Paper Menu >>
Journal Menu >>
 New Journal of Glass and Ceramics, 2011, 1, 125-129 doi:10.4236/njgc.2011.13018 Published Online October 2011 (http://www.SciRP.org/journal/njgc) Copyright © 2011 SciRes. NJGC 125 The Effect of Mixed Iodide Salts on the Conductivity Behavior in Ag2O-V2O5-B2O3 Superionic Glass System Poonam Sharma1, D. K. Kanchan1*, Nirali Gondaliya1,2, Meenakshi Pant1, Manish S. Jayswal1 1Solid State Ionics & Glass Laboratory, Department of Physics, Faculty of Science, The M. S. University of Baroda, Vadodara (Gu- jarat) India; 2Department of Engg Physics, SVMIT, Bharuch, Gujarat, India. Email: *d_k_kanchan@yahoo.com Received June 14th, 2011; revised July 19th, 2011; accepted August 23th, 2011. ABSTRACT A superionic mixed metal iodide glass system [(PbI2 –CuI ) - Ag2O- V2O5- B2O3] has been prepared by splat quenching technique. The prepared glass samples are characterized by X-ray diffraction (XRD) and differential scanning calo- rimetry (DSC). The effect of mixed metal iodide salts concentration on electrical properties has been investigated by the complex impedance spectroscopy (CIS). AC conductivity analysis is carried out in the frequency range of 2MHz-1Hz at different temperatures and an enhancement in the conductivity with iodide salts is the main feature of the observed re- sults with an anomaly at 20 mole % of PbI2 –CuI. Keywords: Iodide Salts, Ag-Ion Conductors, XRD, Impedance 1. Introduction Super-ionic solids are known as potential candidates for application to electrochemical devices and solid state batteries. Electrical properties of silver oxy-salt based solid super ionic electrolytes with different glass modify- ers and formers have been widely reported in the past [1-3]. Some reports on the replacement of Ag+ ion with other feasible cation like CdI2, PbI2, CuI in the glass electrolytes are also available in literature [3-5]. Many interesting results have been obtained regarding the na- ture of electrical conductivity of these glasses. Instead of directly doping pure AgI in a host glass, some different routes by doping with a suitable MIn type salt in an Ag2O containing glass and thereby forming AgI as a result of exchange reaction between the two, based on Lewis HSAB (Hard and Soft Acids and Bases) principle [6], where a hard acid ion prefers to co-ordinate to a hard base ion and vice-versa are reported to have a favorable effect by increasing the electrical conductivity. Accord- ing to the HSAB theory, Pb+2 ion is a borderline acid and I- is a soft base while Ag+ is a soft acid and O–2 is a bor- derline base, hence an exchange reaction between PbI2 and Ag2O takes place as per the following equation 2Io Pb2 Ag ++ éù é êú ê ëû ë +ù ú û…… ù ú û (1) 2 I 2 AgPb ++ éù é êú ê ëû ë + From the above reaction we see that for one molecule of PbI2 and one molecule of Ag2O, as a result of the ex- change reaction, two AgI and one PbO molecules are formed in the glass network. Damravi et. al. reported the more open volume are created by PbI2 based glass sys- tem than by AgI [7]. However, many reports are avail- able on PbI2 and CuI based glasses, but up to author’s knowledge no report is available on mixed iodide salts in literature. In the presently investigated system, an at- tempt is made to observe the effect of varying mixed metal iodide salts concentration on electrical properties in a silver-vanado-borate glass system. 2. Experimental Sample preparation: PbI2 and CuI doped Silver-Vanado-Borate glasses in the composition of x [(PbI2 – CuI) – (100-x) {2Ag2O:3 (0.7V2O5 – 0.3B2O3}] where 10 ≤ x ≤ 30, in steps of 5, were prepared by the melt-quenching process. Required quantities of materials were weighed according to their molecular weight per- centage and mixed thoroughly by wet grinding method. The mixer is then taken in an alumina crucible in a con- trolled electrical furnace and then the furnace tempera- ture was raised gradually up to 873 K and kept for 2 o 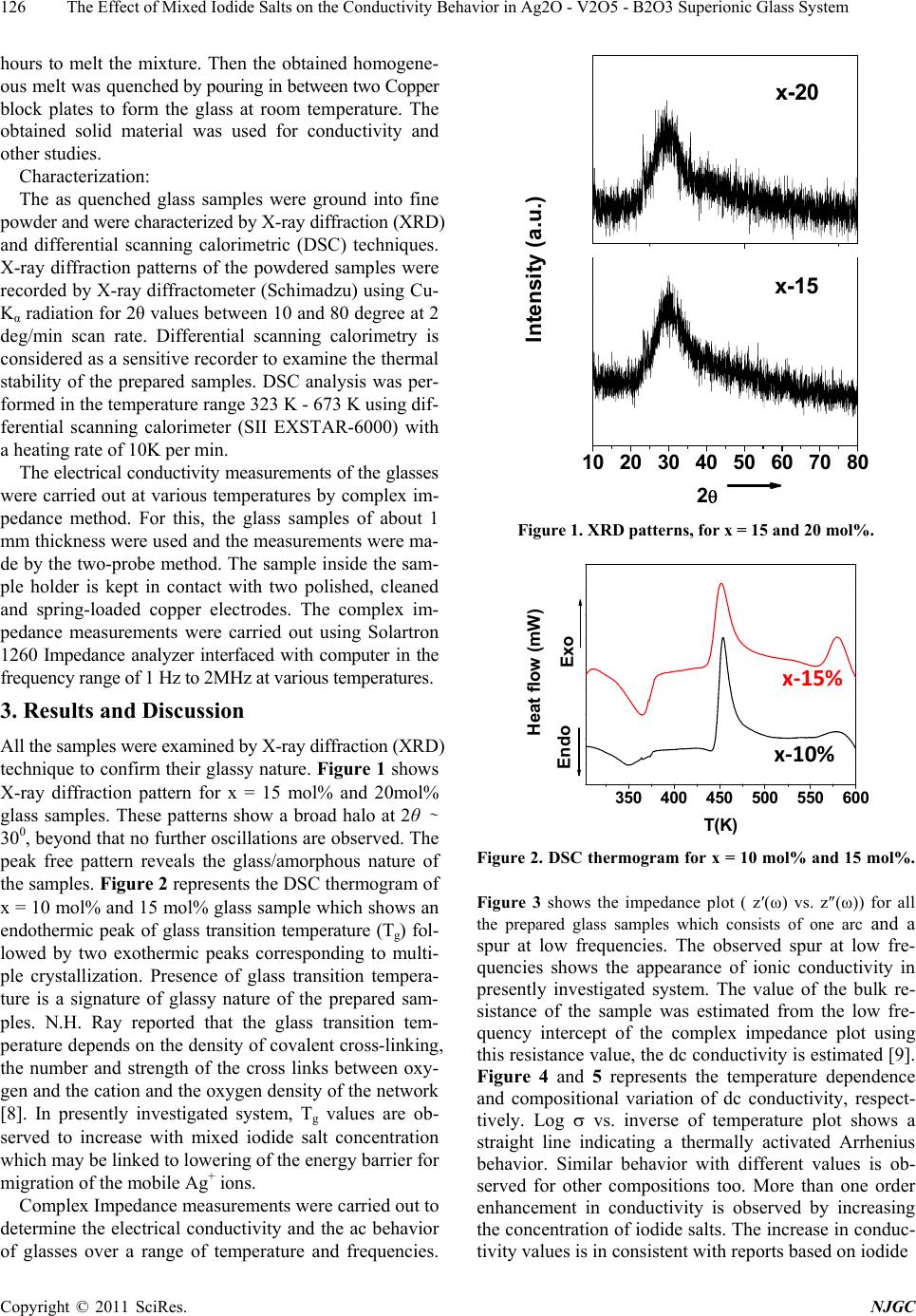 The Effect of Mixed Iodide Salts on the Conductivity Behavior in Ag2O - V2O5 - B2O3 Superionic Glass System 126 hours to melt the mixture. Then the obtained homogene- ous melt was quenched by pouring in between two Copper block plates to form the glass at room temperature. The obtained solid material was used for conductivity and other studies. Characterization: The as quenched glass samples were ground into fine powder and were characterized by X-ray diffraction (XRD) and differential scanning calorimetric (DSC) techniques. X-ray diffraction patterns of the powdered samples were recorded by X-ray diffractometer (Schimadzu) using Cu- Kα radiation for 2θ values between 10 and 80 degree at 2 deg/min scan rate. Differential scanning calorimetry is considered as a sensitive recorder to examine the thermal stability of the prepared samples. DSC analysis was per- formed in the temperature range 323 K - 673 K using dif- ferential scanning calorimeter (SII EXSTAR-6000) with a heating rate of 10K per min. The electrical conductivity measurements of the glasses were carried out at various temperatures by complex im- pedance method. For this, the glass samples of about 1 mm thickness were used and the measurements were ma- de by the two-probe method. The sample inside the sam- ple holder is kept in contact with two polished, cleaned and spring-loaded copper electrodes. The complex im- pedance measurements were carried out using Solartron 1260 Impedance analyzer interfaced with computer in the frequency range of 1 Hz to 2MHz at various temperatures. 3. Results and Discussion All the samples were examined by X-ray diffraction (XRD) technique to confirm their glassy nature. Figure 1 shows X-ray diffraction pattern for x = 15 mol% and 20mol% glass samples. These patterns show a broad halo at 2θ ∼ 300, beyond that no further oscillations are observed. The peak free pattern reveals the glass/amorphous nature of the samples. Figure 2 represents the DSC thermogram of x = 10 mol% and 15 mol% glass sample which shows an endothermic peak of glass transition temperature (Tg) fol- lowed by two exothermic peaks corresponding to multi- ple crystallization. Presence of glass transition tempera- ture is a signature of glassy nature of the prepared sam- ples. N.H. Ray reported that the glass transition tem- perature depends on the density of covalent cross-linking, the number and strength of the cross links between oxy- gen and the cation and the oxygen density of the network [8]. In presently investigated system, Tg values are ob- served to increase with mixed iodide salt concentration which may be linked to lowering of the energy barrier for migration of the mobile Ag+ ions. Complex Impedance measurements were carried out to determine the electrical conductivity and the ac behavior of glasses over a range of temperature and frequencies. 10 20 30 40 50 60 70 80 x-15 2 Intensity (a.u.) x-20 Figure 1. XRD patterns, for x = 15 and 20 mol%. 350 400 450 500 550 600 x‐10% x‐15% Endo Exo Heat flow (mW) T(K) Figure 2. DSC thermogram for x = 10 mol% and 15 mol%. Figure 3 shows the impedance plot ( z′(ω) vs. z″(ω)) for all the prepared glass samples which consists of one arc and a spur at low frequencies. The observed spur at low fre- quencies shows the appearance of ionic conductivity in presently investigated system. The value of the bulk re- sistance of the sample was estimated from the low fre- quency intercept of the complex impedance plot using this resistance value, the dc conductivity is estimated [9]. Figure 4 and 5 represents the temperature dependence and compositional variation of dc conductivity, respect- tively. Log vs. inverse of temperature plot shows a straight line indicating a thermally activated Arrhenius behavior. Similar behavior with different values is ob- served for other compositions too. More than one order enhancement in conductivity is observed by increasing the concentration of iodide salts. The increase in conduc- tivity values is in consistent with reports based on iodide Copyright © 2011 SciRes. NJGC 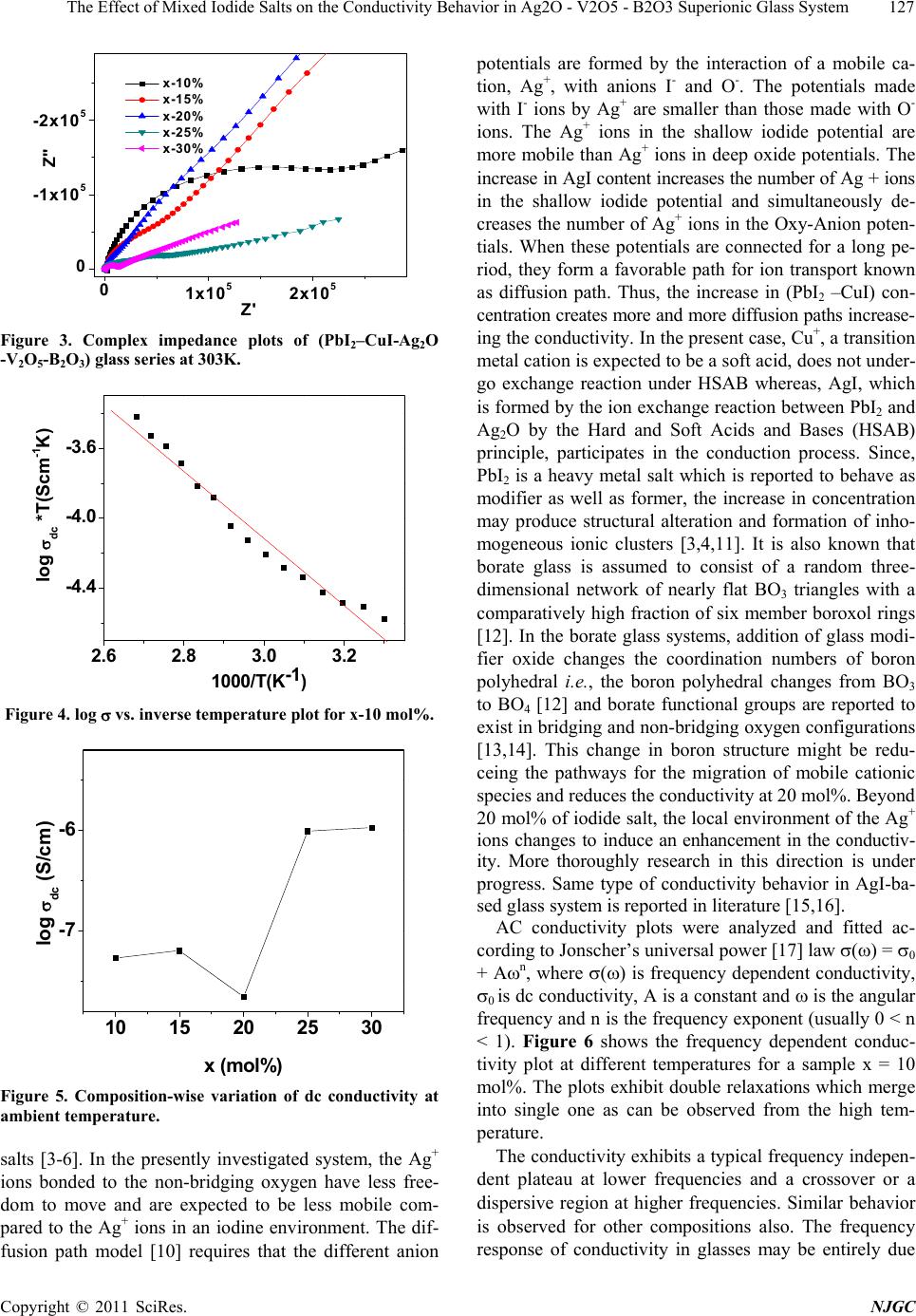 The Effect of Mixed Iodide Salts on the Conductivity Behavior in Ag2O - V2O5 - B2O3 Superionic Glass System127 01x1052x105 0 -1x105 -2x105 x-10% x-15% x-20% x-25% x-30% Z'' Z' Figure 3. Complex impedance plots of (PbI2–CuI-Ag2O -V2O5-B2O3) glass series at 303K. 2.6 2.83.0 3.2 -4.4 -4.0 -3.6 log dc *T(Scm-1K) 1000/T(K-1) Figure 4. log vs. inverse temperature plot for x-10 mol%. 10 15 20 25 30 -7 -6 x ( mol % ) log dc (S/cm) Figure 5. Composition-wise variation of dc conductivity at ambient temperature. salts [3-6]. In the presently investigated system, the Ag+ ions bonded to the non-bridging oxygen have less free- dom to move and are expected to be less mobile com- pared to the Ag+ ions in an iodine environment. The dif- fusion path model [10] requires that the different anion potentials are formed by the interaction of a mobile ca- tion, Ag+, with anions I- and O-. The potentials made with I- ions by Ag+ are smaller than those made with O- ions. The Ag+ ions in the shallow iodide potential are more mobile than Ag+ ions in deep oxide potentials. The increase in AgI content increases the number of Ag + ions in the shallow iodide potential and simultaneously de- creases the number of Ag+ ions in the Oxy-Anion poten- tials. When these potentials are connected for a long pe- riod, they form a favorable path for ion transport known as diffusion path. Thus, the increase in (PbI2 –CuI) con- centration creates more and more diffusion paths increase- ing the conductivity. In the present case, Cu+, a transition metal cation is expected to be a soft acid, does not under- go exchange reaction under HSAB whereas, AgI, which is formed by the ion exchange reaction between PbI2 and Ag2O by the Hard and Soft Acids and Bases (HSAB) principle, participates in the conduction process. Since, PbI2 is a heavy metal salt which is reported to behave as modifier as well as former, the increase in concentration may produce structural alteration and formation of inho- mogeneous ionic clusters [3,4,11]. It is also known that borate glass is assumed to consist of a random three- dimensional network of nearly flat BO3 triangles with a comparatively high fraction of six member boroxol rings [12]. In the borate glass systems, addition of glass modi- fier oxide changes the coordination numbers of boron polyhedral i.e., the boron polyhedral changes from BO3 to BO4 [12] and borate functional groups are reported to exist in bridging and non-bridging oxygen configurations [13,14]. This change in boron structure might be redu- ceing the pathways for the migration of mobile cationic species and reduces the conductivity at 20 mol%. Beyond 20 mol% of iodide salt, the local environment of the Ag+ ions changes to induce an enhancement in the conductiv- ity. More thoroughly research in this direction is under progress. Same type of conductivity behavior in AgI-ba- sed glass system is reported in literature [15,16]. AC conductivity plots were analyzed and fitted ac- cording to Jonscher’s universal power [17] law () = 0 + An, where () is frequency dependent conductivity, 0 is dc conductivity, A is a constant and is the angular frequency and n is the frequency exponent (usually 0 < n < 1). Figure 6 shows the frequency dependent conduc- tivity plot at different temperatures for a sample x = 10 mol%. The plots exhibit double relaxations which merge into single one as can be observed from the high tem- perature. The conductivity exhibits a typical frequency indepen- dent plateau at lower frequencies and a crossover or a dispersive region at higher frequencies. Similar behavior is observed for other compositions also. The frequency response of conductivity in glasses may be entirely due Copyright © 2011 SciRes. NJGC 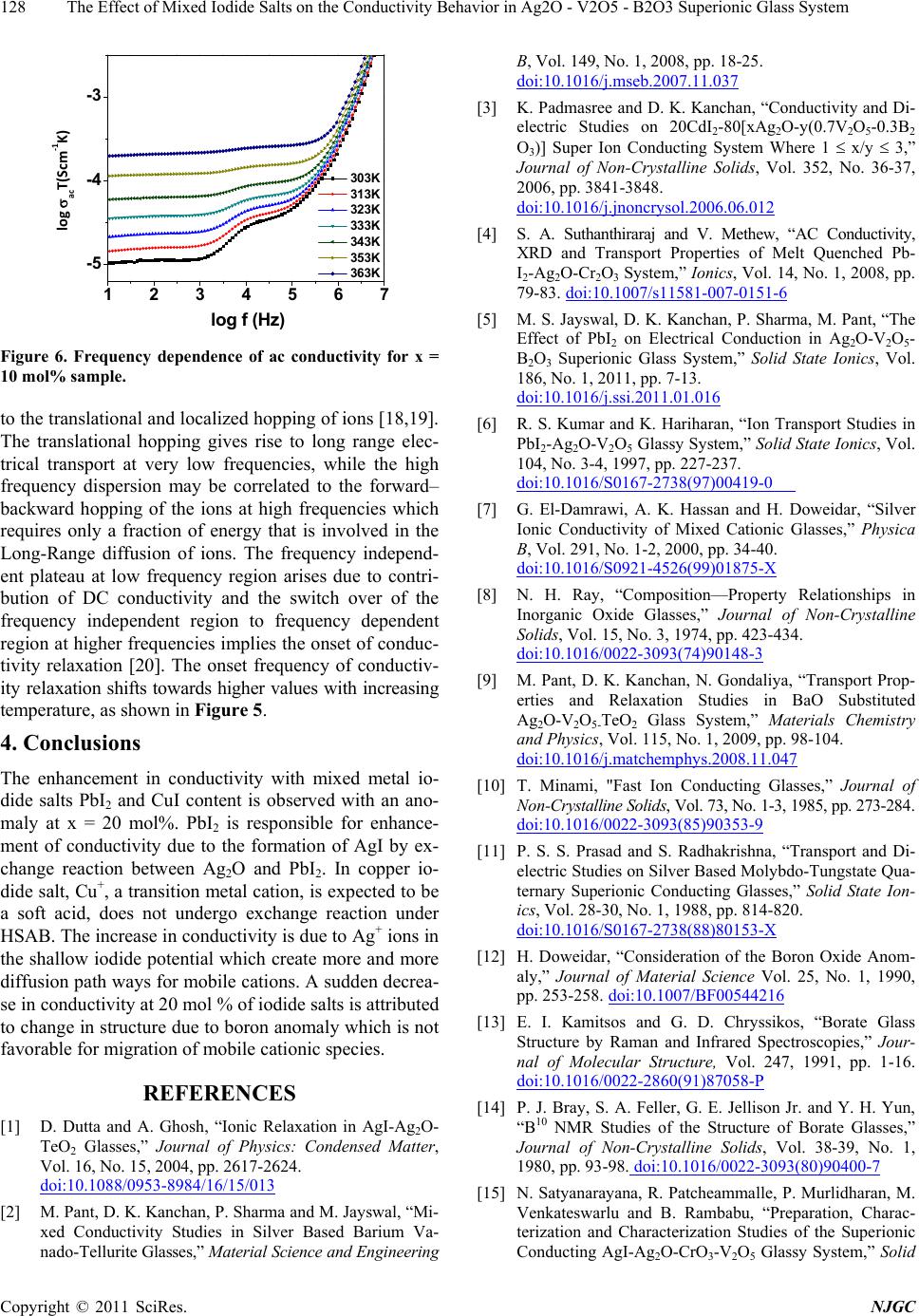 The Effect of Mixed Iodide Salts on the Conductivity Behavior in Ag2O - V2O5 - B2O3 Superionic Glass System 128 123456 -5 -4 -3 7 logacT(Scm‐1K) log f (Hz) 303K 313K 323K 333K 343K 353K 363K Figure 6. Frequency dependence of ac conductivity for x = 10 mol% sample. to the translational and localized hopping of ions [18,19]. The translational hopping gives rise to long range elec- trical transport at very low frequencies, while the high frequency dispersion may be correlated to the forward– backward hopping of the ions at high frequencies which requires only a fraction of energy that is involved in the Long-Range diffusion of ions. The frequency independ- ent plateau at low frequency region arises due to contri- bution of DC conductivity and the switch over of the frequency independent region to frequency dependent region at higher frequencies implies the onset of conduc- tivity relaxation [20]. The onset frequency of conductiv- ity relaxation shifts towards higher values with increasing temperature, as shown in Figure 5. 4. Conclusions The enhancement in conductivity with mixed metal io- dide salts PbI2 and CuI content is observed with an ano- maly at x = 20 mol%. PbI2 is responsible for enhance- ment of conductivity due to the formation of AgI by ex- change reaction between Ag2O and PbI2. In copper io- dide salt, Cu+, a transition metal cation, is expected to be a soft acid, does not undergo exchange reaction under HSAB. The increase in conductivity is due to Ag+ ions in the shallow iodide potential which create more and more diffusion path ways for mobile cations. A sudden decrea- se in conductivity at 20 mol % of iodide salts is attributed to change in structure due to boron anomaly which is not favorable for migration of mobile cationic species. REFERENCES [1] D. Dutta and A. Ghosh, “Ionic Relaxation in AgI-Ag2O- TeO2 Glasses,” Journal of Physics: Condensed Matter, Vol. 16, No. 15, 2004, pp. 2617-2624. doi:10.1088/0953-8984/16/15/013 [2] M. Pant, D. K. Kanchan, P. Sharma and M. Jayswal, “Mi- xed Conductivity Studies in Silver Based Barium Va- nado-Tellurite Glasses,” Material Science and Engineering B, Vol. 149, No. 1, 2008, pp. 18-25. doi:10.1016/j.mseb.2007.11.037 [3] K. Padmasree and D. K. Kanchan, “Conductivity and Di- electric Studies on 20CdI2-80[xAg2O-y(0.7V2O5-0.3B2 O3)] Super Ion Conducting System Where 1 x/y 3,” Journal of Non-Crystalline Solids, Vol. 352, No. 36-37, 2006, pp. 3841-3848. doi:10.1016/j.jnoncrysol.2006.06.012 [4] S. A. Suthanthiraraj and V. Methew, “AC Conductivity, XRD and Transport Properties of Melt Quenched Pb- I2-Ag2O-Cr2O3 System,” Ionics, Vol. 14, No. 1, 2008, pp. 79-83. doi:10.1007/s11581-007-0151-6 [5] M. S. Jayswal, D. K. Kanchan, P. Sharma, M. Pant, “The Effect of PbI2 on Electrical Conduction in Ag2O-V2O5- B2O3 Superionic Glass System,” Solid State Ionics, Vol. 186, No. 1, 2011, pp. 7-13. doi:10.1016/j.ssi.2011.01.016 [6] R. S. Kumar and K. Hariharan, “Ion Transport Studies in PbI2-Ag2O-V2O5 Glassy System,” Solid State Ionics, Vol. 104, No. 3-4, 1997, pp. 227-237. doi:10.1016/S0167-2738(97)00419-0 [7] G. El-Damrawi, A. K. Hassan and H. Doweidar, “Silver Ionic Conductivity of Mixed Cationic Glasses,” Physica B, Vol. 291, No. 1-2, 2000, pp. 34-40. doi:10.1016/S0921-4526(99)01875-X [8] N. H. Ray, “Composition—Property Relationships in Inorganic Oxide Glasses,” Journal of Non-Crystalline Solids, Vol. 15, No. 3, 1974, pp. 423-434. doi:10.1016/0022-3093(74)90148-3 [9] M. Pant, D. K. Kanchan, N. Gondaliya, “Transport Prop- erties and Relaxation Studies in BaO Substituted Ag2O-V2O5-TeO2 Glass System,” Materials Chemistry and Physics, Vol. 115, No. 1, 2009, pp. 98-104. doi:10.1016/j.matchemphys.2008.11.047 [10] T. Minami, "Fast Ion Conducting Glasses,” Journal of Non-Crystalline Solids, Vol. 73, No. 1-3, 1985, pp. 273-284. doi:10.1016/0022-3093(85)90353-9 [11] P. S. S. Prasad and S. Radhakrishna, “Transport and Di- electric Studies on Silver Based Molybdo-Tungstate Qua- ternary Superionic Conducting Glasses,” Solid State Ion- ics, Vol. 28-30, No. 1, 1988, pp. 814-820. doi:10.1016/S0167-2738(88)80153-X [12] H. Doweidar, “Consideration of the Boron Oxide Anom- aly,” Journal of Material Science Vol. 25, No. 1, 1990, pp. 253-258. doi:10.1007/BF00544216 [13] E. I. Kamitsos and G. D. Chryssikos, “Borate Glass Structure by Raman and Infrared Spectroscopies,” Jour- nal of Molecular Structure, Vol. 247, 1991, pp. 1-16. doi:10.1016/0022-2860(91)87058-P [14] P. J. Bray, S. A. Feller, G. E. Jellison Jr. and Y. H. Yun, “B10 NMR Studies of the Structure of Borate Glasses,” Journal of Non-Crystalline Solids, Vol. 38-39, No. 1, 1980, pp. 93-98. doi:10.1016/0022-3093(80)90400-7 [15] N. Satyanarayana, R. Patcheammalle, P. Murlidharan, M. Venkateswarlu and B. Rambabu, “Preparation, Charac- terization and Characterization Studies of the Superionic Conducting AgI-Ag2O-CrO3-V2O5 Glassy System,” Solid Copyright © 2011 SciRes. NJGC  The Effect of Mixed Iodide Salts on the Conductivity Behavior in Ag2O - V2O5 - B2O3 Superionic Glass System Copyright © 2011 SciRes. NJGC 129 State Ionics, Vol. 136-137, 2000, pp. 1097-1100. doi:10.1016/S0167-2738(00)00545-2 [16] R. V .G. K. Sarma and S. Radhakrishna, “Glass Forma- tion and Conductivity Studies in Silver Boromolybdate Systems,” Material Science and Engineering B, Vol. 7, No. 4, 1991, pp. 267-273. doi:10.1016/0921-5107(91)90003-E [17] A. K. Jonscher, “The ‘Universal’ Dielectric Response,” Nature, Vol. 267, 1977, pp. 673-679. [18] N. Baskaran, G. Govindaraj and A. Narayanasamya, “A.c. Con- ductivity and Relaxation Processes in Silver Selenochromate Glass,” Solid State Ionics, Vol. 98, No. 3-4, 1997, pp. 217-227. doi:10.1016/S0167-2738(97)00110-0 [19] S. H. Chung, K. R. Jeffrey, J. R. Stevens and L. Borjes- son, “Dynamics of Silver Ions in (AgI)x-(Ag2O-nB2O3)1-x Glasses: A 109Ag Nuclear Magnetic Resonance Study,” Physical Review B, Vol. 41, No. 10, 1990, pp. 6154-6164. doi:10.1103/PhysRevB.41.6154 [20] B. Deb and A. Ghosh, “Dielectric and Conductivity Re- laxation in AgI Doped Silver Selenite Superionic Glasses,” Journal of Applied Physics, Vol. 108, No. 7, 2010, Article ID: 074104. |

