 Advances in Chemical Engi neering and Science , 2011, 1, 183-190 doi:10.4236/aces.2011.14027 Published Online October 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Formation and Elimination of Pollutant during Sludge Decomposition in the Presence of Cement Raw Material and Other Catalysts Juan A. Conesa, Araceli Gálvez, Ignacio Martín-Gullón, Rafael Font Department of Chemical Engineering, University of Alicante, Alicante, Spain E-mail: ja.conesa@ua.es Recieved January 28, 2011; revised March 25, 2011; accepted May 12, 2011 Abstract The use of a waste-based secondary fuel in clinker kilns is a widely used practice. Nevertheless, specific studies to understand the destruction mechanism of exhaust pollutants in cement raw material (CRM) are limited. This work focuses on the possible catalytic effect of the interaction of exhaust gases from the com- bustion of sewage sludge with various solids beds, including CRM. Catalyst based on vanadium pentoxide and deNOx commercial catalyst, based on Ti/Zr/Pt, were used. The behaviors of volatile compounds, polycyclic aromatic compounds and dioxins and furans are analyzed in the presence or absence of the different materials. Some compounds are produced when interacting the pollutants with the beds, and some others are destroyed. Results show that the presence of CRM at the outlet of the combustion gases is beneficial for the decrease in pollutant emission, confirmed by a catalytic effect of CRM at medium temperatures. Keywords: Dioxin, Polycyclic Aromatic Hydrocarbons, Sewage Sludge, Cement, Emissions 1. Introduction Currently, a common way of eliminating sewage sludge is its combustion in either specifically designed incin- erators or in cement kilns. Incineration is the destruction by thermal oxidation at high temperature to convert waste material into a much smaller inert volume (not dangerous), i.e. the combustion of waste at high tem- perature in the presence of large amounts of oxygen in order to eliminate it. It can be useful to take advantage of these thermal resources while si multaneously eliminatin g waste. This is the case in using waste as an alternative fuel or energy recovery in industrial boilers and furnaces of clinker in the cement industry [1]. The use of alternative fuels has been an established practice in most developed countries for over the last thirty years. This is an activity being developed in Europe with total environmental qua lity since 1975. This activity has both societal and industrial benefits, such as decreasing non-renewable fossil fuel consumption, low- ering the emission of greenhouse gases (75% of green- house gases emissions produced from waste management are due to methane generated in land filling), reducing the amount of waste deposited in landfills, recovering latent energy contained in waste and the reduction of indirect energy costs arising from the use of fossil fuels (payment of allowances for CO2). In 2007, the use of alternative fuels from treated bio- mass in Spain reduced atmospheric CO2 emissions by 300,000 tonnes, equivalent to the emissions of 100,000 cars in one year. The average unit emissions in Spain in 2005-2006 were 860 kg CO2/t clinker against the Euro- pean average of 872 kg CO2/t clinker and the world av- erage of 873 kg CO2/t clinker [2]. In Spain, approximately one third of installed cement furnaces are using alternative fuels, equivalent to a con- sumption of 3 million tonnes of coal. In 2001, the Span- ish cement sector managed almost 52,000 tonnes of waste as alternative fuels, accounting for just over 1% of the theoretical co nsumption of clink er kilns. However, in recent years a significant increase has been realized. In 2007, more than 290,000 t of waste was managed [2]. Nevertheless, absolute amounts are still very small: 72000 t of liquid wastes (oils, solvents, ...); 223,000 t of solid fuels as meat meal (88,000 t), tires (42,000 t), saw- dust (35,000 t), wood (27,00 0 t), WWTP sludge (9000 t),  184 J. A. CONESA ET AL. lightweight plastic (5000 t) and lesser amounts of other waste types. This represents 8% of total fuel used in 2006, but indicating a significant increase in 5 years. In recent literature [3,4] it is shown that while the use of sewage sludge as secondary fuel is beneficial for the reduction in greenhouse gas emissions, no additional health risks for the populatio n derived fr om PCDD/F and metal emissions are estimated. Concerning the properties of the clinker produced by using sludge as fuel, several research groups are working on the properties of the ce- ment (clinker) when mixed with different wastes’ ash. In literature [5] it is shown that the inclusion of ash improve mechanical properties and that mortars fabricated with 10 wt% sewage sludge ash replacement meet the me- chanical requirements of the European standard in terms of early age compressive strength and nominal compres- sive strength. Precedence It is well known that cement kiln exhaust gases are nor- mally clean, and no gas washing processes are necessary. In regular cement plant schemes, the cement raw mate- rial (CRM) is fed to the kiln at the opposite end to the main burner. Combustion gases, together with clinkeri- zation exhaust gases, flow through the whole process counter-currently to solids. This disposition allows a direct contact between the fresh CRM with exhaust gases in a cyclone system, preheating CRM prior to the kiln entrance, thus saving energy. During the cooling of the exhaust gases some pollutants could be produced, such as dioxins by de-novo synthesis [6-8] or other organic compounds from the desorption on CRM [9,10]. Addi- tionally, during the contact of flue gas-CRM in the cy- clones, the elimination of the pollutants could also be possible by the action of CRM through adsorption or catalytic oxidation. There are many papers about the study of the adsorption of organic pollutants in different substances like fly ash, coke or active carbon [11,12] where PCDD/Fs are adsorbed up to 90%, but unfortu- nately, there are not enough scientific references to ana- lyze this fact over other materials. Several works [13-17] report that a catalyst based on V2O5/WO3 supported on TiO2 produced a sharp decrease of organochlorinated compounds, including dioxins and furans (PCDD/Fs) at low temperatures (200˚C - 400˚C). This catalyst is commercially available due to its being commonly used in the SCR process for the elimination of NOx of flue gas stream (DeNOx). Some researchers [17, 18] carried out experiments with a V2O5/TiO2 cata- lyst in relation to PAHs and found that the efficacy for PAHs destruction increases with temperature and the efficacy for PAHs adsorption on the catalyst pellets decreases. With respect to dioxin elimination, some papers have been published on this subject [12,17-21]. The re- sults of these studies revealed that the use of V2O5/TiO2 cata- lysts results in a decrease of PCDD/Fs emissions of 90% - 99% at 200˚C - 300˚C. According to [17], the elimina- tion of dioxins is 98% at 230˚C with 1% adsorption on the catalyst. On the other hand, it has been shown that the adsorption decreases with temperature [18]. In the work presented in this paper, the direct contact of a stream of gases produced during combustion of sewage sludge with a fixed bed of different materials was considered. The analysis of the volatile compounds, se- mivolatile (PAHs) and dioxins and furans resulting in emissions was recorded, in order to analyze the effect of the presence of the different particle beds. This study was conducted in two stages. First, a few preliminary experiments were conducted in the laboratory furnace described elsewhere [22]. In a second stage, a new ex- perimental system was designed, with the aim to cor- roborate some results and to extend the study with the use of new materials and different operating conditions. 2. Material and Methods Dried sewage sludge from a waste water facility in Tar- ragona, Spain was used. In addition, milled cement raw material (CRM), supplied by a cement industry close to the University of Alicante was also used. An exhaustive analysis of the two materials used made, and the results are shown elsewhere [22]. A previous crushing was car- ried out to improve homogenization. Other materials used to directly compare the catalytic over the flue gases are: V2O5—1 wt% of vanadium pentoxide was incorpora- ted to SiO2. It is known the catalytic activity of V2O5 in the removal of NOx emissions in chimneys. In recent years, it has also been proven that reduces the emission of PCDD/Fs [19]. PD—Alumina/Palladium catalyst beads with 1% of active phase provided by Enghelard. CA—Automotive catalyst honeycomb extracted for a car muffler. Table 1 shows the metallic composition of Table 1. Analysis of the automotive catalyst. Oxide Weight% Al 26.64 Si 13.41 Mg 3.61 La 0.72 Fe 0.47 Ce 0.34 Ti 0.26 Zr 0.25 P 0.14 Pt 0.10 Copyright © 2011 SciRes. ACES  J. A. CONESA ET AL. Copyright © 2011 SciRes. ACES 185 this catalyst obtained by X-Ray Fluorescence, where the active metals or promoters seem to be La, Ce and Pt. The equipment used to carry out the present work, this catalyst obtained by X-Ray Fluorescence, where the shown in Figure 1, has been exclusively developed to accurately control the ratio of oxygen in combustion processes. It consists of a moving tubular reactor with the sewage sludge carefully placed along the tube, which is introduced at a precisely controlled speed in a furnace while passing through a constant flow of air. In this way, a constant flow of air and a constant mass rate of sludge were used. This permits to simulate during several min- utes the continuous combustion. The equipment comprises a sample introduction area, the combustion zone, the gas transfer zone and the zone of interaction of gases with the different catalyst, which is separately heated by a blanket heater, simulating the gas temperature in the cyclonic system in a cement plant. Before carrying out the experiments shown in this paper, it was done a comprehensive study on the capacity of the equipment with respect to leaks, reproducibility of expe- riments, and homogeneity of the obtained gas flow. The ratio between carbon monoxide and carbon diox- ide is maintained in all experiments to approximately 0.38, indicating that the combustion process has been carried out very similarly in all runs. In the case of powdery substances, as is the case of V2O5/SiO2 catalyst, quartz wool was intercalated in order to reduce pressure drop. The mass ratio between the amount of sludge used in each experiment and the cata- lyst was 4: 1. During the study, several operating conditions were modified, such as temperature and catalyst nature. Oxy- gen ratio, as defined by [23], was kept constant at λ = 0.14, a sub-stoichiometric value in order to simulate poor areas and poor combustion furnace mixing and therefore mass production of organic contaminants. To achieve these combustion conditions, a sample size of approxi- mately 1200 mg spread over 280 mm was used. The sample introduction rate was 1.8 mm/s and the air flow was 300 ml/min. The temperatures were 850˚C in the combustion oven and 300˚C in the transfer and interact- tion-catalyst line. The particles used in the interaction zone were introduced into tubes of 8 mm internal diame- ter and along the length 10 - 15 mm. Comparing these experimental conditions with that present in the cement plant, it is interesting to note that the temperature in the lab reactor is much lower than that of the plant. Furthermore, the preheater and precalciner stage, from the flue gas, and the quenching of the hot kiln gases before air pollution treatment are not sepa- rately considered. Although the ultimate application of the study is the use of sewage sludge in cement plants, we must take into account that the conditions are not reproducing the industrial plant. The experimental condi- tions, with a very low air supply, are selected in order to maximize the production of pollutants such as PAHs or dioxins, as previously presented [23,24]. On the other hand, temperature of the actual interact- tion of CRM with the hot gases is not known. The inter- action takes place somewhere between the gas exit of the cement kiln (approx. 1500˚C) and the input of the meal solids (approx. 300˚C in the preheater). In the present study an intermediate temperature of 850˚C has been selected. Sampling Sampling was carried out at the end of the interaction zone. Three types of pollutants have been analyzed: - Volatile organic compounds (C1-C6) gases were collected using Tedlar bags and then analyzed by gas chromatograph with flame ignition detector (GC-FID) and with thermal conductivity de tector (GC-TCD). - Semivolatile organic compounds (PAHs): a XAD2 resin w as used as ad sorbe nt and af ter a pro cess of ex trac - tion and concentration was analyzed by high resolution gas chromatography and spectrometry (HRGC/MS). The analysis of semivolatile compounds has focused exclu- sively on the group of 16 polyaromatic hydrocarbons established by the American Environmental Protection Agency (USEPA) as priority pollutants and potential carcinogens. - Dioxins and furans: collected similarly to PAHs with subsequent analysis by HRGC/MS. Method EPA 1613 was used to analyze the samples. 850 ºC Furnace (850 ºC) Adsorbent resin Heating blanket 300ºC Transfer line zone Feed ing zone Waste (sewage sludge) Air input Gases out ut Horizontal actuator (mechanic movement at constant velocity) Figure 1. Scheme of the laboratory e quipme nt used.  186 J. A. CONESA ET AL. 3. Results and Discussion 3.1. Volatile Organic Compounds (VOCs) VOCs analyzed in each of the conditions did not show changes in yields when using any catalyst. Figure 2 shows the results achieved with the different runs con- sidering the sewage sludge combustion without any ma- terial in the bed and with different materials inside the bed. It can be observed that the profile of compounds in all cases is quite similar, and no specific trend can be seen in the elimination of compounds in this range of volatility. This result coincides with that found in the prelimi- nary runs [22] in which smaller-scale experiments al- ready indicated that none of the substances tested caused the removal of hydrocarbons, even heavier substances such as benzene, toluene and xylenes. The main com- pounds are methane, ethylene and benzene, as already shown in the work of [24] and [22]. 3.2. Polycyclic Aromatic Hydrocarbons (PAHs) Table 2 shows the results for the experiments done with the different materials in the bed at the post-combustion zone. The profile of compounds is very similar in all experiments, with naphthalene, acenaphthylene, fluorene and phenanthrene as major compound in all cases, re- gardless of catalyst used. Considering the data, we can observe that the lightest PAHs present relative high yields. The exhaust catalysts from the bed used in the runs were also analyzed for PAHs, and virtually no ad- sorbed hydrocarbons were found. In this way, the power of removing this type of compounds is mainly due to thermal destruction or decay, not adsorption in the bed. In order to analyze the experimental data, it is useful Figure 2. Emission of VOCs. SS: sew age sludge c ombustion; SS + CC: use of automotive honeycomb catalyst; SS + Pd: use o f bead paladium catalyst; SS + CRM: use of cement r aw mate r ial; SS + V2O5: use of Si + V2O5. Table 2. Emission of PAHs. MW (g/mol) SS SS + CCSS + CRM SS + Pd SS + V2O5 Naphthalene 128 2472 3393 1696 1393 1862 Acenaphtylene 152 505 211 385 293 503 Acenaphtene 154 39.8 39.7 24.0 148 25.1 Fluorene 166 178 144 135 149 163 Phenanthrene 188 330 337 215 275 256 Anthracene 188 99.6 123.3 75.4 83.5 107 Fluoranthene 202 58.8 61.5 45.9 45.4 60.9 Pyrene 202 61.2 76.7 51.0 54.4 69.4 Benzo(a)anthracene 228 12.3 32.7 20.8 23.8 32.2 Chrysene 228 24.3 26.4 28.4 24.3 22.4 Benzo(b)fluoranthene 252 7.5 11.2 4.2 5.3 6.2 Benzo(k)fluoranthene 252 1.8 3.3 3.7 6.3 6.2 Benzo(a)pyrene 252 6.5 12.0 7.2 9.1 12.7 Indeno(1,2,3-cd)pyrene 276 3.4 2.5 2.4 2.2 3.1 Dibenz(a,h)anthracene 278 2.4 1.6 1.5 0.7 0.9 Benzo(g,h,i)perylene 276 2.7 1.2 1.8 1.7 1.8 SS = sewa g e sludge; CC = automotive c atalyst; CRM = cement raw material; Pd = palladium b ased catalyst. Copyright © 2011 SciRes. ACES 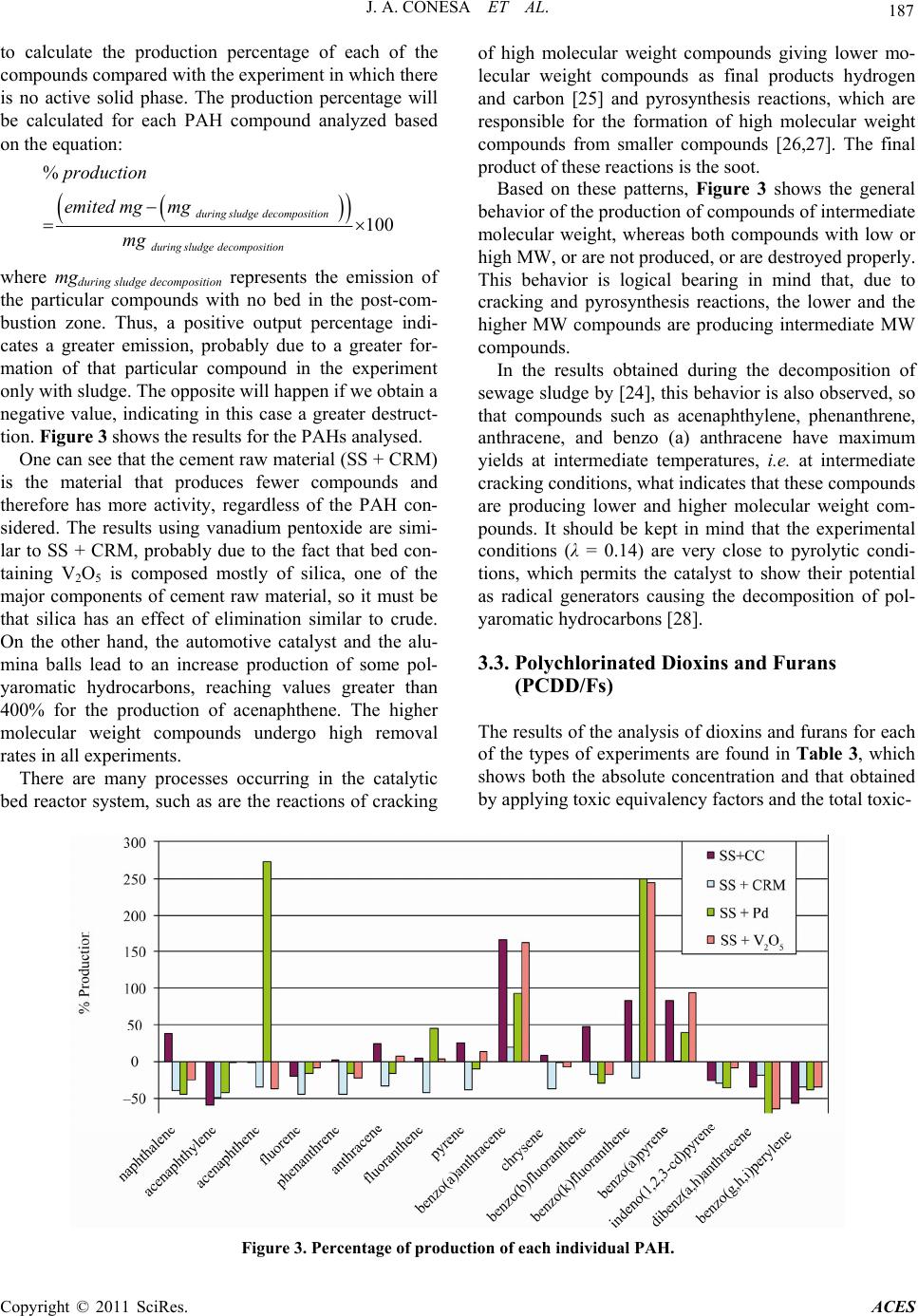 J. A. CONESA ET AL. Copyright © 2011 SciRes. ACES 187 to calculate the production percentage of each of the compounds compared with the experiment in which there is no active solid phase. The production percentage will be calculated for each PAH compound analyzed based on the equation: % 100 during sludgedecomposition duringsludgedecomposition production emited mgmg mg where mgduring sludge decomposition represents the emission of the particular compounds with no bed in the post-com- bustion zone. Thus, a positive output percentage indi- cates a greater emission, probably due to a greater for- mation of that particular compound in the experiment only with sludge. The opposite will happen if we obtain a negative value, indicating in this case a greater destruct- tion. Figure 3 shows the results for the PAHs analysed. One can see that the cement raw material (SS + CRM) is the material that produces fewer compounds and therefore has more activity, regardless of the PAH con- sidered. The results using vanadium pentoxide are simi- lar to SS + CRM, probably due to the fact that bed con- taining V2O5 is composed mostly of silica, one of the major components of cement raw material, so it must be that silica has an effect of elimination similar to crude. On the other hand, the automotive catalyst and the alu- mina balls lead to an increase production of some pol- yaromatic hydrocarbons, reaching values greater than 400% for the production of acenaphthene. The higher molecular weight compounds undergo high removal rates in all experiments. There are many processes occurring in the catalytic bed reactor system, such as are the reactions of cracking of high molecular weight compounds giving lower mo- lecular weight compounds as final products hydrogen and carbon [25] and pyrosynthesis reactions, which are responsible for the formation of high molecular weight compounds from smaller compounds [26,27]. The final product of these reactions is the soot. Based on these patterns, Figure 3 shows the general behavior of the production of compounds of intermediate molecular weight, whereas both compounds with low or high MW, or are not produced, or are destroyed properly. This behavior is logical bearing in mind that, due to cracking and pyrosynthesis reactions, the lower and the higher MW compounds are producing intermediate MW compounds. In the results obtained during the decomposition of sewage sludge by [24], this behavior is also observed, so that compounds such as acenaphthylene, phenanthrene, anthracene, and benzo (a) anthracene have maximum yields at intermediate temperatures, i.e. at intermediate cracking conditions, what indicates that these compounds are producing lower and higher molecular weight com- pounds. It should be kept in mind that the experimental conditions (λ = 0.14) are very close to pyrolytic condi- tions, which permits the catalyst to show their potential as radical generators causing the decomposition of pol- yaromatic hydrocarbons [28]. 3.3. Polychlorinated Dioxins and Furans (PCDD/Fs) The results of the analysis of dioxins and furans for each of the types of experiments are found in Table 3, which shows both the absolute concentration and that obtained by applying toxic equivalency factors and the total toxic- Figure 3. Percentage of production of each individual PAH. 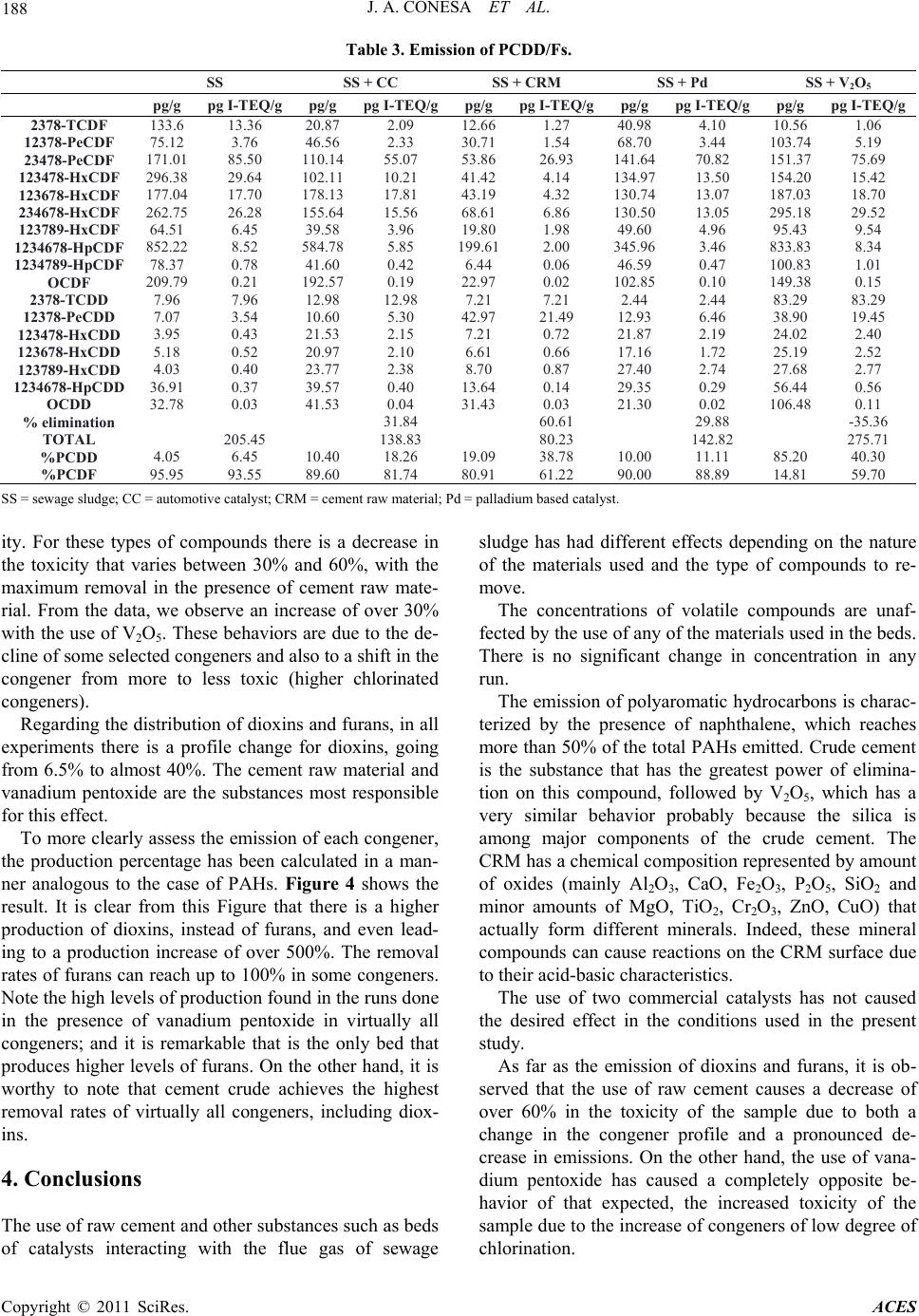 188 J. A. CONESA ET AL. Table 3. Emission of PCDD/Fs. SS = sewa g e sludge; CC = automotive c atalyst; CRM = cement raw material; Pd = palladium b ased catalyst. ity. For these types of compounds there is a decrease in the toxicity that varies between 30% and 60%, with the maximum removal in the presence of cement raw mate- rial. From the data, we observe an increase of over 30% with the use of V2O5. These behaviors are due to the de- cline of some selected congeners and also to a shift in the congener from more to less toxic (higher chlorinated congeners). Regarding the distribution of dioxins and furans, in all experiments there is a profile change for dioxins, going from 6.5% to almost 40%. The cement raw material and vanadium pentoxide are the substances most responsible for this effect. To more clearly assess the emission of each congener, the production percentage has been calculated in a man- ner analogous to the case of PAHs. Figure 4 shows the result. It is clear from this Figure that there is a higher production of dioxins, instead of furans, and even lead- ing to a production increase of over 500%. The removal rates of furans can reach up to 100% in some congeners. Note the high levels of production found in the runs done in the presence of vanadium pentoxide in virtually all congeners; and it is remarkable that is the only bed that produces higher levels of furans. On the other hand, it is worthy to note that cement crude achieves the highest removal rates of virtually all congeners, including diox- ins. 4. Conclusions The use of raw cement and other substances such as beds of catalysts interacting with the flue gas of sewage sludge has had different effects depending on the nature of the materials used and the type of compounds to re- move. The concentrations of volatile compounds are unaf- fected by the use of any of the materials used in the beds. There is no significant change in concentration in any run. The emission of polyaromatic h ydrocarbons is charac- terized by the presence of naphthalene, which reaches more than 50% of the total PAHs emitted. Crude cement is the substance that has the greatest power of elimina- tion on this compound, followed by V2O5, which has a very similar behavior probably because the silica is among major components of the crude cement. The CRM has a chemical composition represented by amount of oxides (mainly Al2O3, CaO, Fe2O3, P2O5, SiO2 and minor amounts of MgO, TiO2, Cr2O3, ZnO, CuO) that actually form different minerals. Indeed, these mineral compounds can cause reactions on the CRM surface due to their acid-basic characteristics. The use of two commercial catalysts has not caused the desired effect in the conditions used in the present study. As far as the emission of dioxins and furans, it is ob- served that the use of raw cement causes a decrease of over 60% in the toxicity of the sample due to both a change in the congener profile and a pronounced de- crease in emissions. On the other hand, the use of vana- dium pentoxide has caused a completely opposite be- havior of that expected, the increased toxicity of the sample due to the increase of congeners of low degree of chlorination. SS SS + CC SS + CRM SS + Pd SS + V2O5 pg/g pg I-TEQ/g pg/g pg I-TEQ/gpg/g pg I-TEQ/gpg/g pg I-TEQ/g pg/g pg I-TEQ/g 2378-TCDF 133.6 13.36 20.87 2.09 12.661.27 40.984.10 10.56 1.06 12378-PeCDF 75.12 3.76 46.56 2.33 30.711.54 68.703.44 103.74 5.19 23478-PeCDF 171.01 85.50 110.14 55.07 53.8626.93 141.6470.82 151.37 75.69 123478-HxCDF 296.38 29.64 102.11 10.21 41.424.14 134.9713.50 154.20 15.42 123678-HxCDF 177.04 17.70 178.13 17.81 43.194.32 130.7413.07 187.03 18.70 234678-HxCDF 262.75 26.28 155.64 15.56 68.616.86 130.5013.05 295.18 29.52 123789-HxCDF 64.51 6.45 39.58 3.96 19.801.98 49.604.96 95.43 9.54 1234678-HpCDF 852.22 8.52 584.78 5.85 199.612.00 345.963.46 833.83 8.34 1234789-HpCDF 78.37 0.78 41.60 0.42 6.44 0.06 46.590.47 100.83 1.01 OCDF 209.79 0.21 192.57 0.19 22.970.02 102.850.10 149.38 0.15 2378-TCDD 7.96 7.96 12.98 12.98 7.21 7.21 2.44 2.44 83.29 83.29 12378-PeCDD 7.07 3.54 10.60 5.30 42.97 21.49 12.936.46 38.90 19.45 123478-HxCDD 3.95 0.43 21.53 2.15 7.21 0.72 21.872.19 24.02 2.40 123678-HxCDD 5.18 0.52 20.97 2.10 6.61 0.66 17.161.72 25.19 2.52 123789-HxCDD 4.03 0.40 23.77 2.38 8.70 0.87 27.402.74 27.68 2.77 1234678-HpCDD 36.91 0.37 39.57 0.40 13.640.14 29.350.29 56.44 0.56 OCDD 32.78 0.03 41.53 0.04 31.430.03 21.300.02 106.48 0.11 % elimination 31.84 60.61 29.88 -35.36 TOTAL 205.45 138.83 80.23 142.82 275.71 %PCDD 4.05 6.45 10.40 18.26 19.0938.78 10.0011.11 85.20 40.30 %PCDF 95.95 93.55 89.60 81.74 80.9161.22 90.0088.89 14.81 59.70 Copyright © 2011 SciRes. ACES 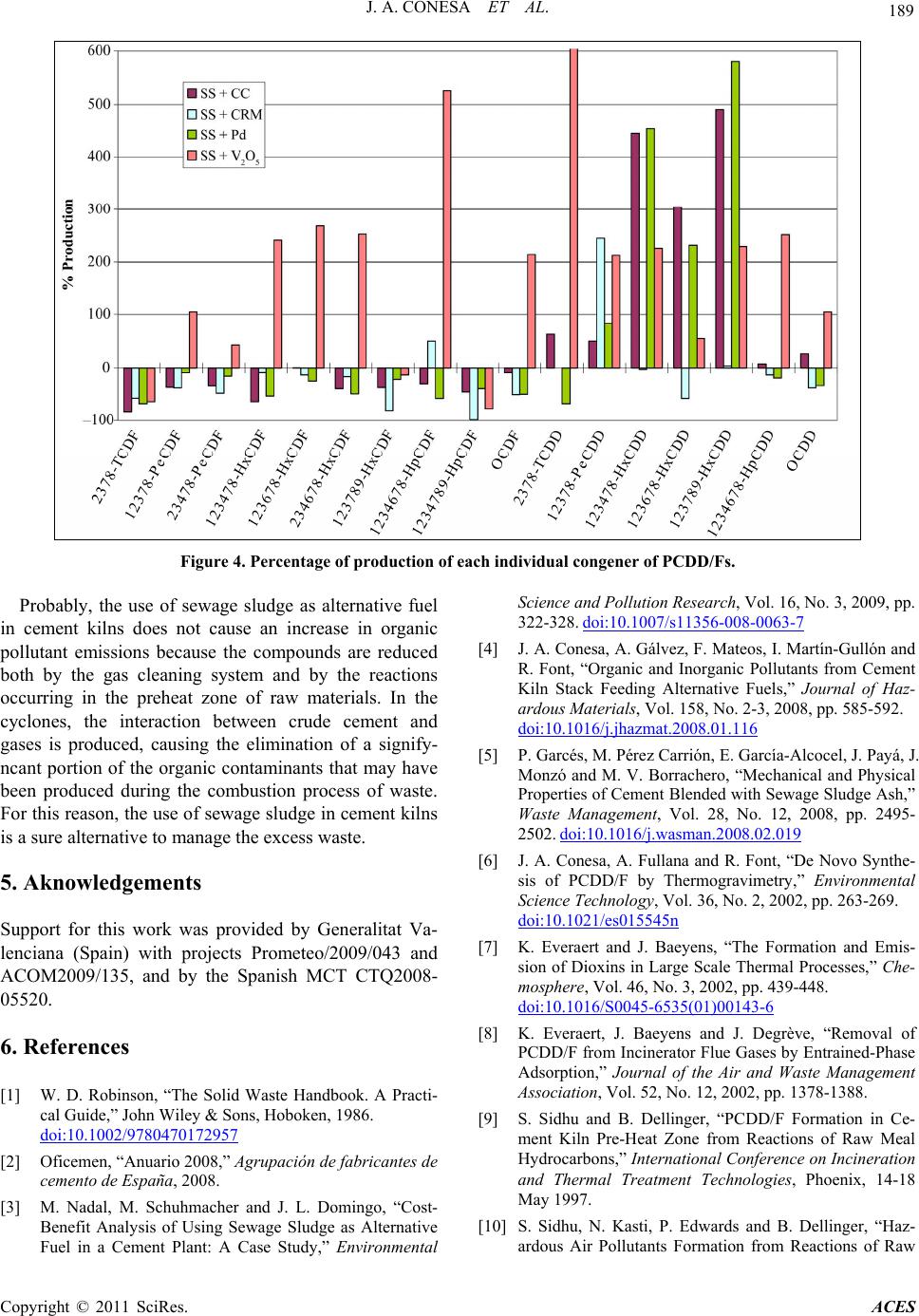 J. A. CONESA ET AL. Copyright © 2011 SciRes. ACES 189 Figure 4. Percentage of production of each individual congener of PCDD/Fs. Probably, the use of sewage sludge as alternative fuel in cement kilns does not cause an increase in organic pollutant emissions because the compounds are reduced both by the gas cleaning system and by the reactions occurring in the preheat zone of raw materials. In the cyclones, the interaction between crude cement and gases is produced, causing the elimination of a signify- ncant portion of the organic contaminants that may have been produced during the combustion process of waste. For this reason, the use of sewage sludge in cement kilns is a sure alternative to manage the excess waste. 5. Aknowledgements Support for this work was provided by Generalitat Va- lenciana (Spain) with projects Prometeo/2009/043 and ACOM2009/135, and by the Spanish MCT CTQ2008- 05520. 6. References [1] W. D. Robinson, “The Solid Waste Handbook. A Practi- cal Guide,” John Wiley & Sons, Hoboken, 1986. doi:10.1002/9780470172957 [2] Oficemen, “Anuario 2008,” Agrupación de fabricantes de cemento de España, 2008. [3] M. Nadal, M. Schuhmacher and J. L. Domingo, “Cost- Benefit Analysis of Using Sewage Sludge as Alternative Fuel in a Cement Plant: A Case Study,” Environmental Science and Pollution Research, Vol. 16, No. 3, 2009, pp. 322-328. doi:10.1007/s11356-008-0063-7 [4] J. A. Conesa, A. Gálvez, F. Mateos, I. Martín-Gullón and R. Font, “Organic and Inorganic Pollutants from Cement Kiln Stack Feeding Alternative Fuels,” Journal of Haz- ardous Materials, Vol. 158, No. 2-3, 2008, pp. 585-592. doi:10.1016/j.jhazmat.2008.01.116 [5] P. Garcés, M. Pérez Carrión, E. García-Alcocel, J. Payá, J. Monzó and M. V. Borrachero, “Mechanical and Physical Properties of Cement Blended with Sewage Sludge Ash,” Waste Management, Vol. 28, No. 12, 2008, pp. 2495- 2502. doi:10.1016/j.wasman.2008.02.019 [6] J. A. Conesa, A. Fullana and R. Font, “De Novo Synthe- sis of PCDD/F by Thermogravimetry,” Environmental Science Technology, Vol. 36, No. 2, 2002, pp. 263-269. doi:10.1021/es015545n [7] K. Everaert and J. Baeyens, “The Formation and Emis- sion of Dioxins in Large Scale Thermal Processes,” Che- mosphere, Vol. 46, No. 3, 2002, pp. 439-448. doi:10.1016/S0045-6535(01)00143-6 [8] K. Everaert, J. Baeyens and J. Degrève, “Removal of PCDD/F from Incinerator Flue Gases by Entrained-Phase Adsorption,” Journal of the Air and Waste Management Association, Vol. 52, No. 12, 2002, pp. 1378-1388. [9] S. Sidhu and B. Dellinger, “PCDD/F Formation in Ce- ment Kiln Pre-Heat Zone from Reactions of Raw Meal Hydrocarbons,” International Conference on Incineration and Thermal Treatment Technologies, Phoenix, 14-18 May 1997. [10] S. Sidhu, N. Kasti, P. Edwards and B. Dellinger, “Haz- ardous Air Pollutants Formation from Reactions of Raw  190 J. A. CONESA ET AL. Meal Organics in Cement Kilns,” Chemosphere, Vol. 42, No. 5-7, 2001, pp. 499-506. doi:10.1016/S0045-6535(00)00222-8 [11] K. Everaert, J. Baeyens and C. Creemers, “Adsorption of Dioxins and Furans from Flue Gases in an entrained Flow or Fixed/Moving Bed Reactor,” Journal of Chemical Technology Biotechnology, Vol. 78, No. 2-3, 2003, pp. 213-219. doi:10.1002/jctb.752 [12] K. Everaert and J. Baeyens, “Removal of PCDD/F from Flue Gases in Fixed or Moving Bed Adsorbers,” Waste Management, Vol. 24, No. 1, 2004, pp. 32-42. doi:10.1016/S0956-053X(03)00136-3 [13] G. C. Bond and S. F. Tahir, “Influence of Phosphorus and Potassium Additives on the Properties of Vanadia/Titania Catalysts,” Catalysis Today, Vol. 10, No. 3, 1991, pp. 393-395. doi:10.1016/0920-5861(91)80021-Z [14] S. Krishnamoorthy, J. P. Baker and M. D. Amiridis, “Ca- talytic Oxidation of 1,2-Dichlorobenzene over V2O5/TiO2- Based Catalysts,” Catalysis Today, Vol. 40, No. 1, 1998, pp. 39-46. doi:10.1016/S0920-5861(97)00117-X [15] S. Krishnamoorthy, J. A. Rivas and M. D. Amiridis, “Catalytic Oxidation of 1,2-Dichlorobenzene over Sup- ported Transition Metal Oxides,” Journal of Catalysis, Vol. 193, No. 2, 2000, pp. 264-272. doi:10.1006/jcat.2000.2895 [16] Y. Liu, Z. Wei, Z. Feng, M. Luo, P. Ying and C. Li, “Oxidative Destruction of Chlorobenzene and o-Dichlo- robenze ne on a Highly Active Cat alyst: Mn Ox/TiO2-Al2O3,” Journal of Catalysis, Vol. 202, No. 1, 2001, pp. 200-204. doi:10.1006/jcat.2001.3284 [17] P. Liljelind, J. Unsworth, O. Maaskant and S. Marklund, “Removal of Dioxins and Related Aromatic Hydrocar- bons from Flue Gas Streams by Adsorption and Catalytic Destruction,” Chemosphere, Vol. 42, No. 5-7, 2001, pp. 615-623. doi:10.1016/S0045-6535(00)00235-6 [18] R. Weber, T. Sakurai and H. Hagenmaier, “Low Tem- perature Decomposition of PCDD/PCDF, Chlorobenze- nes and PAHs by TiO2-Based V2O5-WO3 Catalysts,” Ap- plied Catalysis B: Environmental, Vol. 20, No. 4, 1999, pp. 249-256. doi:10.1016/S0926-3373(98)00115-5 [19] M. Goemans, P. Clarysse, J. Joannès, P. De Clercq, S. Lenaerts, K. Matthys and K. Boels, “Catalytic NOx Re- duction with Simultaneous Dioxin and Furan Oxidation,” Chemosphere, Vol. 54, No. 9, 2004, pp. 1357-1365. doi:10.1016/S0045-6535(03)00255-8 [20] J. Lichtenberger and M. D. Amiridis, “Catalytic Oxida- tion of Chlorinated Benzenes over V2O5/TiO2 Catalysts,” Journal of Catalysis, Vol. 223, No. 2, 2004, pp. 296-308. doi:10.1016/j.jcat.2004.01.032 [21] K. Everaert and J. Baeyens, “Catalytic Combustion of Volatile Organic Compounds,” Journal of Hazardous Materials, Vol. 109, No. 1-3, 2004, pp. 113-139. doi:10.1016/j.jhazmat.2004.03.019 [22] A. Galvez, J. A. Conesa, I. Martin-Gullon and R. Font, “Interaction between Pollutants Produced in Sewage Sludge Combustion and Cement Raw Material,” Chemo- sphere, Vol. 69, No. 3, 2007. pp. 387-394. doi:10.1016/j.chemosphere.2007.05.024 [23] A. Fullana, R. Font, J. A. Conesa and P. Blasco, “Evolu- tion of Products in the Combustion of Scrap Tires in a Horizontal, Laboratory Scale Reactor,” Environmental Science & Technology, Vol. 34, No. 11, 2000, pp. 2092- 2099. doi:10.1021/es990883y [24] A. Fullana, J. A. Conesa, R. Font and S. Sidhu, “Forma- tion and Destruction of Chlorinated Pollutants during Sewage Sludge Incineration,” Environmental Science & Technology, Vol. 38, No. 10, 2004, pp. 2953-2958. doi:10.1021/es034896u [25] B. Dellinger and P. H. Taylor, “Chemical Aspects of Combustion of Hazardous Wastes,” Central European Journal of Public Health, Vol. 6, 1998, pp. 79-87. [26] A. Atal, Y. A. Levendis, J. Carlson, Y. Dunayevskiy and P. Vouros, “On the Survivability and Pyrosynthesis of PAH during Combustion of Pulverized Coal and Tire Crumb,” Combustion and Flame, Vol. 110, No. 4, 1997, pp. 462-478. doi:10.1016/S0010-2180(97)00086-2 [27] H. Richter, T. G. Benish, O. A. Mazyar, W. H. Green and J. B. Howard, “Formation of Polycyclic Aromatic Hy- drocarbons and Their Radicals in a Nearly Sooting Pre- mixed Benzene Flame,” Proceedings of the Combustion Institute, Vol. 28, No. 2, 2000, pp. 2609-2618. doi:10.1016/S0082-0784(00)80679-7 [28] X.-W. Zhang, S.-C. Shen, L. E. Yu, S. Kawi, K. Hi- dajat and K. Y. Simon Ng, “Oxidative Decomposition of Na- phthalene by Supported Metal Catalysts,” Applied Ca- talysis A: General, Vol. 250, No. 2, 2003, pp. 341-352. doi:10.1016/S0926-860X(03)00412-5 Copyright © 2011 SciRes. ACES
|