Paper Menu >>
Journal Menu >>
 New Journal of Glass and Ceramics, 2011, 1, 119-124 doi:10.4236/njgc.2011.13017 Published Online October 2011 (http://www.SciRP.org/journal/njgc) Copyright © 2011 SciRes. NJGC 119 Compressive Strength and Electron Paramagnetic Resonance Studies on Waste Glass Admixtured Cement Ramasamy Gopalakrishnan1, Dharshnamoorthy Govindarajan2 1Department of Physics, SRM University, Kattankulathur, India; 2Department of Physics, Annamalai University, Annamalai Nagar, India. Email: gopalsrmphysics@gmail.com Received July 22th, 2011; revised August 28th, 2011; accepted September 7th, 2011. ABSTRACT The present work reports the effect of waste glass (WG) on the properties of Portland cement through Electron Para- magnetic Resonance (EPR) study. Cement pastes containing 0, 10, and 30% replacement of waste glass with cement and in a water to cement ratio of 0.4 have been prepared. The g factors of Fe(III) and Mn(II) impurities at different hydration ages ha ve been calculated. The decreased gFe values and simultaneous increase in g Mn valu es with increase in replacement % of WG are explained due to retardation of cement hydration. Keywords: EPR, Cement, Waste Glass, Setting Time, Compressive Strength, G-Factor 1. Introduction Cement is an important adhesive used by mankind in all walks of life, ri ght from filling the teeth cavity to th e Sky- Scrapers. Clinker, produced by heating a mixture of limestone and clays at 1500˚C, is composed of various calcium minerals, e.g. calcium silicates, aluminates and ferrites. The principle minerals are tricalcium silicate (C3S), dicalcium silicate (C2S), tricalcium aluminate (C3A) and tetracalcium aluminoferrite (C4AF) [1]. Hy- dration products, formed when cement or clinker miner- als are mixed with specific amount of water, have chemical and physiccal properties that vary with the con- ditions of hydration (time, temperatures, pressure etc.,). The hydration products of cement form a colloidal struc- ture (hydrogel), when cement is hydrated with much ex- cess water. There are many methods, which can be used to analyze the succeeding mechanisms in the cement hydration. They are FTIR, XRD, DTA, SEM, Electrical measurement and dielectric methods [2-6]. The utilization of many industrial byproducts in the construction industry is now well developed as it help s in improving the sustainability in two ways. First, reuse of and will be occupying scarce land resource. Second, it minimizes the degradation of land and the environment as a result of comparatively less digging. “Recycling” is an All-Prevailing practice now as it conserves the planet’s resources. The construction industry has shown great gains in recycling industrial by products and waste, in- cluding waste glass ( WG). Glass is produced in many forms including packaging or container glass (bottles and jars), flat glass (windows and windscreens), bulb glass (TV screens, monitors etc.), all of which have a limited life in the form they are pro- duced and need to be reused/recycled to avoid environ- mental problems that would be created if they were to be stockpiled or send to landfill. The finely ground waste glass is a mineral additive in cement is another promising direction for waste glass recycling. The use of recycled waste glass in Portland cement has attracted a lot of interest worldwide due to the increased disposal costs and environmental concerns. Being amor- phous and containing relatively large quantities of silicon, aluminium, calcium, glass is, in theory, pozzolanic or even cementitious in nature when it is finely ground. Thus, it can be used as a cement replacement in Portland cement. Recently, many studies have focused on the uses of was- tes glasses as aggregates for cement concrete or as ce- ment replacement [7-9]. Recently some attempts have been made to use the waste glasses as raw siliceous materials for the production of Portland cement [10-12]. Studies have been done on the possibility of reusing waste glasses as asphalt additive or rode filler [13]. Electron Paramagnetic Resonance (EPR) is an ideal 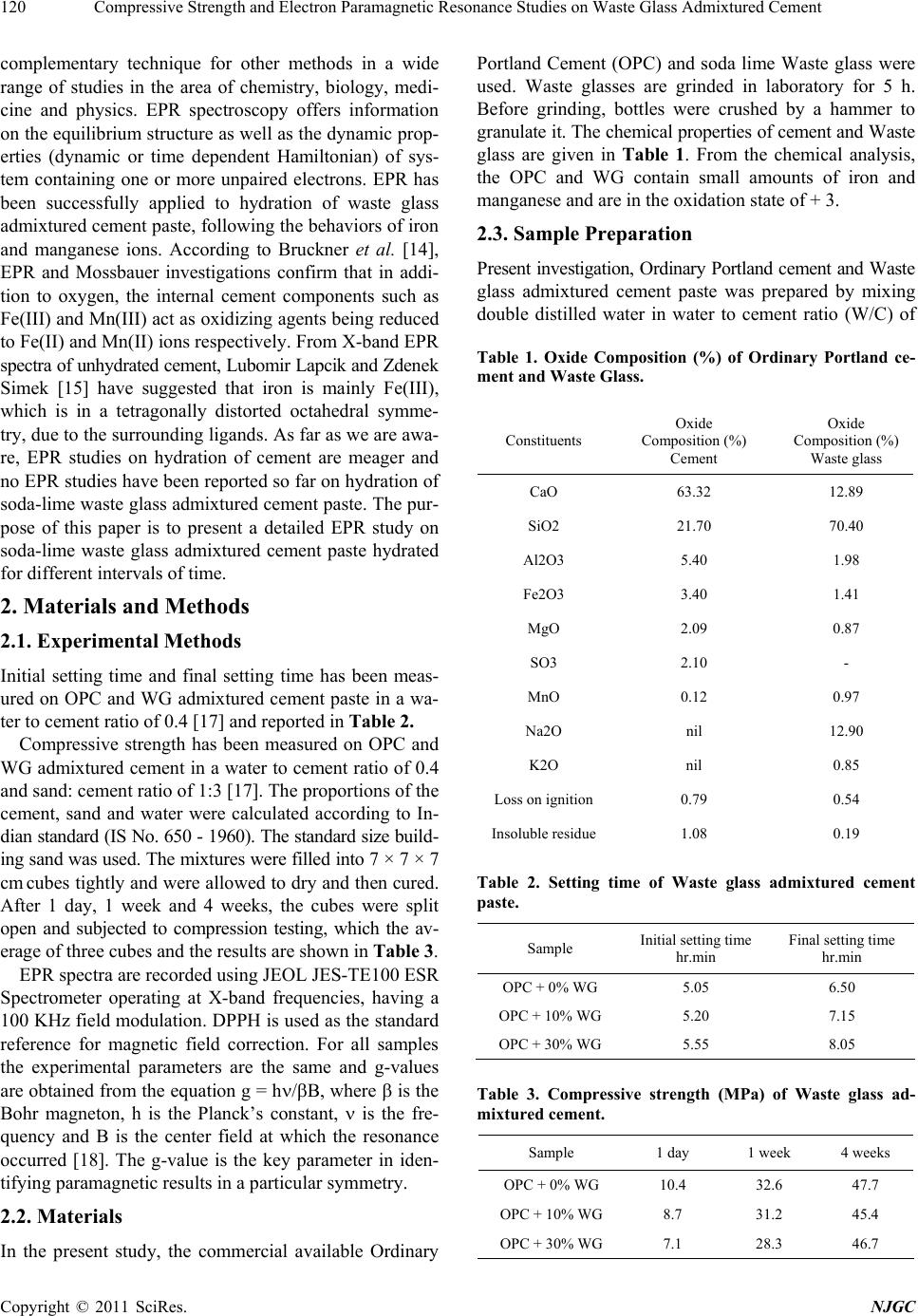 Compressive Strength and Ele c tron Paramagnetic Resonance Studies on Waste Glass Admixtured Cement 120 complementary technique for other methods in a wide range of studies in the area of chemistry, biology, medi- cine and physics. EPR spectroscopy offers information on the equilibrium structure as well as the dyn amic prop- erties (dynamic or time dependent Hamiltonian) of sys- tem containing one or more unpaired electrons. EPR has been successfully applied to hydration of waste glass admixtured cement paste, following the behaviors of iron and manganese ions. According to Bruckner et al. [14], EPR and Mossbauer investigations confirm that in addi- tion to oxygen, the internal cement components such as Fe(III) and Mn(III) act as oxidizing agents being reduced to Fe(II) and Mn ( II) ion s resp ectiv ely. From X- ban d EPR spectra of unhydrated cement, Lubomir Lapcik and Zden ek Simek [15] have suggested that iron is mainly Fe(III), which is in a tetragonally distorted octahedral symme- try, due to th e surroun ding liga nds. As fa r as we are aw a- re, EPR studies on hydration of cement are meager and no EPR studies have been reported so far on hydration of soda-lime waste glass admixtured cement paste. The pur- pose of this paper is to present a detailed EPR study on soda-lime waste glass admixtured cement paste hydrated for differe nt inter vals o f time. 2. Materials and Methods 2.1. Experimental Methods Initial setting time and final setting time has been meas- ured on OPC and WG admixtured cement paste in a wa- ter to cement ratio of 0.4 [17] and reported in Table 2. Compressive strength has been measured on OPC and WG admixtured cement in a water to cement ratio of 0.4 and sand: cement ratio of 1:3 [17]. The proportions of the cement, sand and water were calculated according to In- di an standard (IS No. 650 - 1960). The standard size buil d- ing sand was used. The mixtures were filled into 7 × 7 × 7 cm cubes tightly and were allowed to dry and then cured. After 1 day, 1 week and 4 weeks, the cubes were split open and subjected to compression testing, which the av- erage of three cubes and the results are shown in Table 3. EPR spectra are recorded using JEOL JES-TE100 ESR Spectrometer operating at X-band frequencies, having a 100 KHz field modulation. DPPH is used as the standard reference for magnetic field correction. For all samples the experimental parameters are the same and g-values are obtained from the equation g = h/B, wh ere is the Bohr magneton, h is the Planck’s constant, is the fre- quency and B is the center field at which the resonance occurred [18]. The g-value is the key parameter in iden- tifying paramagnetic results in a particular symmetry. 2.2. Materials In the present study, the commercial available Ordinary Portland Cement (OPC) and soda lime Waste glass were used. Waste glasses are grinded in laboratory for 5 h. Before grinding, bottles were crushed by a hammer to gr anulate it. The chemical properties of cement and Waste glass are given in Table 1. From the chemical analysis, the OPC and WG contain small amounts of iron and manganese and are in the oxidation state of + 3. 2.3. Sample Preparation Present investigation, Ordinary Portland cement and Waste glass admixtured cement paste was prepared by mixing double distilled water in water to cement ratio (W/C) of Table 1. Oxide Composition (%) of Ordinary Portland ce- ment and Waste Glass. Constituents Oxide Composition (%) Cement Oxide Composition (%) Waste glass CaO 63.32 12.89 SiO2 21.70 70.40 Al2O3 5.40 1.98 Fe2O3 3.40 1.41 MgO 2.09 0.87 SO3 2.10 - MnO 0.12 0.97 Na2O nil 12.90 K2O nil 0.85 Loss on ignition 0.79 0.54 Insoluble residue1.08 0.19 Table 2. Setting time of Waste glass admixtured cement paste. Sample Initial setting time hr.min Final setting time hr.min OPC + 0% WG 5.05 6.50 OPC + 10% WG 5.20 7.15 OPC + 30% WG 5.55 8.05 Table 3. Compressive strength (MPa) of Waste glass ad- mixtured cement. Sample 1 day 1 week 4 weeks OPC + 0% WG 10.4 32.6 47.7 OPC + 10% WG 8.7 31.2 45.4 OPC + 30% WG 7.1 28.3 46.7 Copyright © 2011 SciRes. NJGC 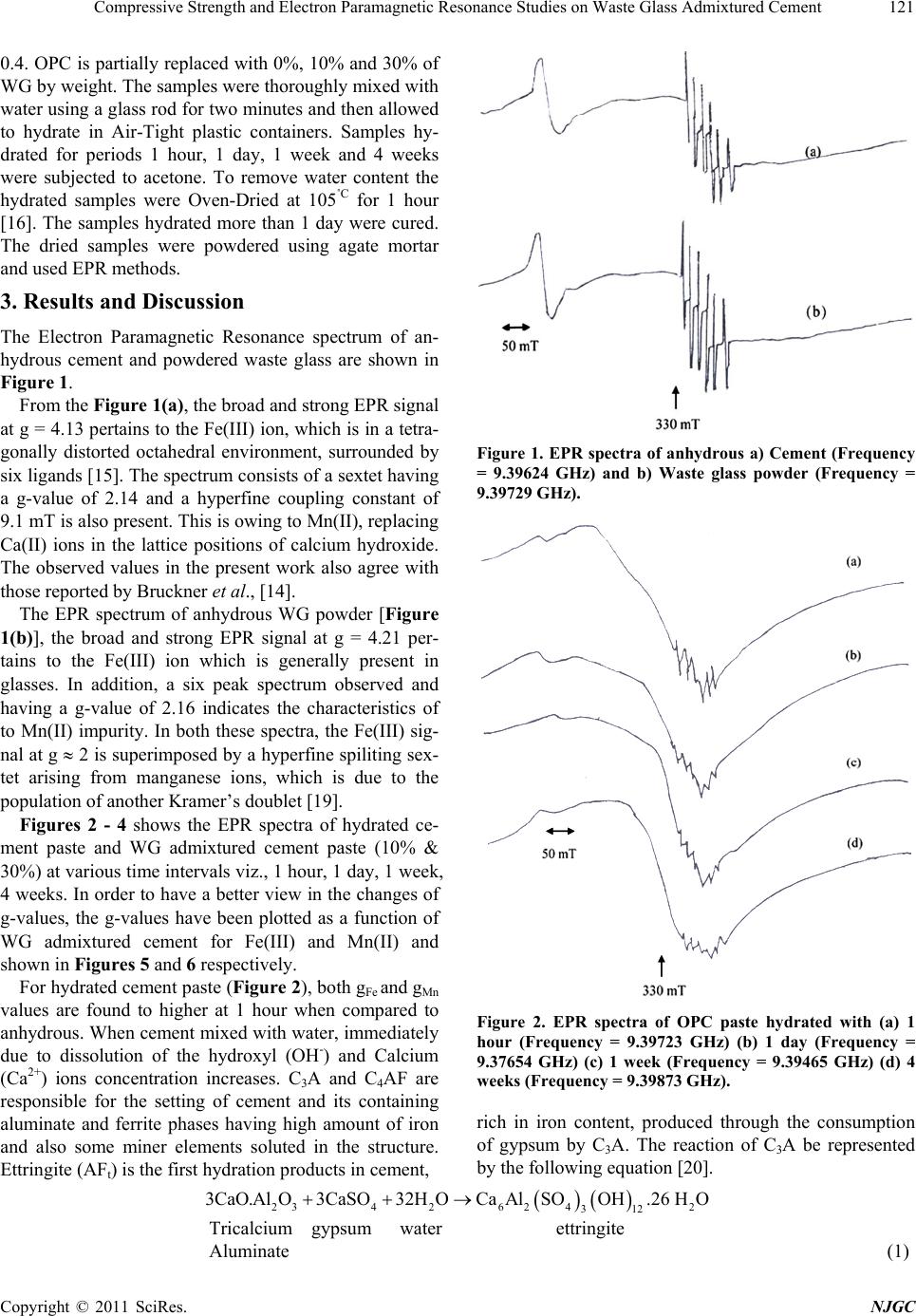 Compressive Strength and Ele c tron Paramagnetic Resonance Studies on Waste Glass Admixtured Cement Copyright © 2011 SciRes. NJGC 121 0.4. OPC is partially replaced with 0%, 10% and 30% of WG by weight. The samples were thorough ly mixed w ith water using a glass rod for two minutes and then allowed to hydrate in Air-Tight plastic containers. Samples hy- drated for periods 1 hour, 1 day, 1 week and 4 weeks were subjected to acetone. To remove water content the hydrated samples were Oven-Dried at 105˚C for 1 hour [16]. The samples hydrated more than 1 day were cured. The dried samples were powdered using agate mortar and used EPR methods. 3. Results and Discussion The Electron Paramagnetic Resonance spectrum of an- hydrous cement and powdered waste glass are shown in Figure 1. From the Figure 1(a), the broad and strong EPR signal at g = 4.13 pertains to the Fe(III) ion, which is in a tetra- gonally distorted octahedral environment, surrounded by six ligands [15]. The spectrum consists of a sextet having a g-value of 2.14 and a hyperfine coupling constant of 9.1 mT is also present. This is owing to Mn(II), replacing Ca(II) ions in the lattice positions of calcium hydroxide. The observed values in the present work also agree with those reported by Bruckner et al., [14]. Figure 1. EPR spectra of anhydrous a) Cement (Frequency = 9.39624 GHz) and b) Waste glass powder (Frequency = 9.39729 GHz). The EPR spectrum of anhydrous WG powder [Figure 1(b)], the broad and strong EPR signal at g = 4.21 per- tains to the Fe(III) ion which is generally present in glasses. In addition, a six peak spectrum observed and having a g-value of 2.16 indicates the characteristics of to Mn(II) impurity. In both these spectra, the Fe(III) sig- nal at g 2 is superimposed by a hyperfine spiliting sex- tet arising from manganese ions, which is due to the population of another Kramer’s doublet [19]. Figures 2 - 4 shows the EPR spectra of hydrated ce- ment paste and WG admixtured cement paste (10% & 30%) at various time intervals viz., 1 hour, 1 day, 1 week, 4 weeks. In order to have a better view in the changes of g-values, the g-values have been plotted as a function of WG admixtured cement for Fe(III) and Mn(II) and shown in Figures 5 and 6 respectively. For hydrated cement paste (Figure 2), both gFe and gMn values are found to higher at 1 hour when compared to anhydrous. When cement mixed with water, immediately due to dissolution of the hydroxyl (OH-) and Calcium (Ca2+) ions concentration increases. C3A and C4AF are responsible for the setting of cement and its containing aluminate and ferrite phases having high amount of iron and also some miner elements soluted in the structure. Ettringite (AFt) is the first hydration products in cement, Figure 2. EPR spectra of OPC paste hydrated with (a) 1 hour (Frequency = 9.39723 GHz) (b) 1 day (Frequency = 9.37654 GHz) (c) 1 week (Frequency = 9.39465 GHz) (d) 4 weeks (Frequency = 9.39873 GHz). rich in iron content, produced through the consumption of gypsum by C3A. The reaction of C3A be represented by the following equation [20]. 23426 242 312 3CaO.A lO3CaSO32HOCaAlSOOH.26 HO Tricalcium gypsum water ettringite Aluminate (1) 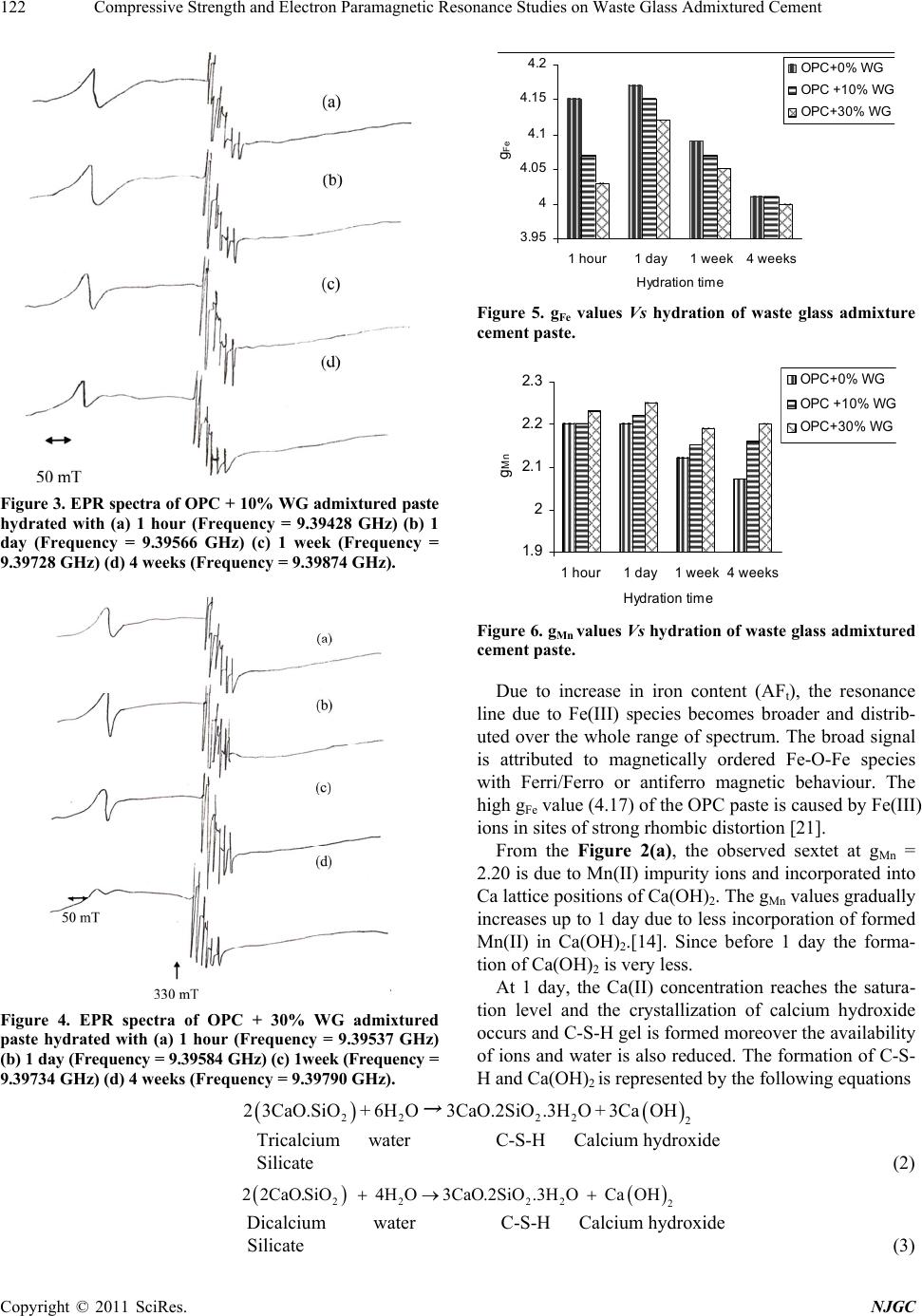 Compressive Strength and Ele c tron Paramagnetic Resonance Studies on Waste Glass Admixtured Cement 122 Fi gure 3. EPR spectra of OPC + 10% WG admixtured paste hydrated with (a) 1 hour (Frequency = 9.39428 GHz) (b) 1 day (Frequency = 9.39566 GHz) (c) 1 week (Frequency = 9.39728 GHz) (d) 4 weeks (Frequency = 9.39874 GHz). Figure 4. EPR spectra of OPC + 30% WG admixtured paste hydrated with (a) 1 hour (Frequency = 9.39537 GHz) (b) 1 day (Frequency = 9.39 584 GHz) (c) 1w eek (Frequency = 9.39734 GHz) (d) 4 weeks (Frequency = 9.39790 GHz). 3.95 4 4.05 4.1 4.15 4.2 1 hour 1 day1 week4 weeks Hydrat ion time g Fe OPC+0% WG O P C + 10% W G O P C+30% WG Figure 5. gFe values Vs hydration of waste glass admixture cement paste. 1.9 2 2.1 2.2 2.3 1 hour 1 day1 week4 week s Hydration t ime g Mn OPC+0% WG OP C +10% WG O P C+ 30% WG Figure 6. gMn values Vs hydration of waste glass admixtured cement paste. Due to increase in iron content (AFt), the resonance line due to Fe(III) species becomes broader and distrib- uted over the whole range of spectrum. The broad signal is attributed to magnetically ordered Fe-O-Fe species with Ferri/Ferro or antiferro magnetic behaviour. The high gFe value (4.17) of the OPC paste is caused by Fe(III) ions in sites of strong rhombic distortion [21]. From the Figure 2(a), the observed sextet at gMn = 2.20 is due to Mn(II) impurity ions and incorporated into Ca lattice positions of Ca(OH)2. The gMn values gradually increases up to 1 day due to less incorporation of formed Mn(II) in Ca(OH)2.[14]. Since before 1 day the forma- tion of Ca( OH )2 is very less. At 1 day, the Ca(II) concentration reaches the satura- tion level and the crystallization of calcium hydroxide occurs and C-S-H gel is formed moreover the availability of ions and water is also reduced. The formation of C-S- H and Ca(OH)2 is represented by the following equations 2222 2 23CaO.SiO + 6HO3CaO.2SiO.3HO+ 3CaOH → Tricalcium water C-S-H Calcium hydroxide Silicate (2) 2222 2 22CaO.SiO 4HO3CaO.2SiO.3HO CaOH Dicalcium water C-S-H Calcium hydroxide Silicate (3) Copyright © 2011 SciRes. NJGC  Compressive Strength and Ele c tron Paramagnetic Resonance Studies on Waste Glass Admixtured Cement123 The C-S-H gel is one of the major strength rendering components of the hydrated cement and it possesses sparse dispersibility, numerous interior structural effects and coarse particle surfaces. After 1 day, the availability of Fe(III) and Mn(II) ions are reduced and hence the g- values have gradually decreased. This is because these two ions in cements are responsible for the kinetics of solidification and hardening of the main silicate compo- nent. This may b e that the mobility of this particular sili- cate aggregate is reflected in the vigorous changes in polycrystalline quasi-isotropic character of electron paramagnetic spectra of Fe(III), Fe(II) ions to the anisot- ropic [15]. Also the decreased g-factor values reflect the structural changes of Fe(III) ions to Fe(II) ions. These characteristics generally decrease the g-values of Fe and Mn but increase the strength of cement up to 4 weeks. From the Figures 3 and 4, it is observed that WG ad- mixtured cement also follows the same fashion as that of OPC. In WG admixtured cement paste exhibit a lower gFe then that of the plain cement paste, the lowest being ob- served for the mixture with the highest amount of WG (30%). This is due to well kno wn retarding effect of WG. The WG admixtured cement showed longer setting times than the pure cement. Since, as the increase of WG con- tent reduces the cement in the mix (cement dilution). As a result, hydration process slows down and hence the volume of hydration products is less causing settin g time to increase. At all hydration periods, the WG admixtured cement paste shows higher gMn values than the plain cement paste. This is attributed to the red uction in the amount of Ca(OH)2 retarded by the clinker phases because of the reduced cement content, leading to less incorporation of formed Mn(II) in Ca(OH)2. This effect is reflected in EPR spectrum (Figure 3 and 4) that as replacement % of WG increase in cement, the intensity of the Mn(II) signal is also increases. Table 3 depicts the compressive strength of waste glass powder modified cement pastes at different hy- drated period. The compressive strength decrease with increase in WG powder contents, the reason being the reduction in cement content and increase porosity. At early age no secondary growth of C-S-H gel, because of the poor pozzolanic reaction between Ca(OH)2 and WG. 4. Conclusions The effect of Waste glass powder on the proper ties of Port- land cement is studied through Electron Paramagnetic Resonance with different hydrated periods. The follow- ing conclusions emerge: 1) The results indicate that EPR studies can be effect- tively used as a powerful tool in delineating the com- plexities of chemical reactions in cement hydration and to detect very small concentrations of Fe(III) and Mn(II) ions present in cement. 2) Setting time and compressive strength results is a confirmation of the retarding effect of WG in the hydra- tion of the Portland cement. 3) The use WG for cement makes it possible to solve some environmental problems well but it may be consid- ered after studying about its long term reactions, shrink- age properties, Alkali-Silica reaction, Porosity and adhe- sive capacity. REFERENCES [1] L. P. Aldridge, “Accuracy and Precision of Phase Analy- sis in Portland Cement by Bogue, Microscopic and X-ray Diffraction Methods,’’ Cement Concrete Research, Vol. 12, 1982, pp. 381-398. doi:10.1016/0008-8846(82)90087-4 [2] M. F. Rojas, “Study of Hydrated Phases Present in a MK-Lime System Cured at 60˚C and 60 Months of Reac- tion,” Cement Concrete Research, Vol. 36, 2006, pp. 827-831. doi:10.1016/j.cemconres.2006.01.001 [3] M. Oriol and J. Pera, “Pozzolanic Activity of Metakaolin Under Microwave Treatment,” Cement Concrete Re- search, Vol. 25, No. 2, 1995, pp. 265-270. doi:10.1016/0008-8846(95)00007-0 [4] N. J. Coleman and W. R Mcwhinnie, “The Solid State Chemistry of Metakaolin Blended Ordinary Portland Ce- ment,” Journal of Materials Science, Vol. 35, 2000, pp. 2701-2710. doi:10.1023/A:1004753926277 [5] C. A. Love, I. G. Richardson and A. R. Brough, “Compo- sition and Structure of C-S-H in White Portland Cement- 20% Metakaolin Pastes Hydrated at 25˚C,” Cement Con- crete Rese asrch, Vol. 37, No. 2, 2007, pp. 109-117. [6] S. Barathan, D. Govindarajan, G. Sivakumar and K. Raghu, “Microwave Study of Hydration of Cement with Different Waters,” Indian Journal of Pure and Applied Physics, Vol. 44, 2006, pp. 334-338. [7] W. W. J. Chan and C. M. L. Wu, “Durability of Concrete with High Cement Replacement,” Cement Concrete Re- search, Vol. 30, No. 6, 2000, pp. 865-879. doi:10.1016/S0008-8846(00)00253-2 [8] S. Goberis and V. Antonovich, “Influence of Sodium Silicate Amount on the Setting Time and Exo Tempera- ture of a Complex Binder Consisting of High Alumina Cement, Liquid Glass and Metallurgical Slag,” Cement Concrete Research, Vol. 34, 2004, pp. 1939-1941. doi:10.1016/j.cemconres.2004.01.004 [9] K. Sobolev, P. Turker, S. S. leva and G. Iscioglu, “Utili- zation of Waste Glass in Eco Cement: Strength Properties and Microstructural Observation,” Waste Management, Vol. 27, No. 7, 2007, pp. 971-976. doi:10.1016/j.wasman.2006.07.014 [10] J. Mohamad Terro, “Properties of Concrete Made with Recycled Crushed Glass at Elevated Temperature,” Build- ing and Environment, Vol. 41, No. 5, 2006, pp. 633-639. doi:10.1016/j.buildenv.2005.02.018 Copyright © 2011 SciRes. NJGC  Compressive Strength and Ele c tron Paramagnetic Resonance Studies on Waste Glass Admixtured Cement 124 [11] S. B. Park and B. C. Lee, “Studies on Expansion Proper- ties in Mortar Containing Waste Glass and Fibres,” Ce- ment Concrete Research, Vol. 34, No. 7, 2004, pp. 1145- 1152. [12] I. B. Topcu and M. Canbaz, “Properties Containing Waste Galss,” Ce me nt Concrete Research , Vol. 34, No. 2, 2004, pp. 267-274. doi:10.1016/j.cemconres.2003.07.003 [13] R. L Sharma and S. P. Pandey, “Influence of Mineral Additives on the Hydration Characteristics of Ordinary Portland Cement,” Cement Concrete Research, Vol. 29, No. 9, 1999, pp. 1525-1529. doi:10.1016/S0008-8846(99)00104-0 [14] A. Bruckner, R. Luck, W. Wieker, A. Winkler, C. An- dreae and H. Mehner, “Investigation of Redox Reactions Proceeding During the Hardening Process of Sulfide Con- taining Cement,” Cement Concrete Research, Vol. 22, 1992, pp. 1116-1169. doi:10.1016/0008-8846(92)90045-W [15] Jr. L. Lapcik and Z. Simek, “Electroparamagnetic Reso- nance Study of Dry Cements,” Cement Concrete Re- search, Vol. 26, No. 2, 1996, pp. 237-242. doi:10.1016/0008-8846(95)00216-2 [16] N. Voglis, G. Kakali, S. Chaniotaki and S. Tsivilis, “Portland-Limestone Cements: Their Properties and Hy- dration Compared to Those of Other Composite Cements,” Cement Concrete Research, Vol. 27, 2005, pp. 191-196. doi:10.1016/j.cemconcomp.2004.02.006 [17] M. S. Shetty, “Concrete Technology,” S. Chand and Co. Ltd., New Delhi, 2004. [18] A. J. Weil, R. J. Bolton and E. J. Wertz, “Electron Para- magnetic Resonance,” Elementary Theory and Practical Applications, John Wiley & Sons, New York, 1994. [19] P. S. Rao, “Single Crystal EPR Study of Fe(III) Doped Magnesium Potassium Tutton’s Salt. Part 3,” Spectro- chimica Acta A, Vol. 52, No. 9, 1996, pp. 1127-1134. doi:10.1016/0584-8539(95)01637-6 [20] M. Y. A. Mollah, W. Yu, R. Schennach and D. L. Cocke, “A Fourier Transform Infrared Spectroscopic Investiga- tion of the Early Hydration of Portland Cement and the Influence of Sodium Lignosulfonate,” Cement Concrete Research, Vol. 30, No. 2, 2000, pp. 267-273. doi:10.1016/S0008-8846(99)00243-4 [21] P. S. Rao and S. Subramanian, “Single Crystal E.P.R Studies of Some First Row Transition Ions in Hex-Aimi- Dazole Zinc(II) Dichloride Tetrahydrate,” Molecular Ph- ysics, Vol. 54, No. 2, 1985, pp. 415-427. doi:10.1080/00268978500100321 Cement nomenclature: C = CaO; S = SiO2; H = H2O; C3S = 3CaO.SiO2; C2S = 2CaO.SiO2; C3A = 3CaO.Al2O3; C4AF = 4CaO.Al2O3.Fe2O3; CH = Ca(OH)2 Copyright © 2011 SciRes. NJGC |

