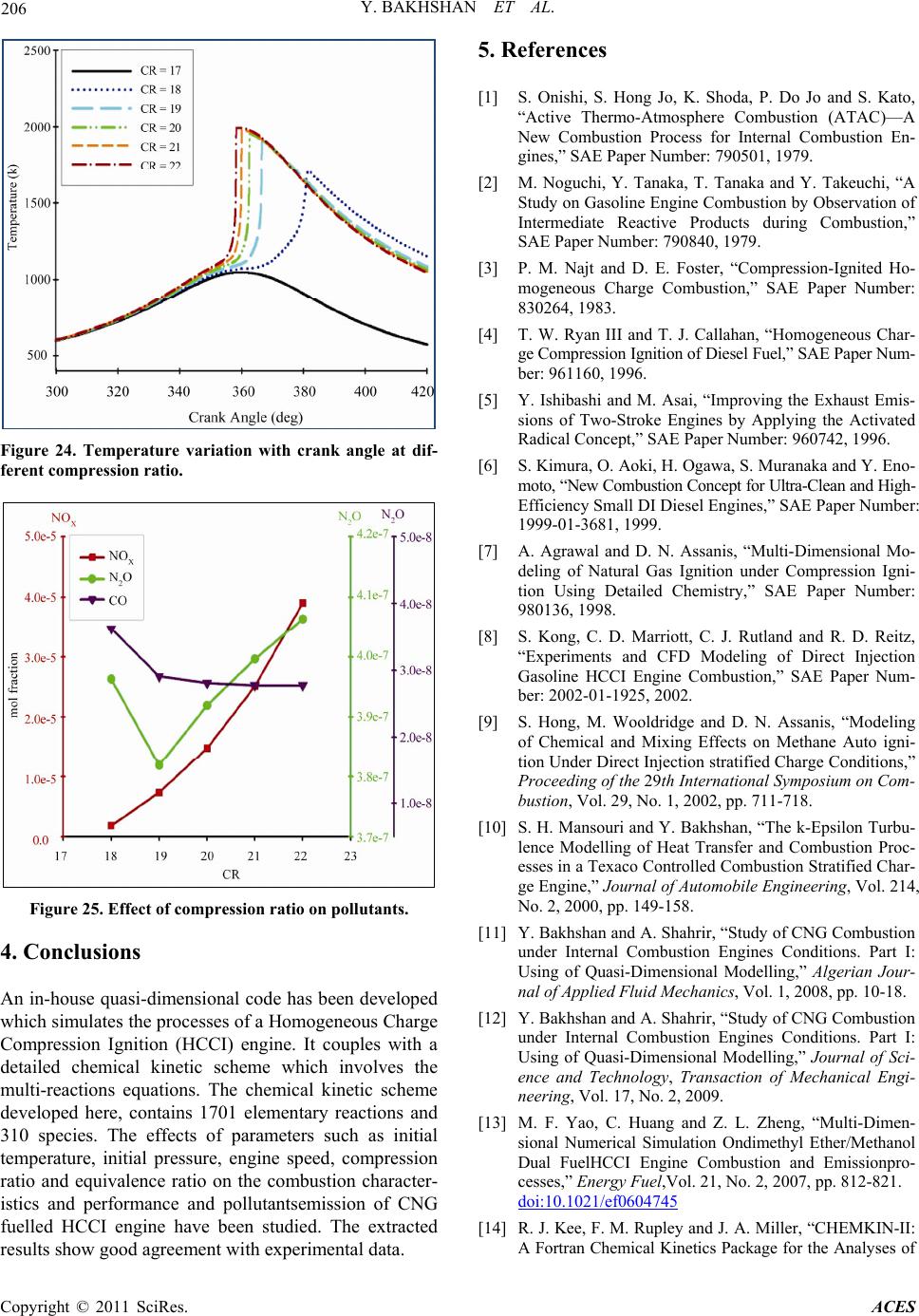
Figure 24. Temperature variation with crank angle at dif-
ferent compression ratio .
Figure 25. Effect of compression ratio on pollutants.
4. Conclusions
An in-house quasi-dimensional code has been developed
which simulates the processes of a Homogeneous Charge
Compression Ignition (HCCI) engine. It couples with a
detailed chemical kinetic scheme which involves the
multi-reactions equations. The chemical kinetic scheme
developed here, contains 1701 elementary reactions and
310 species. The effects of parameters such as initial
temperature, initial pressure, engine speed, compression
ratio and equivalence ratio on the combustion character-
istics and performance and pollutantsemission of CNG
fuelled HCCI engine have been studied. The extracted
results show good agreement with experimental data.
5. References
[1] S. Onishi, S. Hong Jo, K. Shoda, P. Do Jo and S. Kato,
“Active Thermo-Atmosphere Combustion (ATAC)—A
New Combustion Process for Internal Combustion En-
gines,” SAE Paper Number: 790501, 1979.
[2] M. Noguchi, Y. Tanaka, T. Tanaka and Y. Takeuchi, “A
Study on Gasoline Engine Combustion by Observation of
Intermediate Reactive Products during Combustion,”
SAE Paper Number: 790840, 1979.
[3] P. M. Najt and D. E. Foster, “Compression-Ignited Ho-
mogeneous Charge Combustion,” SAE Paper Number:
830264, 1983.
[4] T. W. Ryan III and T. J. Callahan, “Homogeneous Char-
ge Compression Ignition of Diesel Fuel,” SAE Paper Num-
ber: 961160, 1996.
[5] Y. Ishibashi and M. Asai, “Improving the Exhaust Emis-
sions of Two-Stroke Engines by Applying the Activated
Radical Concept,” SAE Paper Number: 960742, 1996.
[6] S. Kimura, O. Aoki, H. Ogawa, S. Muranaka and Y. Eno-
moto, “New Combustion Concept for Ultra-Clean and High-
Efficiency Small DI Diesel Engines,” SAE Paper Number:
1999-01-3681, 1999.
[7] A. Agrawal and D. N. Assanis, “Multi- Dimensional Mo-
deling of Natural Gas Ignition under Compression Igni-
tion Using Detailed Chemistry,” SAE Paper Number:
980136, 1998.
[8] S. Kong, C. D. Marriott, C. J. Rutland and R. D. Reitz,
“Experiments and CFD Modeling of Direct Injection
Gasoline HCCI Engine Combustion,” SAE Paper Num-
ber: 2002-01-1925, 2002.
[9] S. Hong, M. Wooldridge and D. N. Assanis, “Modeling
of Chemical and Mixing Effects on Methane Auto igni-
tion Under Direct Injection stratified Charge Conditions,”
Proceeding of the 29th International Symposium on Com-
bustion, Vol. 29, No. 1, 2002, pp. 711-718.
[10] S. H. Mansouri and Y. Bakhshan, “The k-Epsilon Turbu-
lence Modelling of Heat Transfer and Combustion Proc-
esses in a Texaco Controlled Combustion Stratified Char-
ge Engine,” Journal of Automobile Engineering, Vol. 214,
No. 2, 2000, pp. 149-158.
[11] Y. Bakhshan and A. Shahrir, “Study of CNG Combustion
under Internal Combustion Engines Conditions. Part I:
Using of Quasi-Dimensional Modelling,” Algerian Jour-
nal of Applied Fluid Mechanics, Vol. 1, 2008, pp. 10-18.
[12] Y. Bakhshan and A. Shahrir, “Study of CNG Combustion
under Internal Combustion Engines Conditions. Part I:
Using of Quasi-Dimensional Modelling,” Journal of Sci-
ence and Technology, Transaction of Mechanical Engi-
neering, Vol. 17, No. 2, 2009.
[13] M. F. Yao, C. Huang and Z. L. Zheng, “Multi-Dimen-
sional Numerical Simulation Ondimethyl Ether/Methanol
Dual FuelHCCI Engine Combustion and Emissionpro-
cesses,” Energy Fuel,Vol. 21, No. 2, 2007, pp. 812-821.
doi:10.1021/ef0604745
[14] R. J. Kee, F. M. Rupley and J. A. Miller, “CHEMKIN-II:
A Fortran Chemical Kinetics Package for the Analyses of
Copyright © 2011 SciRes. ACES