Paper Menu >>
Journal Menu >>
 New Journal of Glass and Ceramics, 2011, 1, 105-111 doi:10.4236/njgc.2011.13015 Published Online October 2011 (http://www.SciRP.org/journal/njgc) Copyright © 2011 SciRes. NJGC 105 Sol-Gel Processing of Silica Nuclear Waste Glasses Andrzej Deptuła1, Magdalena Miłkowska1, Wiesława Łada1, Tadeusz Olczak1, Danuta Wawszczak1, Tomasz Smolinski1, Fabio Zaza2, Marcin Brykala1, Andrzej G. Chmielewski1, Kenneth C. Goretta3 1Institute of Nuclear Chemistry and Technology (INCT), Warsaw, Poland; 2Italian National Agency for New Technologies, Energy and Environment (ENEA), CR Casaccia, Rome, Italy; 3Asian Office of Aerospace Research and Development, Japan. Email: a.deptula@ichtj.waw.pl Received June 30th, 2011; revised August 9th, 2011; accepted August 21th, 2011. ABSTRACT A complex Sol-Gel process has been used for synthesis of silica glasses designed to contain high-level nuclear wastes. Cs, Sr, Co, and Nd (generically denoted Me) were used, the last as surrogate for actinides. Gels in the form of powders and sintered compacts were prepared by hydrolysis and polycondensation of tetraethoxide/Me nitrate solutions, which contained ascorbic acid a s a catalyst. Thermal treatment stud ies were conducted on the resulting gels. Transformation to final products was studied by thermog ravimetric analysis, infrared sp ectroscopy, and X-ray diffraction. Prelimina ry testing of Me leaching was also completed in quiescent water. Only a single dense form was resistant to leaching. Keywords: Sol-Gel, Silica Glass, Nuclear Waste, Thermal Treatment 1. Introduction Vitrification of hazardous nuclear wastes has been shown to be a viable technological alternative for effecttive ma- nagement of spent fuel and radioactive waste. The advan- tages of the method are that a large number of elements that can be incorporated into the glass and a highly dura- ble and small-volume waste is produced [1,2]. The prop- erties of silica glasses, including good durability and mechanical strength and ability to incorporated large concentrations of metallic dopants, make them ideal can- didates for matrices for nuclear waste storage [3,4]. The most significant disadvantage of silica glasses for such use is their high processing temperature of ~ 2000˚C. Sin- tering of sol-gel-derived glasses can be accomplished at much lower temperatures, and sol-gel techniques have been successfully used for preparation of porous glass hosts for nuclear wastes [3,4]. Appropriately prepared, sintered ceramic bodies can have higher stabilities and be more resistant to leaching than are many melt-processed glasses [5,6]. In the present work, our proprietary complex sol-gel process (CSGP) [7-9] has been adapted to synthesize silica glasses capable of incorporating significant con- centrations of high-level nuclear wastes. The heavy met- als (denoted Me) Cs, Sr, Co, and, as a surrogate for acti- nides, Nd, were incorporated into silica glasses and the resulting waste forms were characterized and tested for leaching response. 2. Experimental Details A detailed flow-chart for the preparation Cs-, Sr-, Co-, and Nd-doped (10 mole%) silica gels is shown in Figure 1. Gels in the form of powders or monoliths were pre- pared by hydrolysis and subsequent polycondensation of tetraethoxide/Me nitrate solutions containing ascorbic acid (ASC) as a catalyst, instead of the HCl or NH4OH that are routinely used for catalysis in glass synthesis. ASC has not, to the best of our knowledge, been used previ- ously in this type of processing of a nuclear waste glass. Its use has been shown to decrease remarkably the time needed to synthesize gels [7,9]. Thermogravimetric analysis (TG) and differential ther- mal analysis (DTA) were conducted in air with a Hun- garian MOM (Budapest, Hungary) [8,9]. The heating rate was 10˚C /h. All resulting products were analyzed by X-ray diffract- tion (XRD) with a Rigaku Miniflex diffractometer (To- kyo, Japan). Cu-Kα radiation was used. The tube output voltage was 30 kV the tube output current was 15 mA, the angular spread was 2θ = 3˚ - 90˚, with steps of 0.02˚ and a scanning rate of 2˚/min. Raw data were smoothed by the Savitzky method, background was eliminated by the Sonnevelt method, and Kα2 was eliminated. Infrared measurements were conducted with a Perkin Elmer Mo- del 983 Spectrometer (Waltham, MA). The potassium bromide pellet technique was adopted. Leaching tests were conducted on 2 g of material. Powder 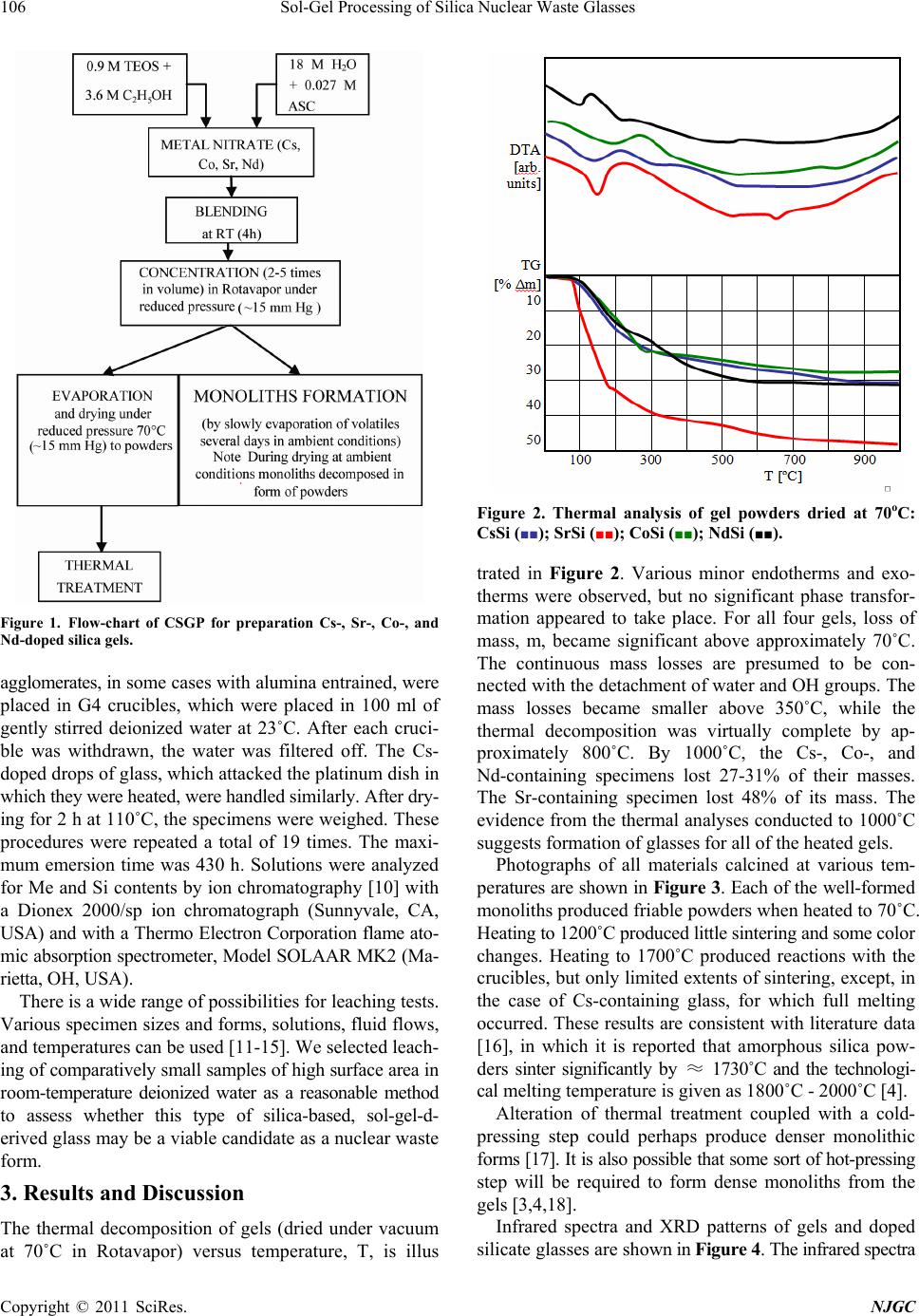 Sol-Gel Processing of Silica Nuclear Waste Glasses 106 Figure 1. Flow-chart of CSGP for preparation Cs-, Sr-, Co-, and Nd-doped silica gels. agglomerates, in some cases with alumina entrained, were placed in G4 crucibles, which were placed in 100 ml of gently stirred deionized water at 23˚C. After each cruci- ble was withdrawn, the water was filtered off. The Cs- doped drops of glass, which attacked the platinum dish in which they were heated, were handled similarly. After dry- ing for 2 h at 110˚C, the specimens were weighed. These procedures were repeated a total of 19 times. The maxi- mum emersion time was 430 h. Solutions were analyzed for Me and Si contents by ion chromatography [10] with a Dionex 2000/sp ion chromatograph (Sunnyvale, CA, USA) and with a Thermo Electron Corporation flame ato- mic absorption spectrometer, Model SOLAAR MK2 (Ma- rietta, OH, USA). There is a wide range of possibilities for leaching tests. Various specimen sizes and forms, solutions, fluid flows, and temperatures can be used [11-15]. We selected leach- ing of comparatively small samples of high surface area in room-temperature deionized water as a reasonable method to assess whether this type of silica-based, sol-gel-d- erived glass may be a viable candidate as a nuclear waste form. 3. Results and Discussion The thermal decomposition of gels (dried under vacuum at 70˚C in Rotavapor) versus temperature, T, is illus Figure 2. Thermal analysis of gel powders dried at 70oC: CsSi (■■); SrSi (■■); CoSi (■■); NdSi (■■). trated in Figure 2. Various minor endotherms and exo- therms were observed, but no significant phase transfor- mation appeared to take place. For all four gels, loss of mass, m, became significant above approximately 70˚C. The continuous mass losses are presumed to be con- nected with the detachment of water and OH groups. The mass losses became smaller above 350˚C, while the thermal decomposition was virtually complete by ap- proximately 800˚C. By 1000˚C, the Cs-, Co-, and Nd-containing specimens lost 27-31% of their masses. The Sr-containing specimen lost 48% of its mass. The evidence from the thermal analyses conducted to 1000˚C suggests formation of glasses for all of the heated gels. Photographs of all materials calcined at various tem- peratures are shown in Figure 3. Each of the well-formed monoliths produced friable powders when heated to 70˚C. Heating to 1200˚C produced little sintering and some color changes. Heating to 1700˚C produced reactions with the crucibles, but only limited extents of sintering, except, in the case of Cs-containing glass, for which full melting occurred. These results are consistent with literature data [16], in which it is reported that amorphous silica pow- ders sinter significantly by ≈ 1730˚C and the technologi- cal melting temperature is given as 1800˚C - 2000˚C [4]. Alteration of thermal treatment coupled with a cold- pressing step could perhaps produce denser monolithic forms [17]. It is also possible that some sort of hot-pressing step will be required to form dense monoliths from the gels [3,4,18]. Infrared spectra and XRD patterns of gels and doped silicate glasses are shown in Figure 4. The infrared spectra Copyright © 2011 SciRes. NJGC  Sol-Gel Processing of Silica Nuclear Waste Glasses Copyright © 2011 SciRes. NJGC 107 Figure 3. Photographs of materials obtained from sols according procedure described in Figure 1, heated for 2 h at tempera- tures shown. 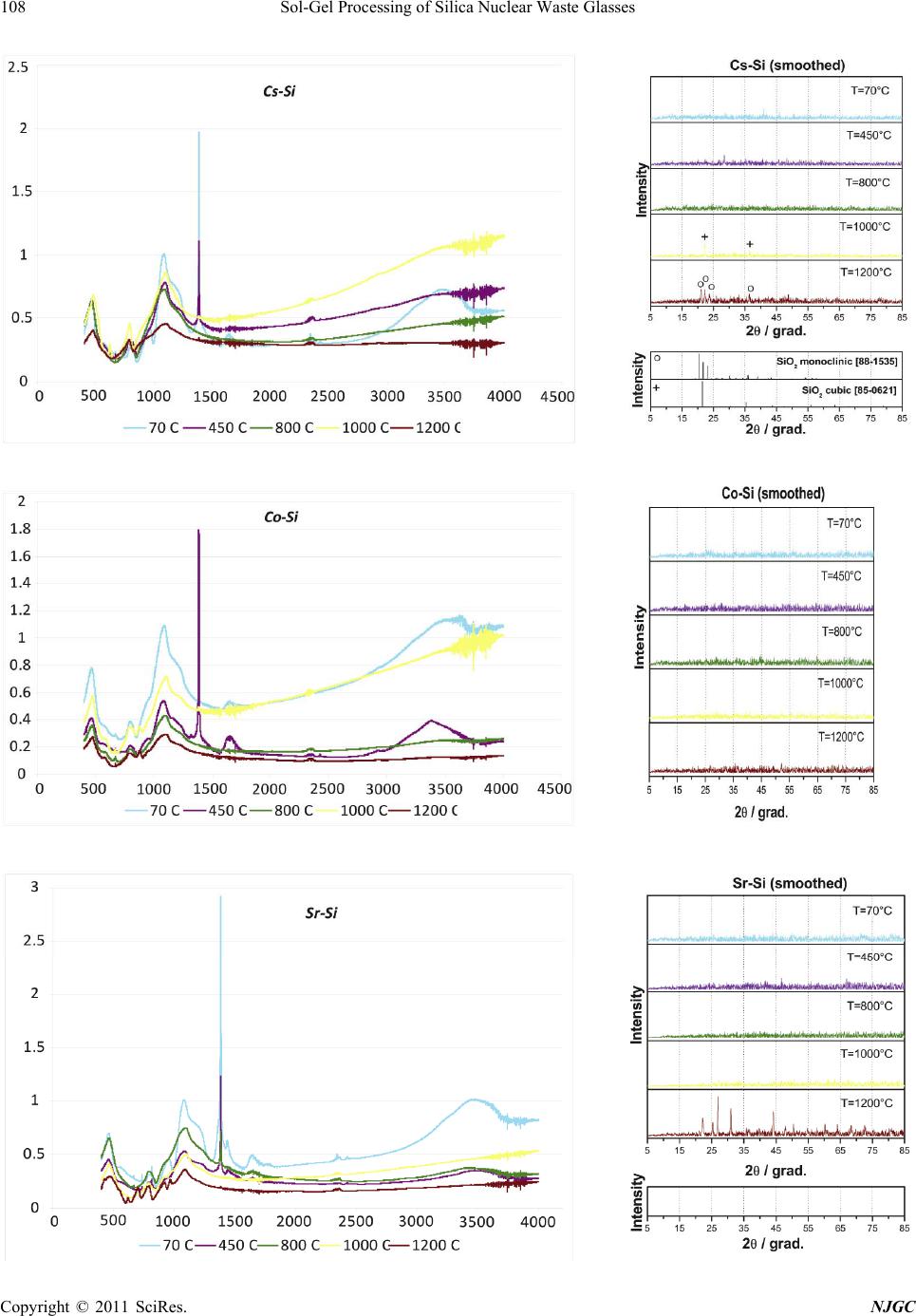 Sol-Gel Processing of Silica Nuclear Waste Glasses 108 Copyright © 2011 SciRes. NJGC 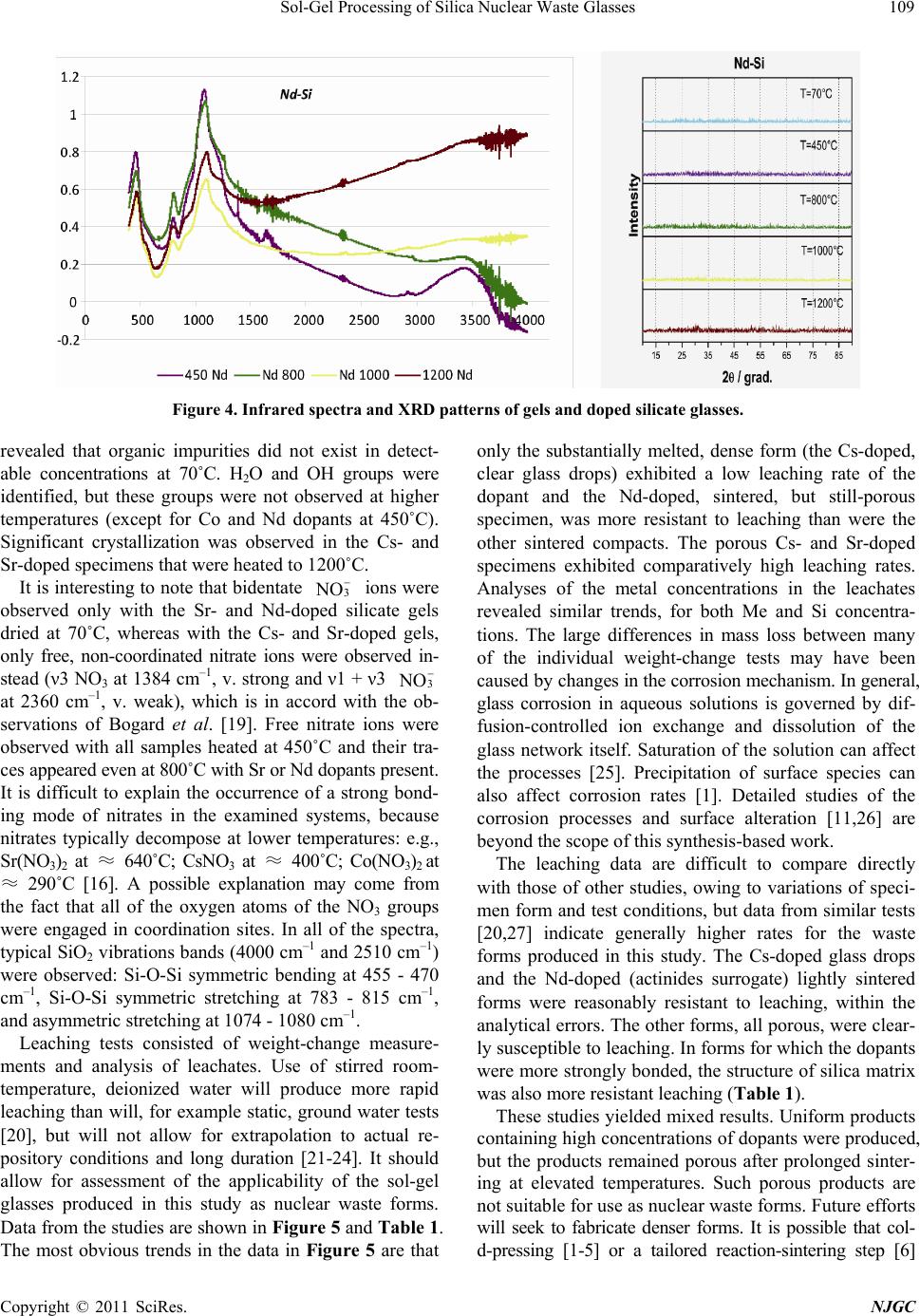 Sol-Gel Processing of Silica Nuclear Waste Glasses Copyright © 2011 SciRes. NJGC 109 Figure 4. Infrared spectra and XRD patterns of gels and doped silicate glasses. revealed that organic impurities did not exist in detect- able concentrations at 70˚C. H2O and OH groups were identified, but these groups were not observed at higher temperatures (except for Co and Nd dopants at 450˚C). Significant crystallization was observed in the Cs- and Sr-doped specimens that were heated to 1200˚C. only the substantially melted, dense form (the Cs-doped, clear glass drops) exhibited a low leaching rate of the dopant and the Nd-doped, sintered, but still-porous specimen, was more resistant to leaching than were the other sintered compacts. The porous Cs- and Sr-doped specimens exhibited comparatively high leaching rates. Analyses of the metal concentrations in the leachates revealed similar trends, for both Me and Si concentra- tions. The large differences in mass loss between many of the individual weight-change tests may have been caused by changes in the corrosion mechanism. In general, glass corrosion in aqueous solutions is governed by dif- fusion-controlled ion exchange and dissolution of the glass network itself. Saturation of the solution can affect the processes [25]. Precipitation of surface species can also affect corrosion rates [1]. Detailed studies of the corrosion processes and surface alteration [11,26] are beyond the scope of this synthesis-based work. It is interesting to note that bidentate 3 N O ions were observed only with the Sr- and Nd-doped silicate gels dried at 70˚C, whereas with the Cs- and Sr-doped gels, only free, non-coordinated nitrate ions were observed in- stead (ν3 NO3 at 1384 cm–1, v. strong and ν1 + ν3 3 N O at 2360 cm–1, v. weak), which is in accord with the ob- servations of Bogard et al. [19]. Free nitrate ions were observed with all samples heated at 450˚C and their tra- ces appeared even at 800˚C with Sr or Nd dopants present. It is difficult to explain the occurrence of a strong bond- ing mode of nitrates in the examined systems, because nitrates typically decompose at lower temperatures: e.g., Sr(NO3)2 at ≈ 640˚C; CsNO3 at ≈ 400˚C; Co(NO3)2 at ≈ 290˚C [16]. A possible explanation may come from the fact that all of the oxygen atoms of the NO3 groups were engaged in coordination sites. In all of the spectra, typical SiO2 vibrations bands (4000 cm–1 and 2510 cm–1) were observed: Si-O-Si symmetric bending at 455 - 470 cm–1, Si-O-Si symmetric stretching at 783 - 815 cm–1, and asymmetric stretching at 1074 - 1080 cm–1. The leaching data are difficult to compare directly with those of other studies, owing to variations of speci- men form and test conditions, but data from similar tests [20,27] indicate generally higher rates for the waste forms produced in this study. The Cs-doped glass drops and the Nd-doped (actinides surrogate) lightly sintered forms were reasonably resistant to leaching, within the analytical errors. The other forms, all porous, were clear- ly susceptible to leaching. In forms for which the dopants were more strongly bonded, the structure of silica matrix was also more resistant leaching (Table 1). Leaching tests consisted of weight-change measure- ments and analysis of leachates. Use of stirred room- temperature, deionized water will produce more rapid leaching than will, for example static, ground water tests [20], but will not allow for extrapolation to actual re- pository conditions and long duration [21-24]. It should allow for assessment of the applicability of the sol-gel glasses produced in this study as nuclear waste forms. Data from the studies are shown in Figure 5 and Table 1. The most obvious trends in the data in Figure 5 are that These studies yielded mixed results. Uniform products containing high concentrations of dopants were produced, but the products remained porous after prolonged sinter- ing at elevated temperatures. Such porous products are not suitable for use as nuclear waste forms. Future efforts will seek to fabricate denser forms. It is possible that col- d-pressing [1-5] or a tailored reaction-sintering step [6] 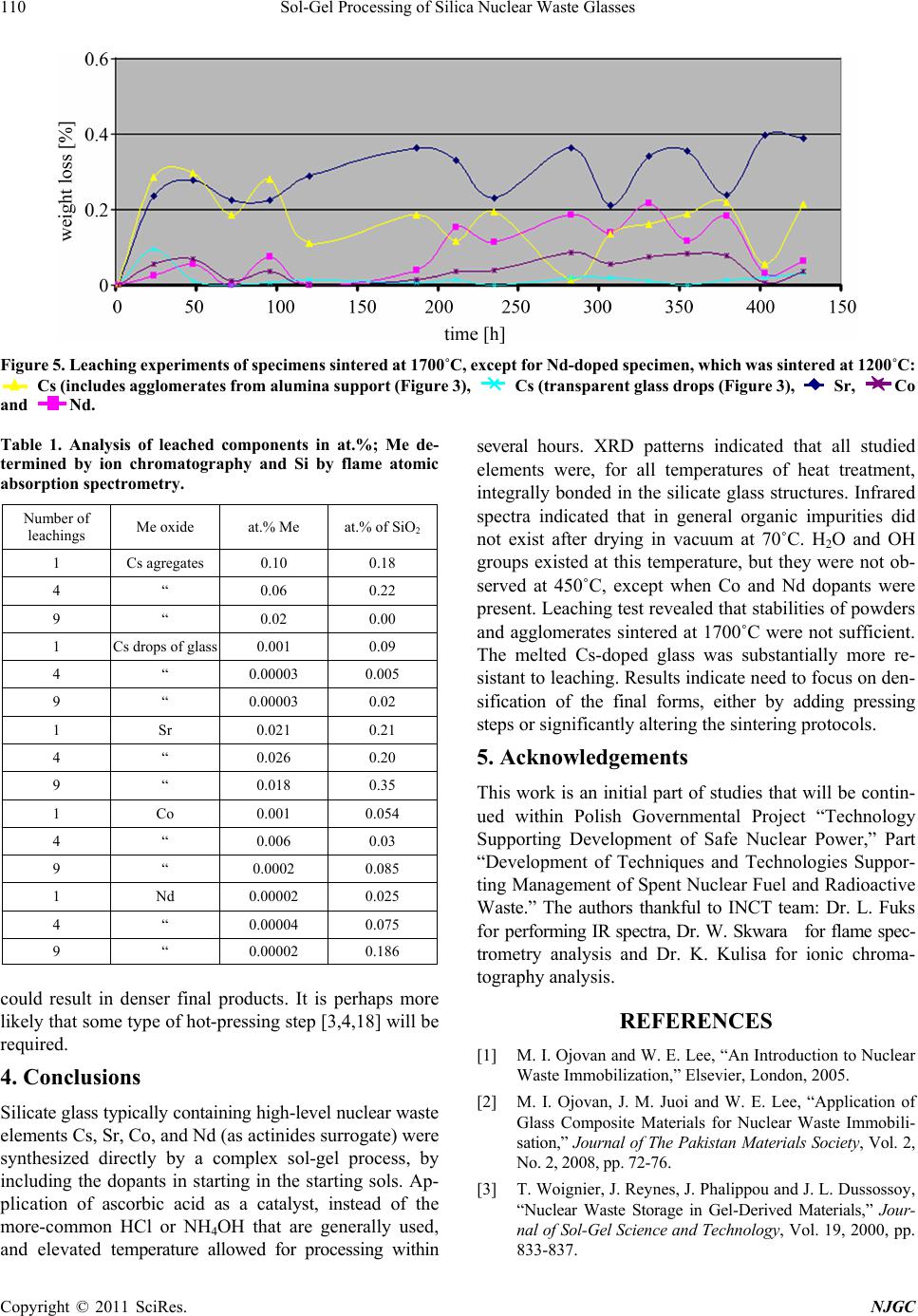 Sol-Gel Processing of Silica Nuclear Waste Glasses 110 Figure 5. Leaching experiments of specimens sintered at 1700˚C, except for Nd-doped specimen, which was sintered at 1200˚C: Cs (includes agglomerates from alumina support (Figure 3), Cs (transparent glass drops (Figure 3), Sr, Co and Nd. Table 1. Analysis of leached components in at.%; Me de- termined by ion chromatography and Si by flame atomic absorption spectrometry. Number of leachings Me oxide at.% Me at.% of SiO2 1 Cs agregates 0.10 0.18 4 “ 0.06 0.22 9 “ 0.02 0.00 1 Cs drops of glass 0.001 0.09 4 “ 0.00003 0.005 9 “ 0.00003 0.02 1 Sr 0.021 0.21 4 “ 0.026 0.20 9 “ 0.018 0.35 1 Co 0.001 0.054 4 “ 0.006 0.03 9 “ 0.0002 0.085 1 Nd 0.00002 0.025 4 “ 0.00004 0.075 9 “ 0.00002 0.186 could result in denser final products. It is perhaps more likely that some type of hot-pressing step [3,4,18] will be required. 4. Conclusions Silicate glass typically containing high-level nuclear waste elements Cs, Sr, Co, and Nd (as actinides surrogate) were synthesized directly by a complex sol-gel process, by including the dopants in starting in the starting sols. Ap- plication of ascorbic acid as a catalyst, instead of the more-common HCl or NH4OH that are generally used, and elevated temperature allowed for processing within several hours. XRD patterns indicated that all studied elements were, for all temperatures of heat treatment, integrally bonded in the silicate glass structures. Infrared spectra indicated that in general organic impurities did not exist after drying in vacuum at 70˚C. H2O and OH groups existed at this temperature, but they were not ob- served at 450˚C, except when Co and Nd dopants were present. Leaching test revealed that stabilities of powders and agglomerates sintered at 1700˚C were not sufficient. The melted Cs-doped glass was substantially more re- sistant to leaching. Results indicate need to focus on den- sification of the final forms, either by adding pressing steps or significantly altering the sintering protocols. 5. Acknowledgements This work is an initial part of studies that will be contin- ued within Polish Governmental Project “Technology Supporting Development of Safe Nuclear Power,” Part “Development of Techniques and Technologies Suppor- ting Management of Spent Nuclear Fuel and Radioactive Waste.” The authors thankful to INCT team: Dr. L. Fuks for performing IR spectra, Dr. W. Skwara for flame spec- trometry analysis and Dr. K. Kulisa for ionic chroma- tography analysis. REFERENCES [1] M. I. Ojovan and W. E. Lee, “An Introduction to Nuclear Waste Immobilization,” Elsevier, London, 2005. [2] M. I. Ojovan, J. M. Juoi and W. E. Lee, “Application of Glass Composite Materials for Nuclear Waste Immobili- sation,” Journal of The Pakistan Materials Societ y, Vol. 2, No. 2, 2008, pp. 72-76. [3] T. Woignier, J. Reynes, J. Phalippou and J. L. Dussossoy, “Nuclear Waste Storage in Gel-Derived Materials,” Jour- nal of Sol-Gel Science and Technology, Vol. 19, 2000, pp. 833-837. Copyright © 2011 SciRes. NJGC  Sol-Gel Processing of Silica Nuclear Waste Glasses111 [4] T. Woignier, J. Reynes, J. Phalippou and J. L. Dussossoy, “Sintered Silica Aerogel: A Host Matrix for Long Life Nuclear Wastes,” Journal of Non-Crystalline Solids, Vol. 225, 1998, pp. 353-357. doi:10.1016/S0022-3093(98)00052-0 [5] D. R. Clarke, “Ceramic Materials for the Immobilization of Nuclear Waste,” Annual Review of Materials Science, Vol. 13, 1983, pp. 191-218. doi:10.1146/annurev.ms.13.080183.001203 [6] W. L. Gong, W. Lutze and R. C. Ewing, “Reaction sin- tered glass: A Durable Matrix for Spinel-Forming Nu- clear Waste Compositions,” Journal of Nuclear Materials, Vol. 278, No. 1, 2000, pp. 73-84. doi:10.1016/S0022-3115(99)00226-3 [7] A. Deptula, W. Lada, T. Olczak, M. T. Lanagan, S. E. Dorris, K. C. Goretta and R. B. Poeppel, “Method for Preparing High-Temperature Superconductors,” Polish Pa- tent 172618, 1997. [8] A. Deptula, J. Chwastowska, W. Lada, T. Olczak, D. Wawszczak, E. Sterlinska, B. Sartowska and K. C. Gor- etta, “Sol-Gel-Derived Hydroxyapatite and Its Applica- tion to Sorption of Heavy Metals,” Advances in Science and Technology, Vol. 45, 2006, pp. 2198-2203. doi:10.4028/www.scientific.net/AST.45.2198 [9] A. Deptula, W. Lada, T. Olczak, D. Wawszczak, M. Bry- kala, F. Zaza and K. C. Goretta, “Novel Sol-Gel Synthe- sis of LiMn2O4 and LiNixCo1-xO2 Powders,” Advances in Science and Technology, Vol. 63, 2010, pp. 14-23. doi:10.4028/www.scientific.net/AST.63.14 [10] P. R. Haddad, “Comparison of Ion Chromatography and Capillary Electrophoresis for the Determination of Inor- ganic Ions,” Journal of Chromatography A, Vol. 770, No. 1-2, 1997, pp. 281-290. doi:10.1016/S0021-9673(96)01085-0 [11] J. A. Stone, “An Overview of Factors Affecting the Lea- chability of Nuclear Waste Forms,” Nuclear and Chemi- cal Waste Management, Vol. 2, No. 2, 1981, pp. 113-118. doi:10.1016/0191-815X(81)90026-7 [12] A. Barkatt, E. E. Saad, R. Adiga, W. Sousanpour, Al. Barkatt, M. A. Adel-Hadadi, J. A. O'Keefe and S. Al- terescu, “Leaching of Natural and Nuclear Waste Glasses in Sea Water,” Applied Geochemistry, Vol. 4, No. 6, 1989, pp. 593-603. doi:10.1016/0883-2927(89)90069-3 [13] W. L. Ebert, “Effects of the Glass Surface Area/Solution Volume Ratio on Glass Corrosion: A Critical Review,” ANL-94/34, Argonne National Laboratory, 1994. [14] J. R. Martinez, E. Espericueta and G. Ortega-Zarzosa, “Effect of Aging of Chlorophyll Species Embeded in Sil- ica Xerogels Matrix,” New Journal of Glass and Ceram- ics, Vol. 1, No. 1, 2011, pp. 7-12. [15] L. Werme, I. K. Björner, G. Bart, H. U. Zwicky, B. Gram- bow, W. Lutze, R. C. Ewing and C. Magrabi, “Chemical corrosion of highly radioactive borosilicate nuclear waste glass under simulated repository conditions,” Journal of Materials Research, Vol. 5, No. 5, 1990, pp. 1130-1146. doi:10.1557/JMR.1990.1130 [16] E. M. Rabinovich, “Preparation of Glass by Sintering,” Journal of Materials Science, Vol. 20, No. 12, 1985, pp. 4259-4297. doi:10.1007/BF00559317 [17] J. Phalippou, M. Prassas and J. Zarzycki, “Crystallization of Gels and Glasses Made from Hot-Pressed Gels,” Journal of Non-Crystalline Solids, Vol. 48, No. 1, 1982, pp. 17-30. doi:10.1016/0022-3093(82)90243-5 [18] M. Nishioka, S. Hirai, K. Yanagisawa and N. Yamasaki, “Solidification of Glass Powder with Simulated High- Level Radioactive Waste During Hydrothermal Hot-Pres- sing”, Journal of the American Ceramic Society, Vol. 73, No. 2, 1990, pp. 317-322. doi:10.1111/j.1151-2916.1990.tb06512.x [19] J. S. Bogard, S. A. Johnson, R. Kumar and P. T. Cun- ningham, “Quantitative Analysis of Nitrate Ion in Ambi- ent Aerosols by Fourier-Transform Infrared Spectroscopy,” Environmental Science and Technology, Vol. 16, No. 3, 1982, pp. 136-140. doi:10.1021/es00097a004 [20] A. N. Sharaf El-Deen, M. M. El-Dessouky, M. A. Helmy, M. Wagdy, A. Raouf and M. I. El-Dessouky, “Charac- terization and Leach Investigation of Sodium Silicate Matrices Used for Immobilization of Radioactive Waste,” in M. F. Barakat, Ed., Proceedings of Second Arab Con- ference on the Peaceful Uses of Atomic Energy, Cairo, 5-9 November 1994, pp. 375-391. [21] G. Malow, W. Lutze and R. C. Ewing, “Alteration Effects and Leach Rates of Basaltic Glasses: Implications for the Long-Term Stability of Nuclear Waste Form Borosilicate Glasses,” Journal of Non-Crystalline Solids, Vol. 67, 1984, pp. 305-321. doi:10.1016/0022-3093(84)90156-X [22] L. Werme, I. K. Björner, G. Bart, H. U. Zwicky, B. Gram- bow, W. Lutze, R. C. Ewing and C. Magrabi, “Chemical Corrosion of Highly Radioactive Borosilicate Nuclear Waste Glass Under Simulated Repository Conditions,” Journal of Materials Research, Vol. 5, No. 5, 1990, pp. 1130-1146. doi:10.1557/JMR.1990.1130 [23] G. Leturcq, G. Berger, T. Advocat and E. Vernaz, “Initial and Long-Term Dissolution Rates of Aluminosilicate Glasses Enriched with Ti, Zr and Nd,” Chemical Geology, Vol. 160, 1999, pp. 39-62. doi:10.1016/S0009-2541(99)00055-8 [24] J. Sterpenich and G. Libourel, “Using Stained Glass Win- dows to Understand the Durability of Toxic Waste Ma- trices,” Chemical Geology, Vol. 174, 2001, pp. 181-193. doi:10.1016/S0009-2541(00)00315-6 [25] M. I. Ojovan, R. J. Hand, N. V. Ojovan and W. E. Lee, “Corrosion of Alkali—Borosilicate Waste Glass K-26 in Non-Saturated Conditions,” Journal of Nuclear Materials, Vol. 340, 2005, pp. 12-24. doi:10.1016/j.jnucmat.2004.10.095 [26] W. L. Gong, L. M. Wang, R. C. Ewing, E. Vernaz, J. K. Bates and W. L. Ebert, “Analytical Electron Microscopy Study of Surface Layers Formed on the French SON68 Nuclear Waste Glass during Vapor Hydration at 200˚C,” Journal of Nuclear Materials, Vol. 254, 1998, pp. 249-265. doi:10.1016/S0022-3115(97)00349-8 [27] P. R. Aravind, L. Sithara, P. Mukundan, P. Krishna Pillai and K. G. K. Warrier, “Silica Alcogels for Possible Nu- clear Waste Confinement—A Simulated Study,” Materi- als Letters, Vol. 61, No. 11-12, 2007, pp. 2398-2401. doi:10.1016/j.matlet.2006.09.022 Copyright © 2011 SciRes. NJGC |

