 Pharmacology & Pharmacy, 2011, 2, 322-331 doi:10.4236/pp.2011.24041 Published Online October 2011 (http://www.SciRP.org/journal/pp) Copyright © 2011 SciRes. PP Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. A Possible Molecular Target? David Crouzier1, Jean-Claude Debouzy1, Florian Nachon2, Dominique Debouzy3, Guy Lallement4, Frédéric Canini5 1Effets Biologiques des Rayonnements, IRBA/CRSSA, La Tronche, France; 2Unité d’Enzymologie IRBA/CRSSA, La Tronche, France; 3Université Pierre Mendes France, St. Martin d’Hères, France; 4Département de Toxicologie, IRBA/CRSSA, La Tronche, France; 5Unité de Neurophysiologie du Stress, IRBA/CRSSA, La Tronche, France. Email: david.crouzier@wanadoo.fr Received August 17th, 2011; revised September 13th, 2011; accepted September 28th, 2011. ABSTRACT Metyrapone (2-methyl-1,2-di-3-pyridyl-1-propanone) is a drug largely used as inhibitor of glucocorticoid synthesis. Although its binding to various proteins has been well indentified, its accurate molecular mechanism of action remains unknown. Therefore, the interactions of metyrapone (MET) with various membrane components such as phospholipids, cholesterol, their corresponding polar heads and a model serine containing peptide have been investigated by NMR and ESR methods. It was found that neither cholesterol nor most of the phospholipids tested, nor dimyristin exhibit any in- teraction with MET, except phosphatidylserine (DMPS). Furthermore, only serine bearing polar head (O-phosphoser- ine) showed an association with MET (stoechiometry 1:1, Kd = 3200 M-1). As similar observations were also performed on serine alone and in the presence of the serine containing model peptide, (NASDSDGQDL), a possible implication of these interactions in the binding recognition of MET on serine-containing active site was finally tested and discussed. Keywords: Metyrapone, Membranes, Serine, NMR, ESR, Interactions 1. Introduction In the clinical field, metyrapone (2-methyl-1,2-di-3-pyri- dyl-1-propanone MET), an inhibitor of glucocorticoid synthesis [1], is widely used to treat the Cushing disease [2,3]. Since high blood corticosterone levels have been linked to a depressive mood [4], metyrapone is also used to treat Cushing related mental disturbances [5] and ma- jor depression [6-9]. However, metyrapone exhibits other properties highlighted by preclinical studies that can not be explained by its action on the corticotrope axis. At the physiological level, metyrapone injection is followed by a slight hypothermia [10] not reversed by glucocorticoid supplementation [11], a decreased locomotion [12], an enhanced arousal [13] together with biological signs of stress [14]. The latter does not mask the protective effect of metyrapone against psychological stressors [15,16] and physiological stressors such as brain ischemia [17,18] and excitotoxicity [19]. At the cell level, metyrapone modify the energy metabolism [10] with a decreased use of glucose [20-21] and alters the mitochondrial function- ing [22]. Lastly, metyrapone also modify the detoxifica- tion as it modifies the expression of numerous cyto- chrome P450 such as CYP1A1 and CYP 3A [23]. Besides these integrated levels, the molecular support, i.e. the identification or the molecular target, still failed. Metyrapone profoundly modify the steroid metabolism through its inhibitory activity on the 11β hydroxylase (CYP11B1), [1], 17β hydroxysteroid deshydrogenase (17β-HSD) [24] and the 11 β-HSD [25]. Metyrapone also inhibits P450 [26] in numerous species [27]. Its inhibi- tory effect is not specific as it targets the CYP11B1 [28] and the CYP3A [29], but also other enzymes such as lipooxygenase [30], guanylate cyclase [31] or nitric ox- ide synthetase as a member of cytochrome P450-like hemoprotein [32]. Some works have focused on the mechanisms by which metyrapone interacts with P450. Its fixation on P450 is independent from the redox state of the enzyme and th e availability of oxygen [33,34] and does not modify the conformation of the protein P450A3 [35,36]. Another mechanisms sugg ested was the possible 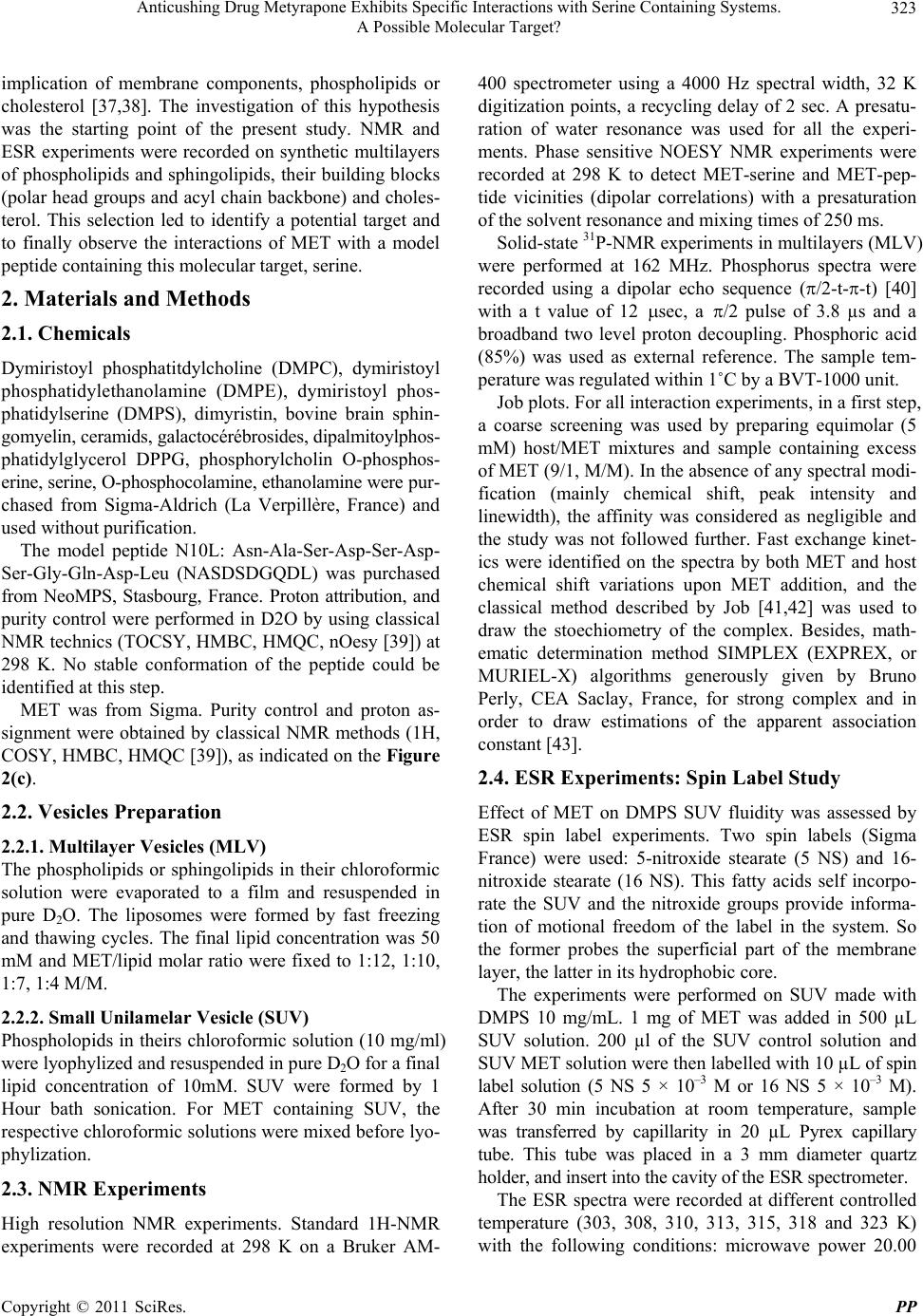 Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. 323 A Possible Molecular Target? implication of membrane components, phospholipids or cholesterol [37,38]. The investigation of this hypothesis was the starting point of the present study. NMR and ESR experiments were recorded on synthetic multilayers of phospholipids and sphingolipids, their building blocks (polar head groups and acyl chain backbone) and choles- terol. This selection led to identify a potential target and to finally observe the interactions of MET with a model peptide containin g this molecular target, serine. 2. Materials and Methods 2.1. Chemicals Dymiristoyl phosphatitdylcholine (DMPC), dymiristoyl phosphatidylethanolamine (DMPE), dymiristoyl phos- phatidylserine (DMPS), dimyristin, bovine brain sphin- gomyelin, ceramids, galactocérébrosides, dipalmitoylphos- phatidylglycerol DPPG, phosphorylcholin O-phosphos- erine, serine, O-phosphocolamine, ethanolamine were pu r - chased from Sigma-Aldrich (La Verpillère, France) and used without purification. The model peptide N10L: Asn-Ala-Ser-Asp-Ser-Asp- Ser-Gly-Gln-Asp-Leu (NASDSDGQDL) was purchased from NeoMPS, Stasbourg, France. Proton attribution, and purity control were performed in D2O by using classical NMR technics (TOCSY, HMBC, HMQC, nOesy [39]) at 298 K. No stable conformation of the peptide could be identified at this step. MET was from Sigma. Purity control and proton as- signment were obtained by classical NMR methods (1H, COSY, HMBC, HMQC [39]), as indicated on the Figure 2(c). 2.2. Vesicles Preparation 2.2.1. Multilayer Vesicles (MLV) The phospholipids or sphingolipids in their chloroformic solution were evaporated to a film and resuspended in pure D2O. The liposomes were formed by fast freezing and thawing cycles. The final lipid concentration was 50 mM and MET/lipid molar ratio were fixed to 1:12, 1:10, 1:7, 1:4 M/M. 2.2.2. Small Unilamelar Vesicle (SUV) Phospholopids in theirs chloroformic solution (10 mg/ml) were lyophylized and resuspended in pure D2O for a final lipid concentration of 10mM. SUV were formed by 1 Hour bath sonication. For MET containing SUV, the respective chloroformic solutions were mixed before lyo- phylization. 2.3. NMR Experiments High resolution NMR experiments. Standard 1H-NMR experiments were recorded at 298 K on a Bruker AM- 400 spectrometer using a 4000 Hz spectral width, 32 K digitization points, a recycling delay o f 2 sec. A presatu- ration of water resonance was used for all the experi- ments. Phase sensitive NOESY NMR experiments were recorded at 298 K to detect MET-serine and MET-pep- tide vicinities (dipolar correlations) with a presaturation of the solvent resonance and mixing times of 250 ms. Solid-state 31P-NMR experiments in multilayers (MLV) were performed at 162 MHz. Phosphorus spectra were recorded using a dipolar echo sequence (/2-t--t) [40] with a t value of 12 sec, a /2 pulse of 3.8 µs and a broadband two level proton decoupling. Phosphoric acid (85%) was used as external reference. The sample tem- perature was regulated within 1˚C by a BVT-1000 unit. Job plots. For all interaction experiments, in a first step, a coarse screening was used by preparing equimolar (5 mM) host/MET mixtures and sample containing excess of MET (9/1, M/M). In the absence of any spectral modi- fication (mainly chemical shift, peak intensity and linewidth), the affinity was considered as negligible and the study was not followed further. Fast exchange kinet- ics were identified on the spectra by both MET and host chemical shift variations upon MET addition, and the classical method described by Job [41,42] was used to draw the stoechiometry of the complex. Besides, math- ematic determination method SIMPLEX (EXPREX, or MURIEL-X) algorithms generously given by Bruno Perly, CEA Saclay, France, for strong complex and in order to draw estimations of the apparent association constant [43]. 2.4. ESR Experiments: Spin Label Study Effect of MET on DMPS SUV fluidity was assessed by ESR spin label experiments. Two spin labels (Sigma France) were used: 5-nitroxide stearate (5 NS) and 16- nitroxide stearate (16 NS). This fatty acids self incorpo- rate the SUV and the nitroxide groups provide informa- tion of motional freedom of the label in the system. So the former probes the superficial part of the membrane layer, the latter in its hydrophob ic core. The experiments were performed on SUV made with DMPS 10 mg/mL. 1 mg of MET was added in 500 µL SUV solution. 200 µl of the SUV control solution and SUV MET solution were then labelled with 10 µL of spin label solution (5 NS 5 × 10–3 M or 16 NS 5 × 10–3 M). After 30 min incubation at room temperature, sample was transferred by capillarity in 20 µL Pyrex capillary tube. This tube was placed in a 3 mm diameter quartz holder, and insert into the cavity of t he ESR spectrometer. The ESR spectra were recorded at different controlled temperature (303, 308, 310, 313, 315, 318 and 323 K) with the following conditions: microwave power 20.00 Copyright © 2011 SciRes. PP  Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. 324 A Possible Molecular Target? mW, modulation frequency 100 kHz, modulation ampli- tude 2.05 G, receiver gain 6.3 × 105 conversion time 81.92 ms, time constant 81.92 ms. Sweep range was 100 G with a central field value of 3435 G for 5 NS probe, and in the same condition except, modulation amplitude 1.03 G, receiver gain 105 conversion time 40.96 ms, time constant 81.9 2 ms for 16 NS probe. The complete membrane incorporation of the spin la- bels was ascertained by the absence on the spectra of the extremely resolved ESR lines corresponding to free ro- tating markers. 5 NS experimentations: The values of outer and inner hyperfine splitting were measured (2T// and 2T respect- tively), on ESR spectra (Figure 3(b)), and order parame- ter S was calculated following the equation [44]: // // TTC S1.723 T2TC with // C1.4 0.053TT The increase in the order parameter v alue means a de- crease of local membrane fluidity. 16 NS experimentations: The changes in freedom mo- tion of 16 NS were analysed with the calculation of c , the rotational correlation time. c was calculated fol- lowing the formula [45]: 0o1 TcKWh h1 with 10 1 K6.510sG In this formula, W0 is the peak-to-peak line width of the central line; h0 an d h–1 are the peak height of the cen- tral and high-field lines respectively (Figure 3(d)). The increase in the rotational correlation time means a decrease of local membrane fluidity. 3. Results 3.1. 31P-NMR Experiments in MLV A first attempt for MET affinity and interaction screen- ing was performed by using phospholipid dispersions as membrane model. This model allows to observe the structural and dynamics consequences of the presence of MET at the polar head (31P-NMR) by recording NMR spectra under different temperature conditions. Thus the dependence of the interactions with MET following the nature of the polar head group was tested by using dif- ferent phospholipids, i.e. DMPC, sphingomyeline, DMPE, DMPS, DPPG, and also galactocerebrosides and cera- mides. At this point, it is noteworthy to recall several basic principles about 31P-NMR in membranes. 31P-NMR chemical shift (the resonance frequency) depends on the orientation of phosphorus nuclei in the field (shielding). The chemical shift difference between the low field and the hi ghfield edges of a 31P-NMR spec- trum is called Chemical Shift Anisotropy (CSA, ppm) and is directly related to the fluidity—reorientation—at the polar head level where the phosphorus nuclei are lo- cated. On such spectra a mobile phosphorus group gives a single narrow resonance (several Hz) as detected in true solutions or for small structures (micelles), while phos- phorus groups in solid state gives extremely broad con- tributions (CSA values exceeding 100 ppm). Note that membrane fluidity increases (and CSA decreases) with temperature, with a special jump at the transition tem- perature between gel phase and liquid crystal structure (e.g. around 297 K for DMPC [46]). Thus the plot of CSA as a function of temperature provides a good overview of mem- brane dynamics at the polar head level where phosphorus nuclei are located, while the lineshape allows identifying the overall membrane organization (bilayer, hexagonal, isotropic phases). Such plots are presented on the traces of the Figure 1 (top traces). The bottom spectrum (Figure 1, column A) shows the spectrum of pure DMPC dispersion (MLV), typical of an axia11y symmetric powd er pattern, with a chemical shift anisotropy of 65 ppm, classical of a phospholipid (here DMPC) bilayers in their liquid crystalline phase around phase transition (297 K) [47]. As expected for pure DMPC dispersions a CSA de- crease (around 18 - 20 ppm) was me asured on pure DM PC systems by increasing the temperature (and the mem- brane fluidity) with the transition-related jump around 297 K [48]. This was also the case for MET containing MLV (MET/DMPC ratios of 1/10 and up to 1/4 M/M, middle and top traces, column A). Also temperature de- pendence of CSA values was found almost surperim- posed on the entire temperature range Figure 1(c)). Be- sides, no significant isotropic contribution typical of de- tergent effect was observed, thus finally indicating the absence of any interaction with the membrane. Similarly, no interaction with MET was found for cholesterol (up to 20%) containing DMPC dispersions and also for galac- tocerebrosides, ceramides, and DPPG MLV (not shown). Besides, when DMPE systems were used, only a limited increase in the fluidity (i.e. a limited CSA reduction) was observed all over the temperature range, never exceeding 2ppm, even for high MET/DMPE ratios of 1/4. Such was not the case for the spectra recorded under the same conditions on DMPS (Figures 1(b)-(d)) disper- sions in the presence of MET. As expected, pure DMPS dispersions gave typical spectra of bilayer structures (see Copyright © 2011 SciRes. PP  Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. 325 A Possible Molecular Target? Figure 1(b) bottom), with a decrease of the CSA with temperature. The same spectra recorded on MET con- taining DMPS systems resulted in a reduction of the CSA for MET/DMPS molar ratios exceeding R = 1/10 M/M (trace of the Figure 1(d)), while an isotropic contribution appeared for higher R values, with a relative contribution (a) (b) (c) (d) Figure 1. 31P-NMR (a) spectra of DMPC dispersion (50 mM, D2O) at 297 K, pure (bottom trace) and in the presence of MET, molar ratios of R = 1/10 and 1/4, M/M, (middle and top traces, respectively); horizontal bracket represent the CSA; (b) spectra of DMPS dispersion (50 mM, D2O) at 313 K, pure (bottom trace) and in the presence of MET, molar ratios of 1/10 and 1/4, M/M, (middle and top traces, respect- tively; (c) plot of temperature dependence CSA measured on DMPC dispersion spectra of DMPC (○), and in the presence of MET, R = 1/4 M/M (■); (d) plot of temperature dependence CSA measured on DMPS dispersion spectra of DMPS (●), and in the presence of MET, R = 1/10 M/M (□), and R = 1/7 M/M (■); bottom trace (♦) represents the line width of the isotropic contribution observed at R = 1/4 M/M. being developed at the expense of the main structure (Figure 1(b) middle and top traces). Finally, a single line of less than 50 Hz with was exclusively detected for R = 1/4, indicating that the native structure had been com- pletely replaced by very mobile systems of high fluidity, consistent with a detergent effect or micelle formation. At this step, the existence of MET/DMPS bilayers ap- pears as highly probable. In order to obtain more preci- sions, the different building blocks of the phospho- or sphingolipids were considered separately, i.e. the polar headgroups and the diacylglycerol backbone. These ex- periments are the topic of the next section. 3.2. 1H-NMR and ESR of Polar Headgroups and Chains Headgroups. As both polar groups and MET are soluble in D2O, the first step was achieved by recording spectra on equimolar mixtures and compared to those of pure species of O-phosphocholine (OPC), O-phosphoserin (OPS), O-phosphocolamine(OPCO), and sphingosine. A special care was paid to the aromatic resonances of MET, clearly isolated in the 7 - 9 ppm region (see Figure 2(a) for MET structure and nomenclature, bottom trace for the corresponding s pe ctrum of MET). In the case of OPC, and sphingosine, as no detectable spectrum variation was observed both in chemical shift and linewidth even MET was p resent in excess (9 /1 M/M ratios MET/headgroup) it was assumed that no interact- tion occurred and the investigation was not studied fur- ther. OPCO-MET systems only provided minor chemical shift variations that did not allow to propose a precise mode of interaction, stoechiometry or affinity constant (attempts made using Benesi-Hidebrandt method only showed it should not excess 30 M–1). The affinity of MET for OPCO was thus considered as very weak. As significant chemical shift variations were observed on both OPS and MET resonances upon addition of OPS, the continuous variations method was used by recording spectra of different molar ratios MET/OPS, keeping total concentration constant (5 mM, see examples of such spectra on Figure 2). This allowed us to use the method to characterize these interactions, as classically described by Job [41]. In such plots, the curves built using concentration weighted chemical shift variations as a function of the molar fraction show a maximum for the molar fraction corresponding to the stoechiometry, here 0.5, which means a 1/1 association (2B). This also allowed the use of numerical simulations (EXPREX2 [43]) that gave an apparent association constant of 3200 M–1. In order to detect spatial vicinities between MET and Copyright © 2011 SciRes. PP  Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. 326 A Possible Molecular Target? (a) (b) (c) Figure 2. (a) Metyrapone (MET) formula and proton no- menclature (b) Job-Plots built from concentration-weighted chemical shift variations (mM*Hz) as a function of molar fraction (F), for H5 (7.356 ppm, ■), H4 (7.82 ppm, ●), H16 (7.47 ppm, ○) protons of MET or H2” proton of phosphos- erine (4.19 ppm, ▲), 298 K D2O. The total concentrations were kept at 5 mM. Bottom traces with proton numbering below (from bottom to top): aromatic part of the 1H-NMR spectra of MET (bottom trace), and in the presence of O-phosphoserine (corresponding molar ratios MET/OPS of 1:0, 9/1; 3/2 and 1/1, respectively. OPS, nOesy experiments (mixing time of 250 ms) were then recorded on the 1/1 mixture. Hence weak dipolar correlations were found between one of methylenic pro- tons (4.22 ppm) of OPS and H16, H15 and H13 protons of MET, and also between CHα, (4.15 ppm) and H15 not shown). However, molecular dynamics simulations could not lead to propose any stable conformation. Diacylglycerol backbone. In order to observe chains interaction without any polar head contribution, disper- sions of dimyristin and dipalmitin were prepared as for phospholipid MLV. As the 1H spectra recorded on the dispersions containing MET (R = 1/1 M/M) were the single sum of the spectra of pure dispersions and MET recorded separately under the same conditions, it was concluded that no interaction occurred at the molecular level (not shown). 3.3. ESR Experiments Spin label experiments were then realised to investigate the membrane fluidity in different temperature conditions at the chain level. Two probes were separately used, 5 NS gate information about superficial membrane fluidity, while 16 NS concerned the inner membrane region. The overall result (Figures 3(a) and (c)) shows an increase in the mobility of the two probes contribution in the two groups with the temperature increase. As previously de- scribed in SUV DMPS model [47,49], the phase transi- tion in control groups occurs near 313 K. The ESR results were found in full agreement with the experiments recorded by NMR: hence, no interaction with phospholipids and sphingolipids except DMPS was found. In this latter case, an increase in the mobility of the two probes contribution was found when MET was added to DMPS solution. 5 NS results are drawn Figure 3(a). An overhall in- crease of the membrane fluidity and a shift of the phase transition, 5 degrees lower, could be observed in the MET group. In the inner compartment, 16NS results (Figure 3(c)) show a major effect of MET with a total vanishing of phase transition and a strong increase of the membrane between 303 and 313 K. At the highest temperature (above 315 K) the rotational correlation time of the con- trol group reached the same value as MET group. However, a true interaction between free serine and MET was very probable. Under a biological point of view, it was important to know if MET could also have interactions with a serine group engaged in a peptide structure. This was tested by using a reference decapep- tide NASDSDGQDL as target. 3.4. Interactions with the Model Peptide NASDSDGQDL After purity control and attribution of peptide protons using classical NMR methods [50], the same method as before was used on equimolar mixtures of MET/peptide. By comparison with the spectrum of pure MET (Figure 4(a)) significant chemical shift variations were observed on the MET/peptide sample, as shown on Figure 4(a), similar as with OPS/MET reparations. Moreover, the p Copyright © 2011 SciRes. PP  Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. A Possible Molecular Target? Copyright © 2011 SciRes. PP 327 2T H 0 2T // H 1 W 0 Figure 3. ESR spin labeling experiment: (a) temperature dependence of the order parameter (5 NS) for pure DMPS (black diamond full line) and in the presence of MET 2 mg/ml (black square, dashed line); (b) typical 5 NS spectrum parameter used for order parameter estimation are inner (2T) and outer hyperfine (2T//) splitting; (c) temperature dependence of the rotational correlation time (16 NS) for pure DMPC and in the presence of MET 2 mg/ml; (d) typical 16 NS spectrum, pa- rameter used for rotational correlation time was central peak intensity H0, High field peak intensity and the width of the mid-field line W0. nOesy experiment then recorded also showed weak di- polar connectivities, mainly between H15, H6 and sev- eral peptide protons, suggesting vicinities in the 4 - 6 Å. However, whereas MET well exhibited some vicinities with serine groups, others of more strong intensity were also present with Gly and Gln aminoacids. 4. Discussion The present work primarily investigated the interactions of MET with membrane components, phospholipids, sphingolipids or cholesterol, as evoqued in the literature [51]. The use of multibilayers (dispersions prepared by the freezing-thawing method [52]) combined with solid- state 31P-NMR spectroscopy allows to investigate both dynamics and structural collective properties of the bi- layers. Such a method allows to easily obtain a screening the specificity of interactions with a given phospholipid system. In the present case, as preliminary tests performed 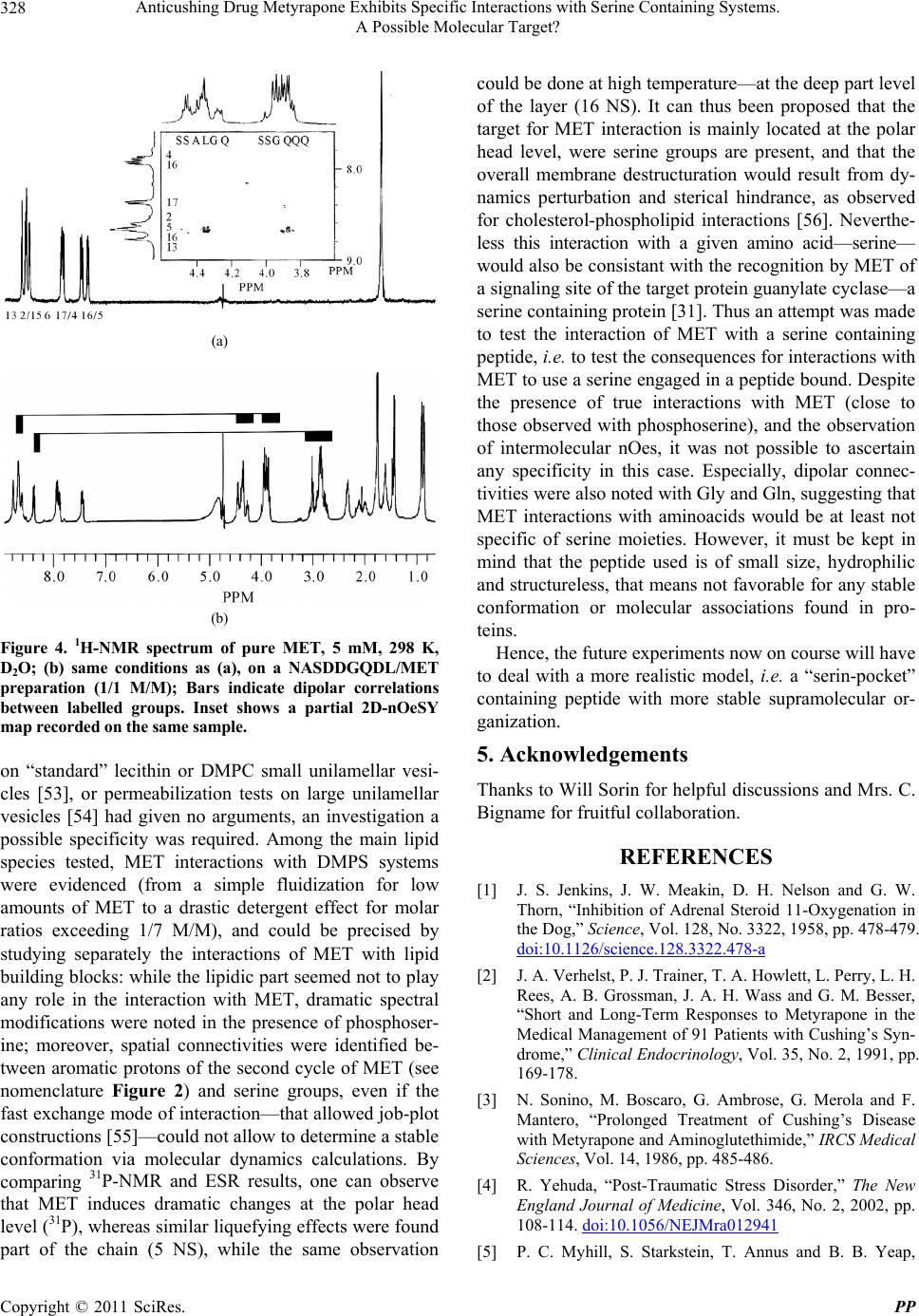 Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. 328 A Possible Molecular Target? (a) (b) Figure 4. 1H-NMR spectrum of pure MET, 5 mM, 298 K, D2O; (b) same conditions as (a), on a NASDDGQDL/MET preparation (1/1 M/M); Bars indicate dipolar correlations between labelled groups. Inset shows a partial 2D-nOeSY map recorded on the same sample. on “standard” lecithin or DMPC small unilamellar vesi- cles [53], or permeabilization tests on large unilamellar vesicles [54] had given no arguments, an investigation a possible specificity was required. Among the main lipid species tested, MET interactions with DMPS systems were evidenced (from a simple fluidization for low amounts of MET to a drastic detergent effect for molar ratios exceeding 1/7 M/M), and could be precised by studying separately the interactions of MET with lipid building blocks: while the lipidic p art seemed not to play any role in the interaction with MET, dramatic spectral modifications were noted in the presence of phosphoser- ine; moreover, spatial connectivities were identified be- tween aromatic protons of the second cycle of MET (see nomenclature Figure 2) and serine groups, even if the fast exchange mode of interaction—that allowed job-plot constructions [55]—cou ld not allow to determine a stab le conformation via molecular dynamics calculations. By comparing 31P-NMR and ESR results, one can observe that MET induces dramatic changes at the polar head level (31P), whereas similar liquefying effects were found part of the chain (5 NS), while the same observation could be done at high temperature—at the deep part level of the layer (16 NS). It can thus been proposed that the target for MET interaction is mainly located at the polar head level, were serine groups are present, and that the overall membrane destructuration would result from dy- namics perturbation and sterical hindrance, as observed for cholesterol-phospholipid interactions [56]. Neverthe- less this interaction with a given amino acid—serine— would also be consistant with the recognition by MET of a signaling site of the target protein guanylate cyclase—a serine containing protein [31]. Thus an attempt was made to test the interaction of MET with a serine containing peptide, i.e. to test the conseque nces for interactions with MET to use a serine engaged in a peptide bound. Despite the presence of true interactions with MET (close to those observed with phosphoserine), and the observation of intermolecular nOes, it was not possible to ascertain any specificity in this case. Especially, dipolar connec- tivities were also noted with Gly and Gln, suggesting that MET interactions with aminoacids would be at least not specific of serine moieties. However, it must be kept in mind that the peptide used is of small size, hydrophilic and structureless, that means not favorable for any stable conformation or molecular associations found in pro- teins. Hence, the future experiments now on course will have to deal with a more realistic model, i.e. a “serin-pocket” containing peptide with more stable supramolecular or- ganization. 5. Acknowledgements Thanks to Will Sorin for helpful discu ssions and Mrs. C. Bigname for fruitful collaboration. REFERENCES [1] J. S. Jenkins, J. W. Meakin, D. H. Nelson and G. W. Thorn, “Inhibition of Adrenal Steroid 11-Oxygenation in the Dog,” Science, Vol. 128, No. 3322, 1958, pp. 478-479. doi:10.1126/science.128.3322.478-a [2] J. A. Verhelst, P. J. Trainer, T. A. Howlett, L. Perry, L. H. Rees, A. B. Grossman, J. A. H. Wass and G. M. Besser, “Short and Long-Term Responses to Metyrapone in the Medical Management of 91 Patients with Cushing’s Syn- drome,” Clinical Endocrinology, Vol. 35, No. 2, 1991, pp. 169-178. [3] N. Sonino, M. Boscaro, G. Ambrose, G. Merola and F. Mantero, “Prolonged Treatment of Cushing’s Disease with Metyrapone and Aminoglutethimide,” IRCS Medical Sciences, Vol. 14, 1986, pp. 485-486. [4] R. Yehuda, “Post-Traumatic Stress Disorder,” The New England Journal of Medicine, Vol. 346, No. 2, 2002, pp. 108-114. doi:10.1056/NEJMra012941 [5] P. C. Myhill, S. Starkstein, T. Annus and B. B. Yeap, Copyright © 2011 SciRes. PP  Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. 329 A Possible Molecular Target? “Reduction in Salivary Cortisol Concentration Correlates with Resolution of Psychosis in Cushing’s Syndrome,” Journal of Neuropsychiatry and Clinical Neuroscience, Vol. 20, No. 1, 2008, pp. 113-115. doi:10.1176/appi.neuropsych.20.1.113 [6] B. E. P. Murphy, V. Dhar, A. M. Ghadirian, G. Choui- nard and R. Keller, “Response to Steroid Suppression in Major Depression Resistant to Antidepressant Therapy,” Journal of Clinical Psychopharmacology, Vol. 11, No. 2, 1991, pp. 121-126. [7] Z. Rogoz, G. Skuza, J. Wójcikowski, W. A. Daniel, A. Wróbel, D. Dudek and A. Zieba, “Effect of Metyrapone Supplementation on Imipramine Therapy in Patients with Treatment Resistant Unipolar Depression,” Polish Jour- nal of Pharmacology, Vol. 56, No. 6, 2004, pp. 849-855. [8] O. M. Wolkowitz and V. I. Reus, “Treatment of Depres- sion with Antiglucocorticoid Drugs,” Psychosomatic Medi- cine, Vol. 61, No. 5, 1999, pp. 698-711. [9] B. E. P. Murphy, “Steroid and Depression,” Journal of Steroid Biochemistry and Molecular Biology, Vol. 38, No. 5, 1991, pp. 537-559. doi:10.1016/0960-0760(91)90312-S [10] R. Werner, “Effect of Metopirone-Ditartrate on Ther- mogenesis in the Guinea-Pig,” Comparative Biochemistry and Physiology Part C, Vol. 90, No. 2, 1988, pp. 445-450. doi:10.1016/0742-8413(88)90025-4 [11] J.-B. Drouet, V. Michel, A. Peinnequin, A. Alonso, N. Fi- dier, R. Maury, A. Buguet, R. Cespuglio and F. Canini, “Metyrapone Blunts Stress-Induced Hyperthermia and Increased Locomotor Activity Independently of Gluco- corticoids and Neurosteroids,” Psychoneuroendocrinology, Vol. 35, No. 9, 2010, pp. 1299-1310. doi:10.1016/j.psyneuen.2010.03.001 [12] F. Canini, S. Brahimia, J.-B. Drouet, V. Michel, A. Alonso, A. Buguet and R. Cespuglio, “Metyrapone Decreases Lo- comotion Acutely,” Neuroscience Letters, Vol. 457, No. 1, 2009, pp. 41-44. doi:10.1016/j.neulet.2009.03.103 [13] J.-B. Drouet, C. Rousset, R. Maury, V. Michel, A. Buguet, R. Cespuglio and F. Canini, “Single Administration of Metyrapone Modifies Sleep-Wake Patterns in the Rat,” European Journal of Pharmacology, Vol. 652 No. 1-3, 2010, pp. 60-64. doi:10.1016/j.ejphar.2010.10.071 [14] D. Rottlant, S. Ons, J. Carrasco and A. Armario, “Evi- dence That Metyrapone Can Act as a stressor: Effect on Pituitary-Adrenal Hormones, Plasma Glucose and Brain C-Fos Induction,” European Journal of Neuroscience, Vol. 16, No. 4, 2002, pp. 693-700. doi:10.1046/j.1460-9568.2002.02120.x [15] M. Baez, I. Siriczman and M. Volosin, “Corticosterone Is Involved in Foot Shock-In duced Inactivity in Rats,” Physi- ology and Behavior, Vol. 60, No. 3, 1996, pp. 795-801. doi:10.1016/0031-9384(96)00025-X [16] N. Calvo, I. D. Martijena, V. A. Molina and M. Volosin, “Metyrapone Pretreatment Prevents the Behavioral and Neurochemical Sequelae Induced by Stress,” Brain Re- search, Vol. 800, No. 2, 1998, pp. 227-235. doi:10.1016/S0006-8993(98)00515-0 [17] H. J. Krugers, S. Maslam, J. Korf and M. Joëls, “The Corticosterone Synthesis Inhibitor Metyrapone Prevents Hypoxia/Ischemia-Induced Loss of Synaptic Function in the Rat Hippocampus,” Stroke, Vol. 31, No. 5, 2000, pp. 1162-1172. doi:10.1161/01.STR.31.5.1162 [18] V. L. Smith-Swintosky, L. C. Pettigrew, R. M. Sapolsky, C. Phares, S. D. Craddock, S. M. Brooke and M. P. Mattson, “Metyrapone, an Inhibitor of Glucocorticoid Production, Reduces Brain Injury Induced by Focal and Global Ischemia and Seizures,” Journal of Cerebral Blood Flow and Metabolism, Vol. 16, No. 4, 1996, pp. 585-598. doi:10.1097/00004647-199607000-00008 [19] B. A. Stein and R. M. Sapolsky, “Chemical Adre nalectomy Reduces Hippocampal Damage Induced by Kainic Acid,” Brain Research, Vol. 473, No. 1, 1988, pp. 175-180. doi:10.1016/0006-8993(88)90332-0 [20] O. D. Bruno, P. Metzger and W. J. Malaisse, “Inhibitory Effect of Metyrapone on Glucose Utilization by Brain and Muscle and on Insulin Release by the Pancreas,” Acta Endocrinologica, Vol. 70, No. 4, 1972, pp. 710-718. [21] G.-F. Kahl and K. J. Netter, “The Effect of Metyrapone on Cellular Respiration and Microsomal Drug Oxida- tion,” Biochemical Pharmacology, Vol. 19, No. 1, 1970, pp. 27-34. doi:10.1016/0006-2952(70)90326-6 [22] S. M. Blättner, F. Rencurel, M. R. Kaufman and U. A. Meyer, “In the Regulation of Cytochrome P450 Genes, Phenobarbital Targets LKB1 for Necessary Activation of AMP-Activated Protein Kinase,” Proceeding of the Na- tional Academy of Science of the United States of Amer- ica, Vol. 104, No. 3, 2007, pp. 1045-1050. [23] J. L. Harvey, A. J. Paine, P. Maurel and M. C. Wright, “Effect of the Adrenal 11-B-Hydroxylase Inhibitor Mety- rapone on Human Hepatic Cytochrome P-450 Expression: Induction of Cytochrome P-450 3A4,” Drug Metabolism and Disposition, Vol. 28, No. 1, 2000, pp. 96-101. [24] C. Parthasarathy, S. Yuvaraj, R. Sivukumar, B. Ravi San- kar and K. Balasubramanian, “Metyrapone-Induced Corti- costerone Deficiency Impairs Glucose Oxidation and Ster- oidogenesis in Leydig Cells of Adult Albino Rats,” Endo- crine Journal, Vol. 49, No. 4, 2002, pp. 405-412. doi:10.1507/endocrj.49.405 [25] G. Bannenberg, H.-J. Martin, I. Bélai and E. Maser, “11- B-Hydroxysteroid Deshydrogenase Type 1: Tissue-Specific Expression and Reductive Meta b olism of Some Anti-Insect Agent Azole Analogue of Metyrapone,” Chemico-Biolo- gical Interactions, Vol. 143-144, 2003, pp. 449-457. doi:10.1016/S0009-2797(02)00183-7 [26] B. Testa and P. Jenner, “Inhibitors of Cytochrome P-450s and Their Mechanism of Action,” Drug Metabolism Re- views, Vol. 12, No. 1, 1981, pp. 1-117. doi:10.3109/03602538109011082 [27] B. Darvas, I. Bélai, A. Fónagy, P. Kulcsár an d M. H. Tag El-Din, “Lethal Disturbances in Larval Development of Neobellieria Bullata Caused by Metyrapone Derivatives,” Pesticide Science, Vol. 32, No. 2, 1991, pp. 133-139. doi:10.1002/ps.2780320202 Copyright © 2011 SciRes. PP  Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. 330 A Possible Molecular Target? [28] L. Roumen, M. P. A. Sanders, K. Pieterse, P. A. J. Hilbers, R. Plate, E. Custers, M. D. Gooyer, J. F. M. Smits, I. Beugels, J. Emmen, H. C. J. Ottenheijm, D. Leysen and J. J. R. Hermans, “Construction of 3D Models of the CYP11B Family as a Tool to Predict Ligand Binding Characteris- tics,” Journal of Computer Aided Molecular Design, Vol. 21, No. 8, 2007, pp. 455-471. doi:10.1007/s10822-007-9128-9 [29] M. Murray, R. M. Sefton, R. Martini and A. M. Butler, “Comparative Induction of CYP3A and CYP2B in Rat Liver by 3-Benzoylpyridine and Metyrapone,” Chemico- Biological Interactions, Vol. 113, No. 3, 1998, pp. 161- 173. doi:10.1016/S0009-2797(98)00017-9 [30] G. Fantuzzi, L. Cantoni, M. Sironi and P. Ghezzi, “In- hibitors of Cytochrome P450 Suppress Tumor Necrosis Factor Production,” Cellular Immunology, Vol. 150, No. 2, 1993, pp. 417-424. doi:10.1006/cimm.1993.1209 [31] U. Förstermann, U. Alheid, J. C. Frölich and A. Mülsch, “Mechanisms of Action of Lipoxygenase and Cyto- chrome P-450-Mono-Oxygenase Inhibitors in Blocking Endothelium-Dependent Vasodilatation,” British Journal of Pharmacology, Vol. 93, No. 3, 1988, pp. 569-578. [32] D. J. Stuehr and M. Ikeda-Saito, “Spectral Characteriza- tion of Brain and Macrophage Nitric Oxide Synthases. Cytochrome P-450-Like Hemeproteins That Contain a Flavin Semiquinone Radical,” Journal of Biological Che- mistry, Vol. 267, No. 29, 1992, pp. 20547-20550. [33] A. G. Hildenbrandt, “The Binding of Metyrapone to Cy- tochrome P-450 and Its Inhibitory Action on Liver Mi- crosomal Mixed-Function Oxidase Reactions,” The Bio- chemical Journal, Vol. 125, No. 2, 1971, pp. 6-8. [34] R. W. Estabrook, D. Y. Cooper and O. Rosenthal, “The Light Reversible Carbon Monoxide Inhibition of the Steroid C21-Hydroxylase System of the Adrenal Cortex,” Biochemische Zeitschrift, Vol. 338, 1963, pp. 741-755. [35] P. A. Williams, J. Cosme, D. M. Vinkovic, A. Ward, H. C. Angove, P. J. Day, C. Vonrhein, I. J. Tickle and H. Jhoti, “Crystal Structures of Human Cytochrome P450 3A4 Bound to Metyrapone and Progesterone,” Science, Vol. 305, No. 5684, 2004, pp. 683-686. doi:10.1126/science.1099736 [36] W. Li, H. Liu, X. Luo, W. Zhu, Y. Tang, J. R. Halpert and H. Jiang, “Possible Pathway(s) of Metyrapone Egress from the Active Site of Cytochrome P450 3A4: A Mo- lecular Dynamics Simulation,” Drug Metabolism and Disposition, Vol. 35, No. 4, 2007, pp. 689-696. doi:10.1124/dmd.106.014019 [37] S. Lupien, R. Richter, S. C. Risen, A. Mirow, J. C. Gillin and R. L. Hauger, “Time Course of the Corticosteroid- Dopaminergic Interaction during Metyrapone and Dexa- methasone Administration,” Psychiatry Research, Vol. 58, No. 1, 1995, pp. 23-35. doi:10.1016/0165-1781(95)02646-E [38] S. Kawato, M. Yamada and T. Kimoto, “Brain Neuros- teroids Are 4th Generation Neuromessengers in the Brain: Cell Biophysical Analysis of Steroid Signal Transduc- tion,” Advances in Biophysics, Vol. 37, 2003, pp. 1-48. doi:10.1016/S0065-227X(03)80002-3 [39] D. Canet, J. C. Boubel an d E. So ul as, “La R MN, C oncepts, Méthodes et Applications,” Dunod, Paris, 2002. [40] E. Dennis and A. Plückthun, “Phosphorus 31P-NMR: Principles and Applications,” Academic Press, London, 1984. [41] P. Job, “Etude Spectrographique de la Formation de Complexes en Solution et de Leur Stabilité,” Comptes Rendus de l’Académie des Sciences, Vol. 180, 1925, pp. 918-930. [42] F. Djedaïni, “Etude par RMN de s Phénomènes d’Inclusion et d’Adaptation Moléculaire dans les Cy clodextrines Natu- relles et les Dérivés Synthéti ques,” PhD Thesis, Université de Paris Sud, Paris, 1991. [43] J. C. Debouzy, A. Gadelle, F. Fauvelle, S. Aous, J. Y. Pailler, E. Gentilhomme, P. Perrin and F. Lhoste, “Is Uranyl Scavenger Hexak is(3,6-Anhydro) Tetrakis(2A,B,D, E-O-Octyl) Cyclomaltohexaose (OCT) Relevant with Bio- systems?” Bollettino Chimico Farmaceutico, Vol. 140, No. 1, 2001, pp. 4-8. [44] B. J. Gaffney, “Pratical Considerations for the Calculation of Order Parameters for Fatty Acid or Phospholipid Spin Labels in Membranes,” In: R. J. Berliner, Ed., Spin La- belling Theory and Applications, Academic Press, New York, 1976, pp. 567-571. [45] A. Gornicki and A. Gutsze, “In Vitro Effects of Ozone on Human Erythrocyte Membranes: An EPR Study,” Acta Biochimica Polonica, Vol. 47, No. 4, 2000, pp. 963-971. [46] S. Mabrey and J. M. Sturtevant, “Investigation of Phase Transitions of Lipids and Lipid Mixtures by Sensitivity Differential Scanning Calorimetry,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 73, No. 11, 1976, pp. 3862-3866. doi:10.1073/pnas.73.11.3862 [47] E. J. Dufourc, C. Mayer, J. Stohrer, G. Althoff and G. Kothe, “Dynamics of Phosphate Head Group in Bio- membranes,” Biophysical Journal, Vol. 61, No. 1, 1992, pp. 42-57. doi:10.1016/S0006-3495(92)81814-3 [48] D. Gorenstein, “31P-NMR: Principles and Applications,” Academic Press, London, 1984. [49] S. Follot, J. C. Debouzy, D. Crouzier, C. Enguehard- Gueiffier, A. Gueiffier, F. Nachon, B. Lefebvre and F. Fau- velle, “Physicochemical Properties and Membrane Inter- actions of Anti-Apoptotic Derivatives 2-(4-Fluoro-phe- nyl)-3-(pyridin-4-yl)imidazo[1,2-a]pyridine Depending on the Hydroxyalkylamino Side Chain Length and Confor- mation: An NMR and ESR Study,” European Journal of Medicinal Chemistry, Vol. 44, No. 9, 2009, pp. 3509- 3518. doi:10.1016/j.ejmech.2008.12.026 [50] K. Wüthrich, “Structure and Dy namics in Proteins of Phar- macological Interest,” Biochemical Pharmacology, Vol. 40, No. 1, 1990, pp. 55-62. doi:10.1016/0006-2952(90)90178-N [51] M. P. Besenicar, A. Bavdek, A. Kladnik, P. Macek and G. Anderluh, “Kinetics of Cholesterol Extraction from Lipid Membranes by Methyl-[Beta ]-Cy clodextrin-A Surface Plas- Copyright © 2011 SciRes. PP  Anticushing Drug Metyrapone Exhibits Specific Interactions with Serine Containing Systems. A Possible Molecular Target? Copyright © 2011 SciRes. PP 331 mon Resonance Approach,” Biochimica et Biophysica Acta, Vol. 1778, No. 1, 2008, pp. 175-184. [52] S. Bernèche, M. Nina and B. Roux, “Molecular Dy namic s Simulation of Melittin in a Dimyristoylphosphatidylcho- line Bilayer Membrane,” Biophysical Journal, Vol. 75, No. 4, 1998, pp. 1603-1618. doi:10.1016/S0006-3495(98)77604-0 [53] J. C. Debouzy, D. Crouzier and E. Flahaut, “Hydrophobic Double Walled Carbon Nanotubes Interaction with Pho- pholipidic Model Membranes: 1H-, 2H-, 31P NMR and ESR Study,” Environmental Toxicology and Pharmacol- ogy, Vol. 30, No. 2, 2010, pp. 147-152. doi:10.1016/j.etap.2010.05.002 [54] B. Cybulska, M. Herve, E. Borowski and C. M. Gary- Bobo, “Effect of the Polar Head Structure of Polyene Macrolide Antifungal Antibiotics on the Mode of Perme- abilization of Ergosterol- and cholesterol-Containing Li- pidic Vesicles Studied by 31P-NMR,” Molecular Phar- macology, Vol. 29, No. 3, 1986, pp. 293-298. [55] J. I. Kaplan and G. Fraenkel, “NMR of exchanging sys- tems,” Academic Press, New York, 1980. [56] J. H. Fuhrhop and J. Koning, “Membranes and Molecular Assemblies: The Sy nkinetics Approach,” Chemi stry TRSo, Cambridge, 1994.
|