Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 1471-1479 doi:10.4236/msa.2011.210198 Published Online October 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 1471 Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation and the Temperature Said Bensaada, Hamoudi Mazouz, Mohamed Tewfik Bouziane Laboratory LARHYSS Université Mohamed Khider, Biskra, Algeria. Email: s.bensaada@univ-biskra.dz Received January 19th, 2011; revised March 17th, 2011; accepted May 9th, 2011. ABSTRACT The study of the discontinuous precipitation reaction and the lamellar precipitate dissolution in the alloy Cu-In system provoked a considerable benefit and has been the subject of many theoretical and experimental investigations. The aim of this work is to make the evidence on the one hand the effect of the plastic deformation on the mechanism of the dis- continuous precipitation reaction such as nucleation, growth and lamellar coarsening and in other hand the effect of temperature on the characteristics and front behavior movement of the opposite reaction (discontinuous dissolution). Different techniques of analysis have been used in this respect such as the optical microscopy, the differential thermal analysis and the microhardness Vickers. The obtained results confirm various works achieved in this field. Keywords: Cu-In Alloy, Discontinuous Precipitation, Dissolution, Plastic Deformation, Temperature 1. Introduction 1.1. Discontinuous Precipitation The precipitation phenomena take a considerable place in metal solutions, because it modifies the alloy properties, sometimes in favourable way leading to the rise of hard- ness and load breaking. The final state of the precipita- tion process is of several phases. Generally the reaction of precipitation consists of the decomposition of a supersaturated solid solution α0 (mother phase) in a mixture of two phases of different compositions [1], according to the following reaction: α0 → α + δ where α is the girl phase, depleted in alloy element and has the same structure as α0 the mother phase and δ the precipitated phase rich in alloy element and can be: A mixed crystal with the same structure, the case of discontinuous precipitation in the alloy system Au-Ni [2]. A mixed crystal with a different structure, the case of the alloy system Pb-Sn [3]. An intermetallic phase, the case of the alloy system Cu-Zn [4]. A liquid phase, the case of the alloy system Pb-Bi [5]. Discontinuous precipitation in the alloy Cu-In system has been the subject of many research tasks [6-8] and the supersaturated solid solutions of this alloy system is de- composed in continuous and discontinuous modes, ap- pearing at high and low temperature respectively. The favourable sites supporting the appearance of the cellular precipitate are the grain boundaries of large orientation [9,10]. The average size of the initial grains has also a considerable influence on the precipitate morphology. The mechanisms of discontinuous precipitation nu- cleation suggested by Fournelle and Clark [11], then by You and Turnbull [9] are most plausible in this alloy system, i.e. it is a transformation related to the grain boundary dynamics. The growth model in this alloy sys- tem is that proposed by Frebel and Schenk [12] and of which the speed growth of the precipitated lamellas de- pends primarily on the speed of ageing annealing and the solute content of this alloy system, Figure 1 [13]. Two types of cellular reactions were observed by Spenger and Mack [14] in this alloy system, one fine and the other coarse, in both cases the lamellas are uniformly distributed. Predel and Gust [15] has shown also the same observation Figure 2, where the formation of the coarse lamella proceeds mainly between two fine lamellas and in both cases the process is controlled by the diffusion on the grain boundaries. The fine lamellas are uniform-  Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation 1472 and the Temperature Figure 1. Growth speed of precipitate during annealing time in the Cu-In alloy system [13]. ly distributed; in contrast the distribution of thick lamel- las is disordered, so there is a competition between the primary reaction of precipitation and the coalescence of the lamellas. In old work one could not observe the coarse lamellas, because probably they appear only after long annealing time. This sam Figure 2. Morphology of lamellar precipitate in Cu-7, 5 at% In alloy, homogenized, quenched and annealing during 3, 5 h at 392˚C [15]. issolution of the lamellate structure β + α (biphasic carried out according e phenomenon was observed in many al- loys such as (Al-Ag [16], Al-Cu [17], Au-Fe [18], Cu-Ag [19, 20], Fe-Zn [21], Fe-Ni-Ti [22], Ni-Sn [23], Pb-Na [24], Cu-Ga [25], Cu-In [26] and Zn-Al [27]. The types of precipitate in this alloy system have the shapes of hem [28], quadratic and fissure [29]. Experimentally, it was shown that a predeformation with the ageing annealing affects considerably the mechanism and the kinetics of precipitation [30]. D. B. Williams [31] affirmed that un- der the influence of the deformation, the speed of con- tinuous precipitation increases consequently, the degree of supersaturation in solute atom decreases, which im- plies a reduction in the driving force of the cellular reac- tion. 1.2. Discontinuous Dissolution The d structure) formed after annealing is to the reaction: β + α → α. This can take place at any temperature higher from 20 to 50˚C at the solvus temperature (critical temperature of solubility) corresponding to the alloy composition. Dis- solution is easy and rapid if this critical temperature is raised, and supported by diffusion of the addition ele- ment in the matrix. The solid solution results after disap- pearance of the precipitate, has however a uniform com- position only if the dissolution heat treatment were suffi- ciently prolonged (of about an hour) at a temperature higher than the solvus temperature, to ensure by diffusion a perfect homogenization (a complete dissolution). The dissolution process is perhaps explained by Fig- ures 3(a) and 3(b) represented on the one hand the dis- continuous precipitation diagram on the grain boundary with α0 is the mother phase (single-phase structure), the β precipitate in the lamellate form and the new phase α, and in other hand by the dissolution of precipitate with grain boundary displacement (GB) according to the reac- tion front (RF) [32]. According to a study made on several alloy systems and among the Al-38 at %Ag alloy based on the micro- hardness measure, the dissolved phase leads to the alloy hardening, it increases the lattice tension due to the pre- cipitate rich in solute by the introduction of this last into the solid solution. In the same way, the fragmentation of the lamellate of the β phase causes the same effect. The mechanical properties of the alloy during the discon- Copyright © 2011 SciRes. MSA 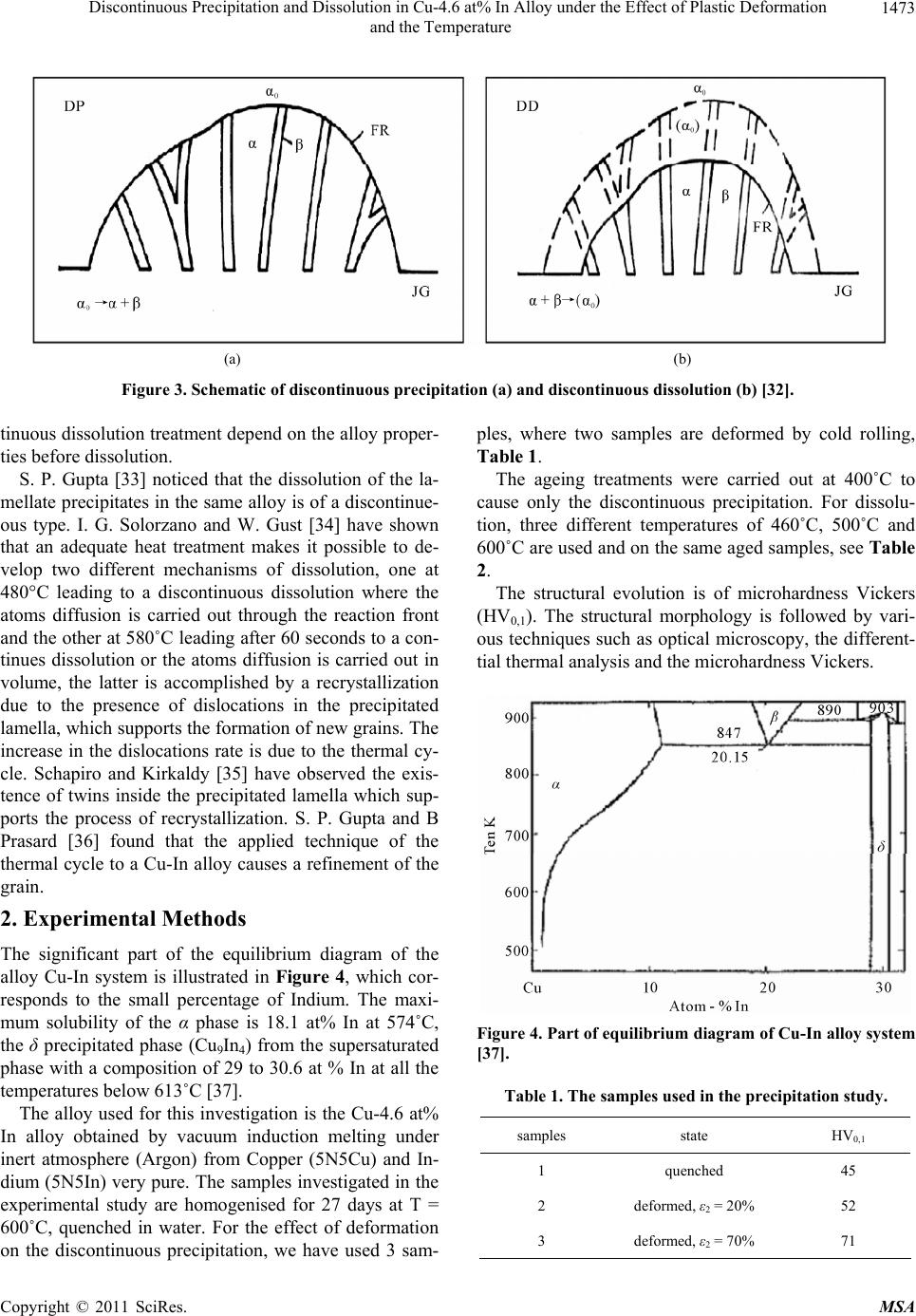 Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation and the Temperature Copyright © 2011 SciRes. MSA 1473 (a) (b) Figure 3. Schetion (b) [32]. tinuous dissolu es before dissolution. y is of a discontinue- ou m diagram of the Figure 4, which cor- nduction melting under in cold rolling, Table 1. sed and on the same aged samples, see Table 2. ermal analysis and the microhardness Vickers. matic of discontinuous precipitation (a) and discontinuous dissolu tion treatment depend on the alloy proper- ples, where two samples are deformed by ti S. P. Gupta [33] noticed that the dissolution of the la- mellate precipitates in the same allo s type. I. G. Solorzano and W. Gust [34] have shown that an adequate heat treatment makes it possible to de- velop two different mechanisms of dissolution, one at 480°C leading to a discontinuous dissolution where the atoms diffusion is carried out through the reaction front and the other at 580˚C leading after 60 seconds to a con- tinues dissolution or the atoms diffusion is carried out in volume, the latter is accomplished by a recrystallization due to the presence of dislocations in the precipitated lamella, which supports the formation of new grains. The increase in the dislocations rate is due to the thermal cy- cle. Schapiro and Kirkaldy [35] have observed the exis- tence of twins inside the precipitated lamella which sup- ports the process of recrystallization. S. P. Gupta and B Prasard [36] found that the applied technique of the thermal cycle to a Cu-In alloy causes a refinement of the grain. 2. Experimental Methods The significant part of the equilibriu alloy Cu-In system is illustrated in responds to the small percentage of Indium. The maxi- mum solubility of the α phase is 18.1 at% In at 574˚C, the δ precipitated phase (Cu9In4) from the supersaturated phase with a composition of 29 to 30.6 at % In at all the temperatures below 613˚C [37]. The alloy used for this investigation is the Cu-4.6 at% In alloy obtained by vacuum i ert atmosphere (Argon) from Copper (5N5Cu) and In- dium (5N5In) very pure. The samples investigated in the experimental study are homogenised for 27 days at T = 600˚C, quenched in water. For the effect of deformation on the discontinuous precipitation, we have used 3 sam- The ageing treatments were carried out at 400˚C to cause only the discontinuous precipitation. For dissolu- tion, three different temperatures of 460˚C, 500˚C and 600˚C are u The structural evolution is of microhardness Vickers (HV0,1). The structural morphology is followed by vari- ous techniques such as optical microscopy, the different- tial th Figure 4. Part of equilibrium diagram of Cu-In alloy system [37]. Table 1. The samples used in the precipitation study. 0,1 samples state HV 1 quenched 45 2 deformed, ε = 20% 52 3 deformed, ε2 = 70% 71 2 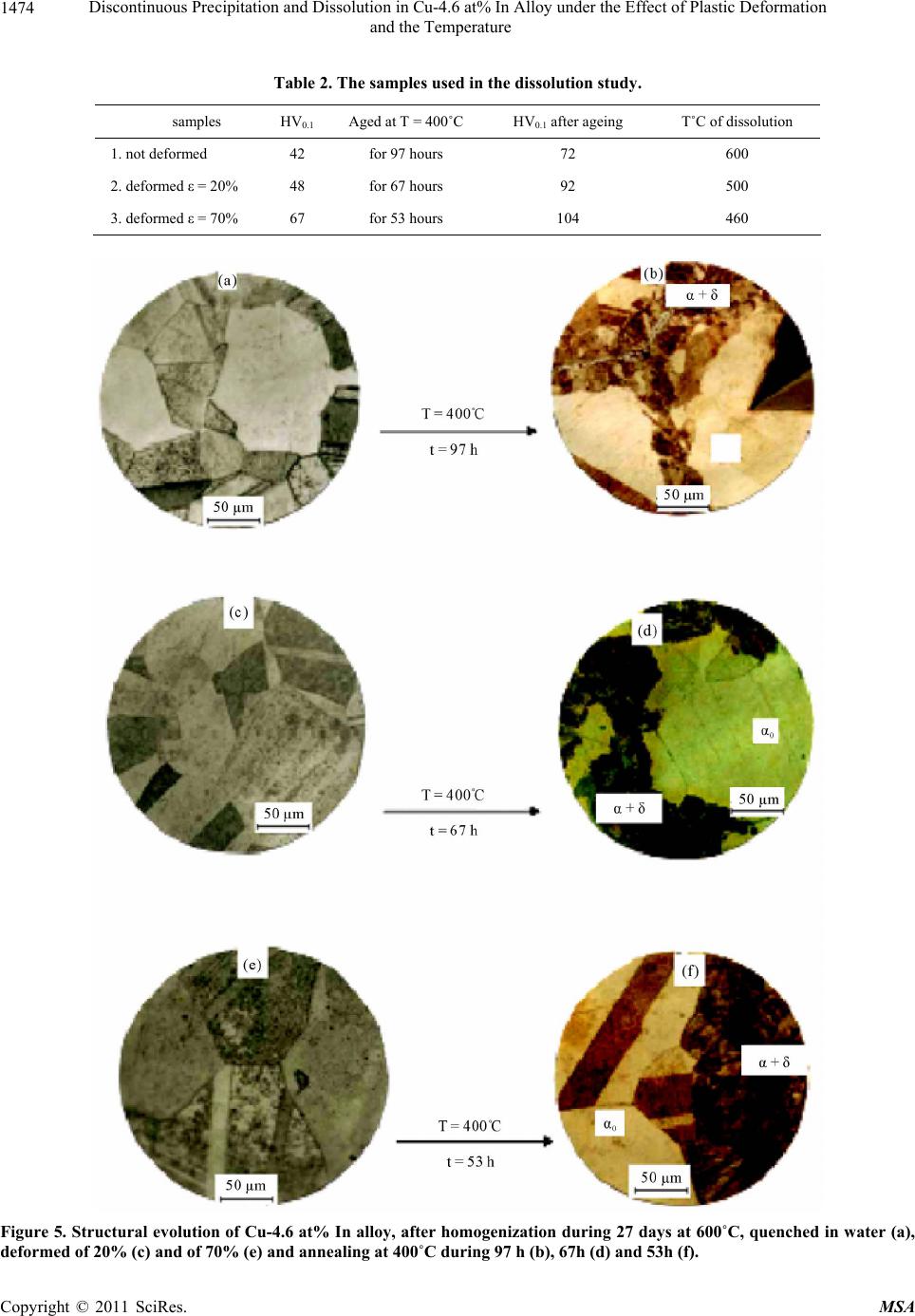 Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation 1474 and the Temperature Table 2. Theples used in the disution stud V0.1 Aged at T = 400˚C HV0.1 after ageing T˚C of dissolution samsoly. samples H 1. not deformed 600 42 for 97 hours 72 2. deform 0% ed ε = 20% 48 for 67 hours 92 500 3. deformed ε = 767 for 53 hours 104 460 Figure 5. Structural evolution of Cu-4.6 at% In alloy, after homogenization during 27 days at 600˚C, quenched in water (a), deformed of 20% (c) and of 70% (e) and annealing at 400˚C during 97 h (b), 67h (d) and 53h (f). Copyright © 2011 SciRes. MSA 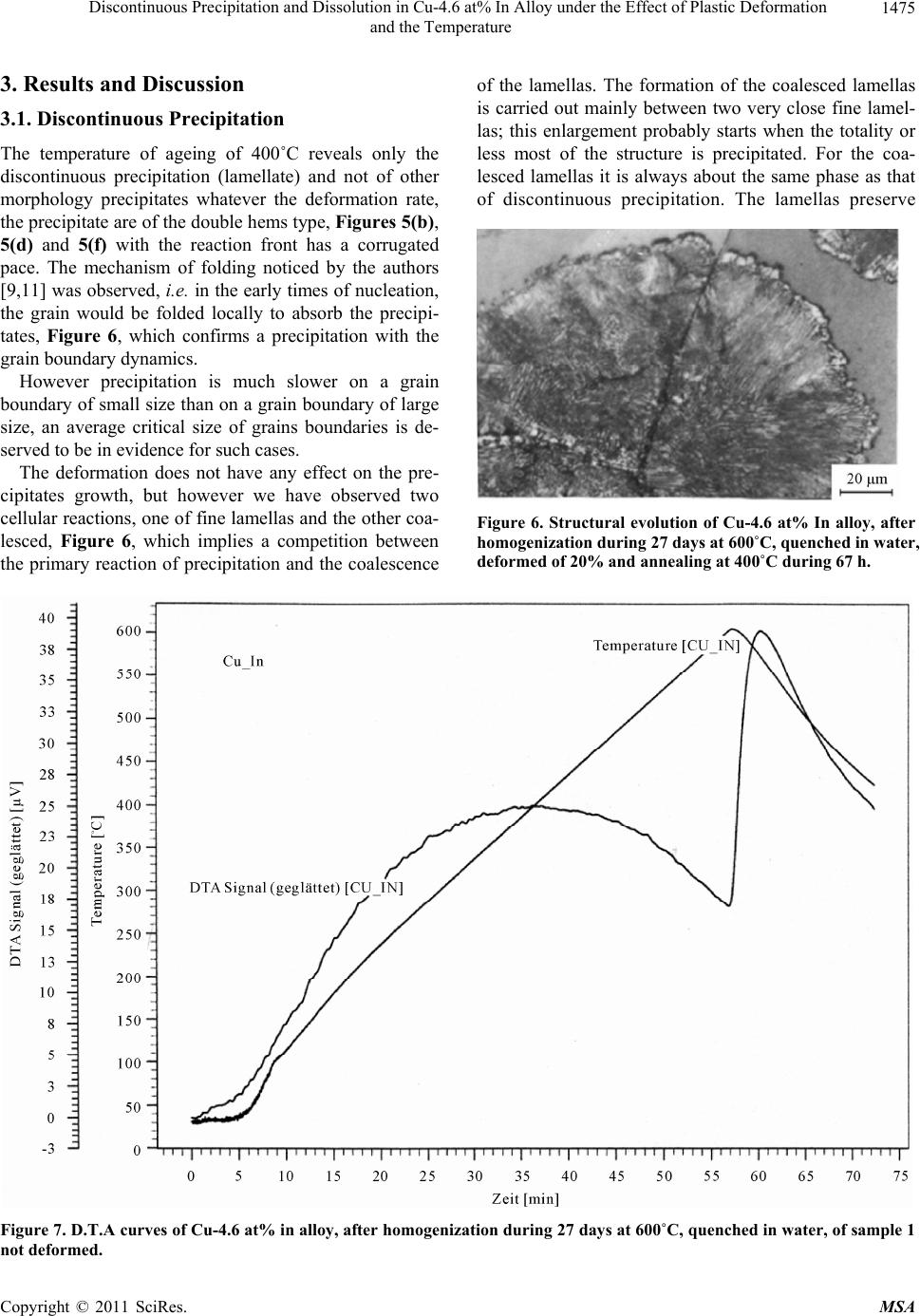 Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation 1475 and the Temperature 3. Results and Discussion 3.1. Discontinuous Precipitation The temperature of ageing of 400˚C reveals only the discontinuous precipitation (lamellate) and not of other morphology precipitates whatever the deformation rate, the precipitate are of the double hems type, Figures 5(b), 5(d) and 5(f) with the reaction front has a corrugated pace. The mechanism of folding noticed by the authors [9,11] was observed, i.e. in the early times of nucleation, the grain would be folded locally to absorb the precipi- tates, Figure 6, which confirms a precipitation with the grain boundary dynamics. However precipitation is much slower on a grain boundary of small size than on a grain boundary of large size, an average critical size of grains boundaries is de- served to be in evidence for such cases. The deformation does not have any effect on the pre- cipitates growth, but however we have observed two cellular reactions, one of fine lamellas and the other coa- lesced, Figure 6, which implies a competition between the primary reaction of precipitation and the coalescence of the lamellas. The formation of the coalesced lamellas is carried out mainly between two very close fine lamel- las; this enlargement probably starts when the totality or less most of the structure is precipitated. For the coa- lesced lamellas it is always about the same phase as that of discontinuous precipitation. The lamellas preserve Figure 6. Structural evolution of Cu-4.6 at% In alloy, after homogenization during 27 days at 600˚C, quenched in water, deformed of 20% and annealing at 400˚C during 67 h. tion during 27 days at 600˚C, quenched in water, of sample 1 Figure 7. D.T.A curves of Cu-4.6 at% in alloy, after homogen not deformed. iza Copyright © 2011 SciRes. MSA  Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation 1476 and the Temperature eir growth; the gr thermic peak confirms the precipitation of a new phase which corresponds to the intermetallic phase Cu9In4. The evolution of the microhardeness, Figure 8 clearly shows the structural hardening of all the samples during discon- tinuous precipitation; however this hardening is rela- tively significant with the rise in the work hardening rate. 3.2. Discontinuous Dissolution their morphology anisotropy during th owth of the lamellas is observed in the deformed than in the not deformed samples, it is a growth in width leading to narrow hems. The differential thermal analysis, Figure 7 which the curve is characterized by a variation of energy during the rise in the temperature and the exo- The various temperatures used during dissolution of pre- cipitates lamellate have developed in two different me- chanisms of dissolution. The temperatures of 600˚C and 500˚C led on the one hand to a dissolution of continues type where the atoms diffusion is carried out in volume (Figures 9(b) and 9(d)) and on the other hand supported the formation of twins after dissolution of precipitates. On the other hand the temperature of 460˚C has shown a discontinuous dissolution of discontinuous type, Figure 9(f), with opposite migration of the reaction front (RF) and where the atoms diffusion is carried out mainly through the grain boundary. However the effect of the temperature on the speed of dissolution is observed, be- cause in comparison with the last two temperatures (600˚C and 500˚C), the process of dissolution in the case of the temperature of 460˚C is relatively slow. The dis- solution of precipitates in the three cases has lead to a fall of the microhardness, Figure 10 . 4. Conclusions The results obtained by different technique of analysis used in this respect are coherent between them and con- firm several work devote to the study of the reaction of precipitation and discontinuous dissolution in the alloy Cu-In system. However the temperature of 400˚C sup- ports only discontinuous precipitation and not other pre- cipitates morphology was observed. The lamellas are of double hems type with a corrugated pace. The presence of the fine lamellas and coalescences confirms the exis- tence of the competition between precipitation and the coalescence of the lamellas. The grain boundary is char- acterized by a crumpling to absorb the precipitate during their movement. The effect of the deformation rate is observed on the precipitation speed during the initial stage; on the other hand no effect was observed on the coalescence of the precipitate. Precipitation is carried out mainly in the coarse grains zones, because the initial av- erage size of th Figure 8. Hardness evolution HV0,1 during annealing time Cu-4.6 at% In alloy, after homogenization during 27 days at 600˚C, quenched in water (a), deformed of 20% (c) and of 70% (e) and annealing at 400˚C during. nisms of precipitation. The appearance of the new inter- metallic phase Cu9In4 led to the hardening of alloy. The effect of the temperature on the mechanism of the disso- lution of the lamellate precipitate has shown on the one hand that dissolution is carried out into two different e grains has an influence on the mecha- Copyright © 2011 SciRes. MSA 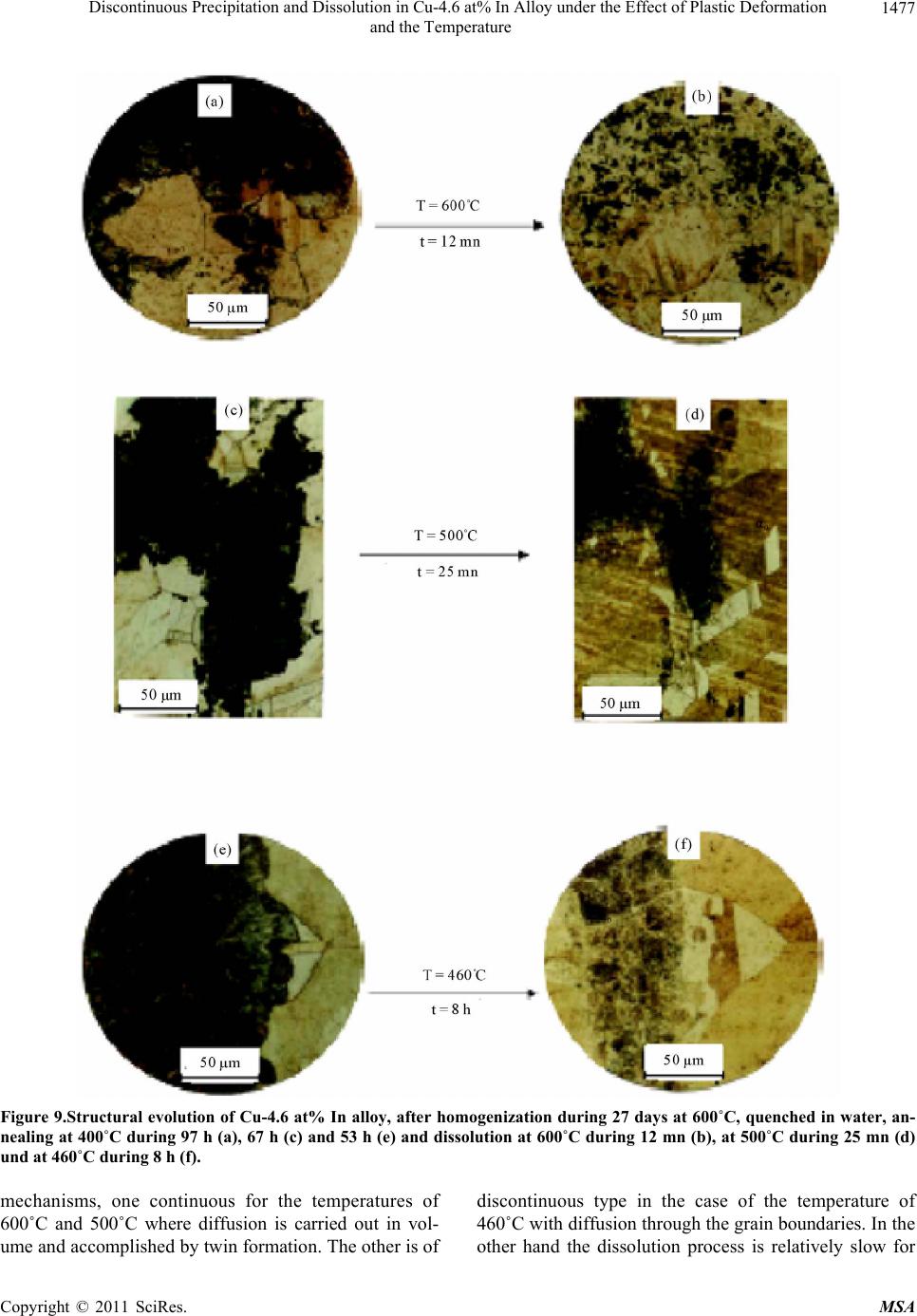 Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation 1477 and the Temperature Figure 9.Structural evolution of Cu-4.6 at% In alloy, after ho nealing at 400˚C during 97 h (a), 67 h (c) and 53 h (e) and dis und at 460˚C during 8 h (f). mo sol genization during 27 days at 600˚C, quenched in wate r, an- ution at 600˚C during 12 mn (b), at 500˚C during 25 mn (d) mechanisms, one continuous for the temperatures of 600˚C and 500˚C where diffusion is carried out in vol- discontinuous type in the case of the temperature of 460˚C with diffusion through the grain boundaries. In the ume and accomplished by twin formation. The other is of other hand the dissolution p is relatively slow for rocess Copyright © 2011 SciRes. MSA 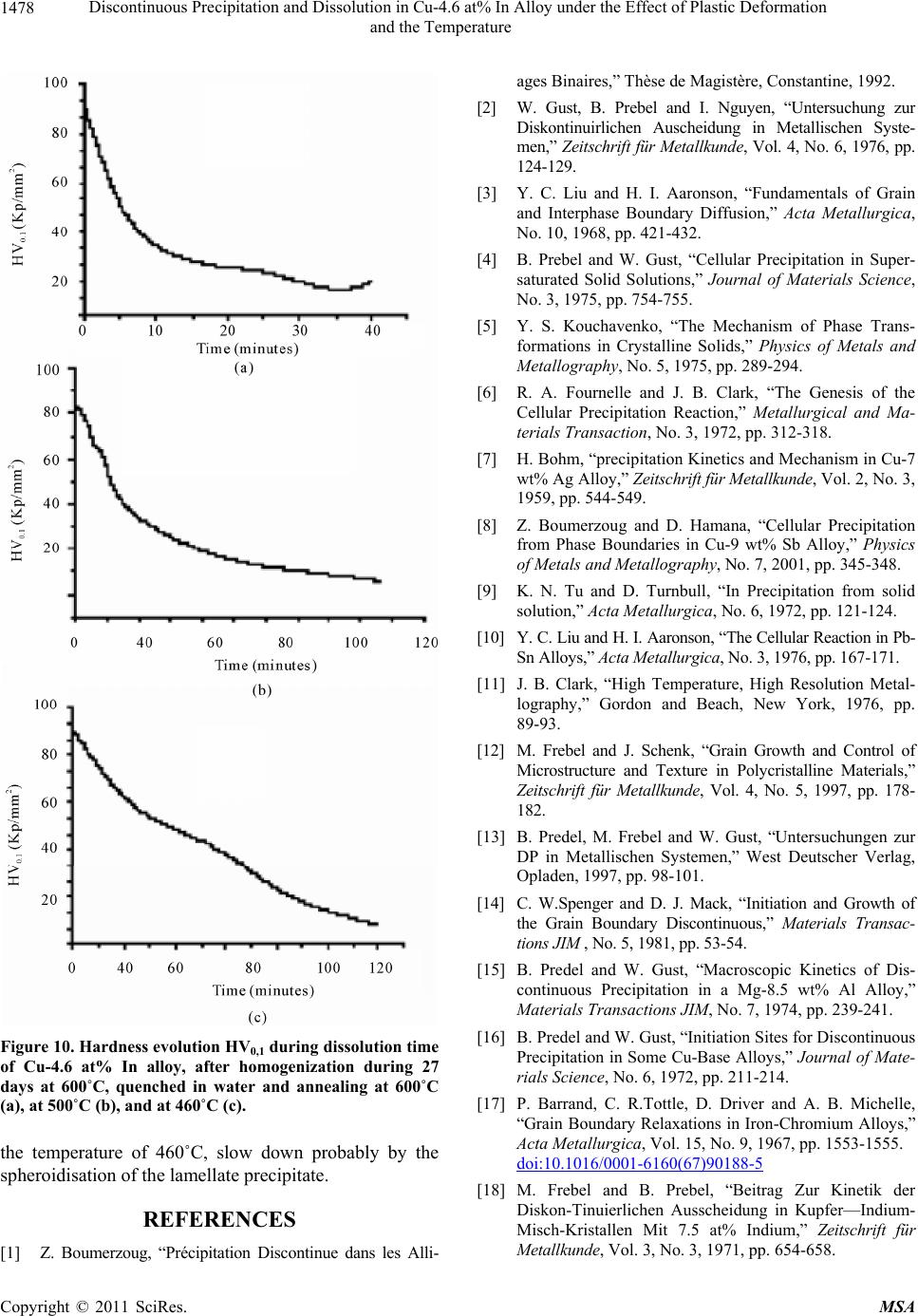 Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation 1478 and the Temperature ages Binaires,” Thèse de Magistère, Constantine, 1992. [2] W. Gust, B. Prebel and I. Nguyen, “Untersuchung zur Diskontinuirlichen Auscheidung in Metallischen Syste- men,” Zeitschrift für Metallkunde, Vol. 4, No. 6, 1976, pp. 124-129. [3] Y. C. Liu and H. I. Aaronson, “Fundamentals of Grain and Interphase Boundary Diffusion,” Acta Metallurgica, No. 10, 1968, pp. 421-432. [4] B. Prebel and W. Gust, “Cellular Precipitation in Super- saturated Solid Solutions,” Journal of Materials Science, No. 3, 1975, pp. 754-755. [5] Y. S. Kouchavenko, “The Mechanism of Phase Trans- formations in Crystalline Solids,” Physics of Metals and Metallography, No. 5, 1975, pp. 289-294. [6] R. A. Fournelle and J. B. Clark, “The Genesis of the Cellular Precipitation Reaction,” Metallurgical and Ma- terials Transaction, No. 3, 1972, pp. 312-318. [7] H. Bohm, “precipitation Kinetics and Mechanism in Cu-7 wt% Ag Alloy,” Zeitschrift für Metallkunde, Vol. 2, No. 3, 1959, pp. 544-549. [8] Z. Boumerzoug and D. Hamana, “Cellular Precipitation from Phase Boundaries in Cu-9 wt% Sb Alloy,” Physics of Metals and Metallography, No. 7, 2001, pp. 345-348. [9] K. N. Tu and D. Turnbull, “In Precipitation from solid solution,” Acta Metallurgica, No. 6, 1972, pp. 121-124. [10] Y. C. Liu and H. I. Aaronson, “The Cellular Reaction in Pb- Sn Alloys,” Acta Metallurgica, No. 3, 1976, pp. 167-171. [11] J. B. Clark, “High Temperature, High Resolution Metal- lography,” Gordon and Beach, New York, 1976, pp. 89-93. [12] M. Frebel and J. Schenk, “Grain Growth and Control of Microstructure and Texture in Polycristalline Materials,” Zeitschrift für Metallkunde, Vol. 4, No. 5, 1997, pp. 178- 182. [13] B. Predel, M. Frebel and W. Gust, “Untersuchungen zur DP in Metallischen Systemen,” West Deutscher Verlag, Opladen, 1997, pp. 98-101. [14] C. W.Spenger and D. J. Mack, “Initiation and Growth of the Grain Boundary Discontinuous,” Materials Transac- tions JIM , No. 5, 1981, pp. 53-54. [15] B. Predel and W. Gust, “Macroscopic Kinetics of Dis- continuous Precipitation in a Mg-8.5 wt% Al Alloy,” Materials Transactions JIM, No. 7, 1974, pp. 239-241. [16] B. Predel and W. Gust, “Initiation Sites for Discontinuous Precipitation in Some Cu-Base Alloys,” Journal of Mate- rials Science, No. 6, 1972, pp. 211-214. Acta Metallurgica, Vol. 15, No. 9, 1967, pp. 1553-1555. Figure 10. Hardness evolution HV0,1 during dissolution time of Cu-4.6 at% In alloy, after homogenization during 27 days at 600˚C, quenched in water and annealing at 600˚C (a), at 500˚C (b), and at 460˚C (c). the temperature of 460˚C, slow down probably by the spheroidisation of the lamellate precipitate. REFERENCES [1] Z. Boumerzoug, “Précipitation Discontinue dans les Alli- [17] P. Barrand, C. R.Tottle, D. Driver and A. B. Michelle, “Grain Boundary Relaxations in Iron-Chromium Alloys,” doi:10.1016/0001-6160(67)90188-5 [18] M. Frebel and B. Prebel, “Beitrag Zur Kinetik der Diskon-Tinuierlichen Ausscheidung in Kupfer—Indium- Misch-Kristallen Mit 7.5 at% Indium,” Zeitschrift für Metallkunde, Vol. 3, No. 3, 1971, pp. 654-658. Copyright © 2011 SciRes. MSA  Discontinuous Precipitation and Dissolution in Cu-4.6 at% In Alloy under the Effect of Plastic Deformation and the Temperature Copyright © 2011 SciRes. MSA 1479 [19] H. Borchers, W. Scharfenberg and R. Zurstege, “Preci- pitation Behavior of α-Solid Solutions of the Fe-Sn System,” Acta Metallurgica, No. 8, 1968, pp. 405-408. [20] W. Leo, “Microstructure and Internal Stresses in Cyclically Deformed Al and Cu Single Crystals,” Zeitschrift für Met- allkunde, Vol. 7, No. 3, 1967, pp. 456-458. [21] B. Predel and M. Frebel, “Explanations of Third Element Effects upon the Growth Kinetics of Discontinuous Precipitation in Cu-In and Cu-Sb Alloys,” Acta Metallur- gica, No. 7, 1972, pp. 1259-1263. doi:10.1016/0001-6160(72)90056-9 [22] G. R. Speich, “Discontinuous Dissolution in Aged Alloy Transactions AIME, Vol. 2, No. 4, 1963, pp. 227-231. [23] M. Frebel, B. Predel and W. Klisa, “concentration Fluctuations and Thermodynamic Properties of Demixing Liquid Binary Alloys,” Zeitschrift für Metallkunde, Vol. 6, No. 3, 1974, pp. 311-316. [24] J. Petermann and E. Hornbogen, “Discontinuous sening of Lamellar Cellular Precipitate in an Austenitic Fe-30 wt% Ni-6 wt% Ti alloy—I. Morphology,” Zeitschrift für Metallkunde, Vol. 4, No. 5, 1968, p. 59, 814. [25] C. W. Spenger and D. J. Mack, “Systematics of the lular Precipitation Reactions,” Metallurgical and Materi- als Transactions, No. 7, 1972, pp. 84-87. [26] C. W. Spenger and D. J. Mack, “Discontinuous Coarseni and Dissolution in an Fe 13.5 at% Zn Solid Solution,” Materials Transactions JIM, No. 3, 1972, pp. 254-258. [27] D.Chethaum and N.Ridley, Materials Transactions JIM No. 5, 1971, pp. 977-982. [28] W. Gust, M. B. Hintz, B. Predel and U. Roll, “Diffusity of Impurities along Migrating and Stationary Grain,” Acta s,” Coar- Cel- ng , Metallurgica, Vol. 28, No. 9, 1980, pp. 1235-1244. doi:10.1016/0001-6160(80)90079-6 [29] W. Gust, B. Predel and U. Roll, “Impurity Diffusion of Al in Ni Single Crystals Studied by Secondary Ion Mass Spectrometry (SIMS),” Acta Metallurgica, Vol. 28, No. 10, 1980, pp. 1395-1398. doi:10.1016/0001-6160(80)90008-5 [30] H. I. Subakino, “The Discontinuous Precipitation Reaction er, Journal of Materials ution,” Anais urnal of d Aluminium-Copper Alloys,” Mate- in Dilute Al-Li Alloys,” Metallography, No. 5, 1984, pp. 371-374. [31] D. B. Williams and E. P. Buthl Science, No. 4, 1981, pp. 153-156. [32] T. H. Chuang, W. Gust and R. A. Fournelle, “Review of Discontinuous Recipitation and Dissol 7cbecimat, UFSC, Florianopolis, 1986, pp. 37-41. [33] S. P. Gupta, “Initiation and Growth of the Grain Bound- ary Discontinuous Precipitation Reaction,” Jo Materials Science, No. 7, 1986, pp. 225-227. [34] I. G. Solarzano and W. Gust, “Microstructural Characteris- tics of Splatquenche rials Science Forum, 1992, pp. 659-664. [35] J. M. Schapiro and J. S. Kirkaldy, “The Kinetics of Dis- continuous Precipitation in Copper-Indium Alloys,” Acta Metallurgica, No. 3, 1968, pp. 1239-1252. [36] S. P. Gupta and B. Prassard, “Morphology and Chemical Nanoanalysis of Discontinuous Precipitation in Mg Al Alloys—I. Regular Growth,” Zeitschrift für Metallkunde, Vol. 3, No. 8, 1987, pp. 91-95. [37] M. Hansen and K. Anderko, “Constitution of Alloys,” McGraw-Hill, New York, 1958, pp. 278-281. |

