Journal of Cancer Therapy
Vol. 3 No. 6A (2012) , Article ID: 25159 , 8 pages DOI:10.4236/jct.2012.326117
Adjuvant Therapy on Cancer of the Lower Rectum. Evaluation of the Effects of Preoperative Radiotherapy on the Prognosis of Patients with Cancer of the Lower Rectum
![]()
1Surgery, Catholic University of Campinas, Campinas, Brazil; 2Surgery, Clinica Reis Neto, Catholic University Campinas, Campinas, Brazil; 3Coloproctology, Clinica Reis Neto, Catholic University Campinas, Campinas, Brazil.
Email: *jareisneto@msn.com
Received September 28th, 2012; revised October 27th, 2012; accepted November 6th, 2012
Keywords: Rectal Cancer; Cancer of the Lower Rectum; Irradiation; Preoperative Radiotherapy; Local Recurrence; Mortality; Survival
ABSTRACT
Aims: The prognosis on treatment of the cancer of the rectum has not changed in the last fifty years. Survival rates of 50% to 55% seems immutable in several published series. The main cause for those results is the high incidence of recurrence, either local or widespread. Local recurrence is directly related to the number of undifferentiated cells and to the grade of wall invasion. Widespread recurrence depends specifically on the lymphatic and vascular spreading. So any kind of treatment that would diminish the number of undifferentiated cells and the size or the tumor wall penetration would certainly decrease the local recurrence rate, lengthening the interval free from cancer and, perhaps, modifying the long term survival rate. Between 1978 and 2009, a total of 538 patients with adenocarcinoma of the lower rectum (from the pectinate line to 10 cm above) were treated by preoperative radiotherapy. Methodology: The same protocol was used in all the patients –400 cGy, 200 cGy/day, during 4 consecutive weeks (anterior and posterior pelvic fields) by means of a Linear Megavoltage Accelerator (25 MeV). Surgery was performed 2 months after completion of the radiotherapy. Results: Statistical analysis of the whole group showed that preoperative radiotherapy does decrease frequency of undifferentiated cells. Moreover, the incidence of local recurrence diminished after irradiation by 3.4%. Preoperative radiotherapy reduces tumor volume (ERUS) and wall invasion, as well as the mortality rate due to local recurrence (2.4%) and alters long-term survival rate (80.1%). Conclusion: Preoperative radiotherapy is really effective in reducing the number of undifferentiated cells and in diminishing the tumor volume and the carcinomatous infiltration of the rectal wall.
1. Introduction
Pre-operative radiotherapy as complementary to surgery, although recognized as effective on controlling the interval-free of rectal cancer, has not been utilized as frequently as expected [1-11].
Several aspects are responsible for this [2-39] but the most important is that there is no world consensus about:
1) The total tumor dose, and the daily dose;
2) Short or long radiotherapy;
3) The target volume;
4) The interval between the completion of the irradiation and surgery;
5) Effects of irradiation on surgery;
6) Which tumor needs irradiation;
7) The effectiveness of preoperative radiotherapy on long-term survival rate.
Nowadays, however, some authors have demonstrated that the association of neo-adjuvant therapy (radiotherapy or radio-chemotherapy) to surgery can improve the interval-free of cancer and alter the long-term survival rate [7,13,24-32].
Preoperative radiotherapy in cancer of the lower rectum has been used since 1975. Previous works defined the target volume and the total tumor dose [27,29]. Analyzing the results obtained in a previous randomized prospective trial [28], it was concluded that the use of preoperative radiotherapy is able to:
• Decrease, significantly, the number of undifferentiated cells;
• Diminish the grade of tumor invasion in the rectal wall;
• Reduce, statistically, the incidence of local recurrence
• Alter long-term survival rate.
In this trial, two groups of patients, randomly allocated, with cancer of the lower rectum were treated surgically following the same oncological parameters, but differing in the use of preoperative radiotherapy in one of the groups. However, frustrated criticism came from the small number of patients (68 patients) involved in the study.
Since then, the number of patients treated by preoperative radiotherapy has risen and the results obtained have been equally registered.
Some new aspects emerged from this prospective study.
The results obtained as to:
• The comparison of the biopsies taken before and after the radiotherapy;
• The evaluation of the cellular undifferentiation and of the Broders’ classification;
• The tumor volume measured before and after radiotherapy by endorectal ultrasound;
• The wall infiltration measured before and after radiotherapy by endorectal ultrasound;
• The analysis of the correlation of these results with the incidence of local recurrence;
• The percentage of long-term survival rate;
have given new insight into the concept of preoperative radiotherapy in patients with cancer of the lower rectum.
2. Methods
From 1978 to 2008, a total of 538 patients with cancer of the lower rectum were submitted to preoperative radiotherapy and all of them had a 5-year follow-up. In this study only patients with rectal adenocarcinoma situated in the lower rectum were included.
There was no gender, race and age distinction.
All patients with tumors located between the pectinate line and 4 cm above it, were submitted to abdominoperineal excision and those with tumors situated between 5 and 10 cm, were treated by a colon-anal stapled anastomoses.
Of the 538 patients, 408 (75.8%) were operated on laparoscopically.
Preoperative dosage of CEA, gamma GT, colonoscopy and abdominal ultrasound were performed in all the patients to stage the tumor. Endorectal ultrasound was performed in 200 patients, size and infiltration of the tumor were thus evaluated before and after the irradiation.
Every patient had biopsies (10) of the tumor taken at the time of the diagnosis and another one after the completion of the radiotherapy, at surgery. The number of undifferentiated cells were exhaustively noted and the results compared, in both biopsies.
Broders’ classification was equally observed and the results obtained, before and after radiotherapy, registered and compared.
Proctoscopy and digital examination were performed at diagnosis and after the end of the irradiation treatment, immediately before surgery, to evaluate tumor extension and wall infiltration.
Preoperative radiotherapy was performed according to the following scheme:
200 cGy/daily for 4 consecutive weeks up to a total of 4000 cGy, by means of a Linear Megavoltage Accelerator (25 MeV), in anterior and posterior pelvic fields. Surgery was performed 7 to 10 days after the conclusion of the radiotherapy.
Postoperative protocol included periodical examination:
• Every 3 months for the first two years, after surgery. Digital examination (or careful perineal palpation) and evaluation of the CEA were performed each time.
• Every 6 months for the next consecutive three years.
Abdominal ultrasound was performed yearly and colonoscopy every 2 or 3 years.
When clinical assessment suggested local or general recurrence, a CT scan or MR was accomplished.
It was considered local recurrence the appearance of perirectal, perineal or anastomotic metastasis.
Pelvic recurrence (bladder, prostate or ovarian) was considered generalized recurrence.
3. Results
Biopsy
Cellular undifferentiation. At diagnosis the tumors were classified as:
286 tumors (53.3%)èhigh grade of cellular undifferentiation181 tumors (33.5%)èmoderate grade of cellular undifferentiation71 tumors (13.2%)èlow grade of cellular undifferentiation.
After the radiotherapy, at surgery, the tumors were classified as:
87 tumors (16.2%)èhigh grade of cellular undifferentiation299 tumors (55.6%)èmoderate grade of cellular undifferentiation152 tumors (28.2%)èlow grade of cellular undifferentiation (Figure 1).
Broders’ classification: At diagnosis the tumors were classified as.
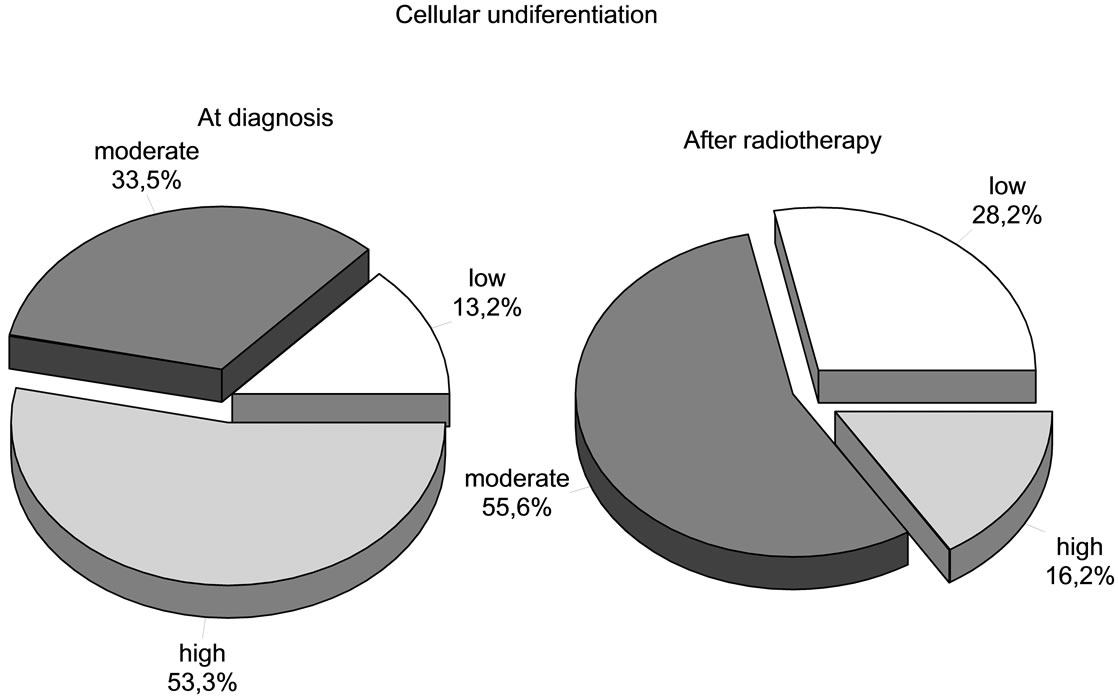
Figure 1. Cellular undifferentiation.
48 (8.8%)→Broders’ I
142 (26.5%)→Broders’ II
206 (38.3%)→Broders’ III
142 (26.5%)→Broders’ IV.
At surgery, after irradiation therapy, the tumors were classified as:
126 (23.5%)→Broders’ I
301 (55.9%)→Broders’ II
95 (17.6%)→Broders’ III
16 (2.9%)→Broders’ IV (Figure 2).
Of the 142 patients considered as Broders IV at diagnosis, 88.2% of them changed to a different classification: 52.6% changed to Broders II, 30,3% modified to Broders I (Figure 3).
Endorectal ultrasound: The endorectal ultrasound (ERUS) evaluation in 20 patients, comparing tumor size before and after radiotherapy (Figure 4), showed that:
■ In 40 patients (20%) the tumor was reduced to a small superficial ulcer;
■ In t120 patients (60%) the tumor receded between 60% to 75%;
■ In 40 patients (20%) the tumor receded between 20% to 30%.
Gross and endoscopic tumor involution: digital evaluation allows recognition of a tumor reduction in all the patients. In 166 patients (31%) the tumor mass receded more than seventy percent of its initial size.
Endoscopically almost all the exophytic tumors were converted into small ulcers or to a whitish pale fibrosis zone. The majority of the ulcer-vegetating tumors after irradiation looked like a large superficial necrotic ulceration.
According to Dukes’ classification, the tumors at surgery were classified as (Figure 5):
58 (10.6%)→Dukes A
290 (53.9%)→Dukes B and
190 (35.5%)→Dukes C.
Of the classified Dukes A tumors, 10 (2.9%) were classified as microscopic tumors.
Local Recurrence: of the 538 patients only 16 (3.0%) had a diagnosis of isolated local recurrence confirmed at the follow-up. Of them, 8 (50%) died of cancer in the first two years after surgery (Figure 6).
Generalized Recurrence: of the patients with a 5-year follow-up, 83 (15.6%) patients presented widespread recurrence:
36 (44.4% of the 83)→lung metastasis
26 (31.1% of the 83)→ liver
13 (15.5% of the 83)→disseminated peritoneal recurrence
08 (8.9% of the 83)→pelvic recurrence.
Long-term follow-up: of the 538 patients, 101 (18.9%) had a 15-years follow-up.
Of the 538 patients with a 5-year follow-up (Figure 7):
230 (80.1% of the 287 patients) were alive, with no signs of recurrence disease
53 (18.5% of the 287 patients) died of cancer
45 (15.7% of the 287 patients) died with generalized
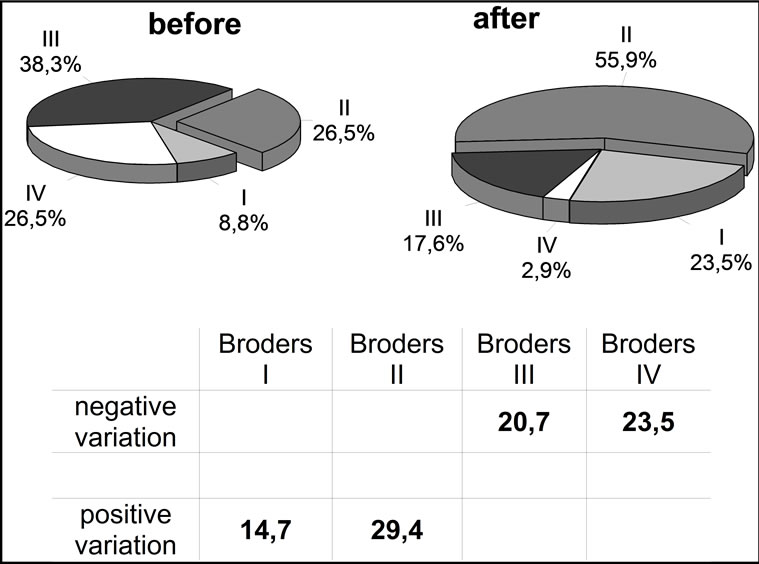
Figure 2. Broders’ classification before and after radiotherapy.
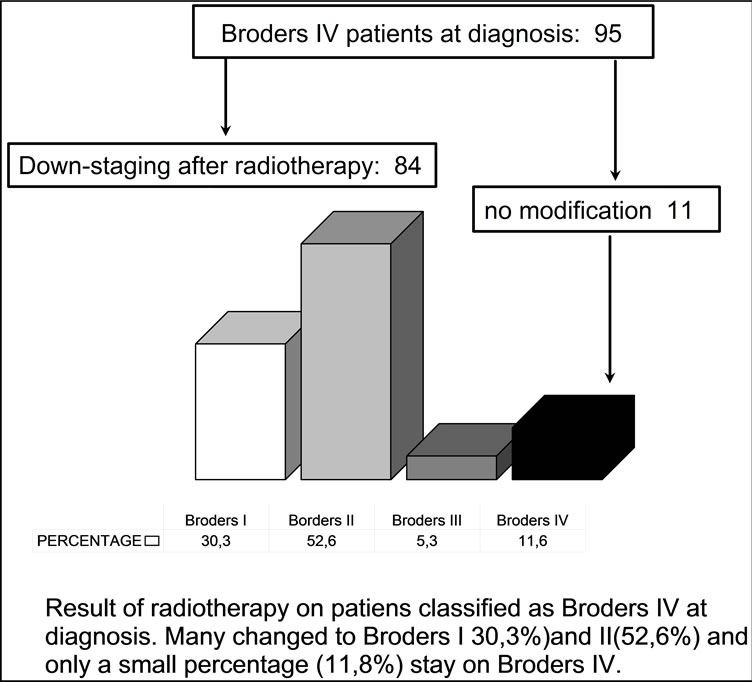
Figure 3. Modification observed in Broders IV patients after irradiation.
recurrence
08 (2.8% of the 287 patients) died with local recurrence
04 (1.4% of the 287 patients) died of other causes.
4. Discussion
Preoperative irradiation for cancer of the lower rectum has been the object of controversies, specially when survival is discussed [2,5,8,9,11,13,15-18,20,21,24-27,30-3436-39] and only a very few randomized statistic papers are able to demonstrate better long-term results [28,35].
Recurrence, especially local recurrence, is responsible for the great majority of deaths in the first two years after surgery [1,7,19]. It is well recognized that the incidence of local recurrence is a direct consequence of the proportion of undifferentiated cells and of the tumoral invasion of the bowel wall [1,7,12,19]. Less undifferentiated and less invasive tumors tend to have better prognosis [19,22, 23,29]. So, hypothetically, any treatment that can reduce
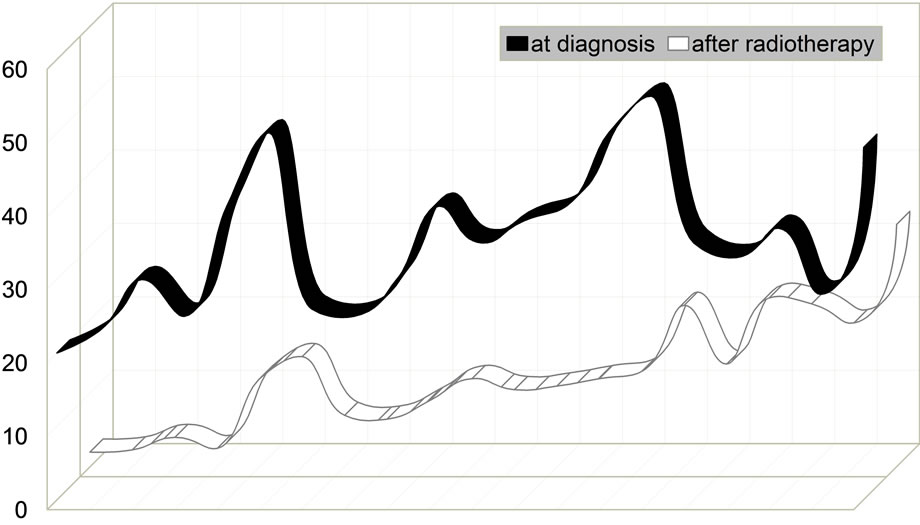
Figure 4. Endorectal ultrasound before and after radiotherapy in 200 patients.
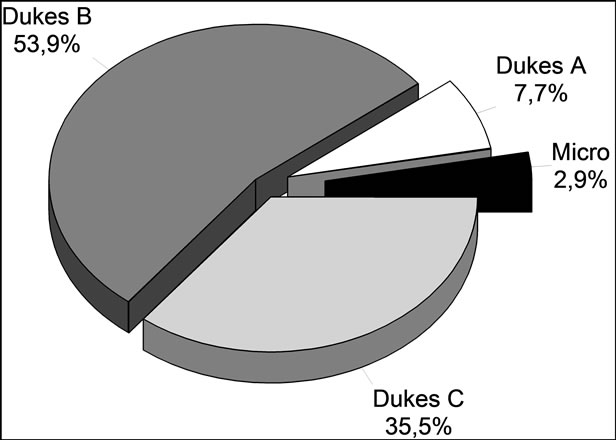
Figure 5. Dukes classification of the tumors after irradiation.
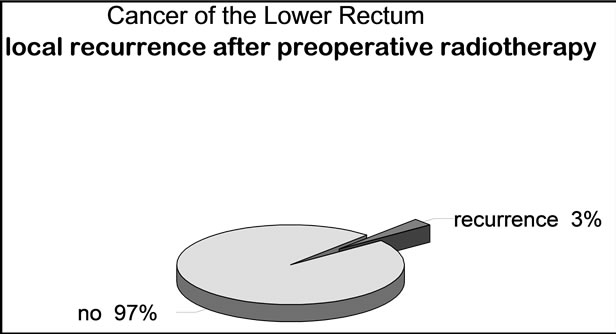
Figure 6. Incidence of local recurrence.
the number of undifferentiated cells will affect the incidence of local recurrence and will certainly increase the long-term survival rate.
In our series the comparison between biopsies taken before and after irradiation showed a very important re-
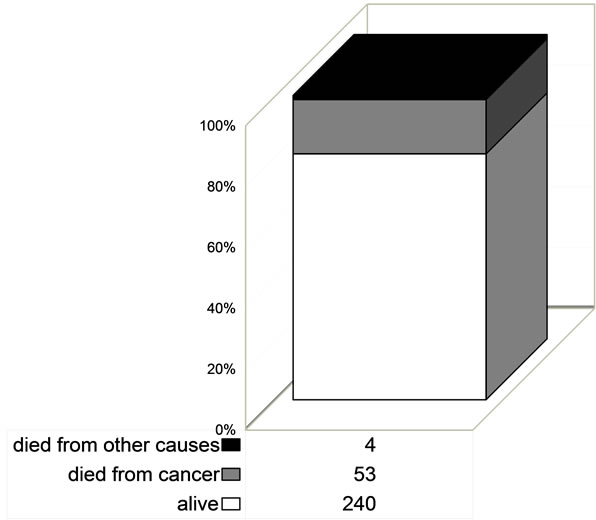
Figure 7. Deaths of patients with 5-year follow-up.
duction in the number of carcinomatous (undifferentiated) cells, statistically increasing the number of differentiated cells.
As a fact, the variation in the number of undifferentiated cells before and after irradiation was significantly proved; the number of tumors with a high grade of undifferentiated cells shows a negative variation after irradiation: 191 (53.3%) patients at diagnosis and 58 (16.2%) patients after radiotherapy, showing a significant statistical reduction in the number of undifferentiated cells (Figure 1).
At the same time, as a corollary, the number of tumors with a low grade of differentiated cells at diagnosis shows an effective increase after irradiation: of 47 (13.2%) patients at diagnosis to 101 (28.2%) patients after irradiation, with a positive variation.
The real significance of this fact is that the preoperative radiotherapy reduced the number of undifferentiated cells, enough to invert the prognosis of local recurrence.
Broders’ classification, in the same biopsy study, revealed a similar aspect: there was a significant statistical reduction in the number of undifferentiated cells. Patients with Broders IV at diagnosis changed to Broders III, II and even I after the irradiation (Figure 3):
05 tumors Broders’ IV (5.3%) became Broders’ III.
50 tumors Broders’ IV (52.8%) changed into Broders’ II.
29 tumors Broders’ IV (30.3%) were reduced to Broders’ I.
11 tumors Broders’ IV (11.8%) were resistant to radiotherapy.
As a matter of fact, the reduction in the number of undifferentiated cells because of preoperative radiotherapy had a significant influence in the incidence of local recurrence.
It is important to notice that the great majority of patients with local recurrence occurred exactly in those patients with tumors considered as resistant to irradiation: patients with high grade of undifferentiated cells at diagnosis that persisted as high grade of undifferentiation at surgery.
Of the 11 patients observed with local recurrence:
• 9 (81.8% of the 11 patients) were Broders’ IV, all of them classified as resistant to irradiation; 8 of them died postoperatively in the first 18 months after surgery and one with anastomotic recurrence underwent an abdomino-perineal excision with a satisfactory response;
• 2 patients were Broders’ IV at diagnosis, reduced to Broders’ III after radiotherapy: one with anastomotic recurrence was submitted to an abdomino-perineal excision with satisfactory response and one underwent a local excision plus with a new radiotherapy treatment.
But the most important fact was that all the 11 patients with local recurrence were classified, at the time of the diagnosis, as having tumors of a high grade of cellular undifferentiation and this cellular aspect was not reduced with radiotherapy. These patients were considered to have radio-resistant tumors.
Generalized recurrence occurred in 45 patients:
• One patient had a tumor classified as Broders’ IV at diagnosis which did not change after radiotherapy;
• 44 (97.7%) had tumors which were classified as having a high grade of cellular differentiation at the time of diagnosis and this cellular aspect did not change after radiotherapy.
All patients with recurrence (56 patients from 358) belonged to the group with high grade of cellular undifferentiation that did not respond to the irradiation treatment: radio resistant tumors.
• 11 (3.0% of the 358 patients) had local recurrence;
• 37 (10.33% of the 358 patients) acquired widespread metastasis.
• 8 (2.2% of the 358 patients) had concomitant metastasis (local and general).
• Those tumors were classified as resistant to irradiation.
Long-term survival rate (5 year follow-up) was of 83.6%: 240 patients out of 287 were alive with no clinical evidence of recurrence after 5 years.
Only 8 patients out of the 11 with local metastasis died of cancer.
Besides the observed fact of a low incidence of local recurrence (3.0%) in irradiated patients, it was also observed that not all the patients died in the first 2 years of follow-up: 27.2% of the patients with local recurrence were alive after a 5 year follow-up. The percentage of deaths with local recurrence was of 2.23%: eight patients in 358.
It is logical to conclude that the irradiation effect, diminishing the number of undifferentiated cells, not only decreased the local recurrence rate but also reduced the mortality rate in this kind of recurrence.
Nonetheless, as mentioned above [1,7,12,19,22-27,35], the incidence of local recurrence depends also on the tumoral infiltration of the rectum wall.
The results observed in this study confirmed that after the irradiation an involution of the tumor size and volume occurred, detectable not only by endorectal ultrasound (ERUS) in 20 patients, but also by digital and endoscopic examinations.
So, it is questionable to confirm, but logical to say, that besides the proven reduction in the number of undifferentiated cells, preoperative irradiation also produces a diminishing of the carcinomatous infiltration of the rectal wall. In a previous study [28], analyzing the results of a randomized analytical trial, comparing two groups of patients with cancer of the lower rectum (adenocarcinoma between the pectinate line and 10 cm above it), one of them submitted to preoperative radiotherapy plus surgery and the other one submitted only to surgery, this fact was also noted on.
In the group submitted to preoperative radiotherapy there was an increased number of B Dukes tumors.
Classifying the tumors observed at diagnosis and after radiotherapy, at surgery, according to cTNM classification it was observed a down-staging in all the patients:
• In 76% of the patients there was a reduction of 20% to 70% of the initial tumor volume;
• In 24% of the patients there was a reduction of 71% to 90% of the initial tumor volume;
• In 2.9% of the patients there was a reduction of the initial tumor to a microscopic lesion.
Undoubtedly, both radiation therapeutic effects—reducing the number of undifferentiated cells and diminishing the tumor volume and the carcinomatous infiltration of the rectal wall—are responsible for the reduction of the local recurrence rate and for a better prognosis of long-term survival rate.
Besides these effects one more very important aspect was noted on: the number of pT3 with negative lymph nodes carcinomatous invasion decreased. Nevertheless, when lymph nodes invasion was detected in patients with pT3 tumors, only less than one third of them showed cancer. In reviewing the anatomo-pathology of pT3 patients selected for an earlier trial [28] this fact was confirmed: patients that received preoperative radiotherapy and had tumors classified as pT3 at surgery there was 26.4% of lymph node cancer invasion, but in the group of patients operated on without preoperative radiotherapy the number of lymph nodes with cancer invasion arose to 47%.
This fact deserves a more objective analysis.
Was this fact responsible for better survival results observed in irradiated patients with pT3? It is very clearly demonstrated [1,7,12,19] that recurrence and survival is directly proportional to the number of lymph nodes invaded: as the incidence of lymphatic invasion decreased, there were better rates of recurrence and survival.
But why this difference?
Not frequently the pathologist demonstrates that some lymphatic with cancer invasion has altered this condition.
Anatomo-pathological studies registering effects of irradiation in lymphatic of the peri-rectal fat (mesorectum) are rare.
However, if this fact is added to the other mentioned effects of the irradiation (on tumor infiltration and carcinomatous cells) there is an explanation for the better observed curative results on C tumors survival rates of patients submitted to preoperative radiotherapy.
REFERENCES
- H. E. Bacon, “Cancer of the Colon, Rectum and Anal Canal,” J.B. Lippincott Co., Philadelphia, 1964.
- I. Balslev, M. Pedersen, P. S. Tegljaerg, et al., “Postoperative Radiotherapy in Rectosigmoid Cancer Dukes B and C: Interim Report from a Randomized Multicentre Study,” British Journal of Cancer, Vol. 46, No. 4, 1982, pp. 551-556. doi:10.1038/bjc.1982.239
- T. Buroker, N. Nigro, J. Correa, V. K. Vaitkevicius, M. Samson and B. Considine, “Combination Preoperative Radiation and Chemotherapy in Adenocarcinoma of the Rectum: Preliminary Report,” Diseases of the Colon & Rectum, Vol. 19, No. 8, 1976, pp. 660-663. doi:10.1007/BF02591003
- A. M. Cohen, L. L. Gunderson and C. E. Welch, “Selective Use of Adjuvant Radiation Therapy in Resectable Colorectal Adenocarcinoma,” Diseases of the Colon & Rectum, Vol. 24, No. 4, 1981, pp. 247-251. doi:10.1007/BF02641869
- B. J. Cummings, W. D. Rider, A. R. Harwood, T. J. Keane and G. M. Thomas, “Radical External Beam Radiation Therapy for Adenocarcinoma of the Rectum,” Diseases of the Colon & Rectum, Vol. 23, No. 1, 1983, pp. 30-36. doi:10.1007/BF02554676
- B. J. Cummings, “Adjuvant Radiation Therapy for Rectal Adenocarcinoma,” Diseases of the Colon & Rectum, Vol. 27, No. 12, 1984, pp. 826-836. doi:10.1007/BF02553949
- S. Drobni and F. Incze, “Surgery of Rectal Cancer,” Akademiai Kiado, Budapest, 1969.
- G. H. Fletcher, “Textbook of Radiotherapy,” Lea & Febiger, Philadelphia, 1980, pp. 704-716.
- A. H. Gama, P. M. S. B. Souza, U. Ribeiro Jr., F. Campos, A. H. S. Souza Jr., W. Nadalin, R. Gansi and J. G. Rodrigues, “Low Rectal Câncer: Impact of Pré-Operative Radiation and Chemotherapy on Surgical Treatment,” Hospital das Clínicas of the University of São Paulo Medical School Experience. In: J. A. Reis Neto, Ed., New Trends in Coloproctology, Revinter, 2000, pp. 423-429.
- J. Gary-Bobo, H. Pujol, C. Solassol, J. L. Broquerie and M. Nguyen, “‘L’ Irradiation Pré-Operatoire du Cancer Rectal: Résultats à 4 ans de 116 Cas,” Bull Cancer, Vol. 66, No. 5, 1979, pp. 461-466.
- B. Glimenius, S. Graffman, L. Pahlman, A. Rimsten and E. Wilander, “Preoperative Irradiation with High Dose Fractionation in Adenocarcinoma of the Rectum and Rectosigmoid,” Acta Radiologica, Vol. 21, No. 6, 1982, pp. 373-379. doi:10.3109/02841868209134315
- J. C. Goligher, “Surgery of the Anus, Rectum and Colon,” 4th ed. London: Bailliere Tindall, 1980.
- L. L. Gunderson, D. E. Dosoretz, S. E. Hedberg, et al., “Low Dose Preoperative Radiation Surgery and Elective Post-Operative Radiation Therapy for Resectable Rectum and Rectosigmoid Carcinoma,” Cancer, Vol. 52, No. 3, 1983, pp. 446-451. doi:10.1002/1097-0142(19830801)52:3<446::AID-CNCR2820520311>3.0.CO;2-H
- C. M. Haskel, “Cancer Treatment,” WB Saunders, Philadelphia, 1980, pp. 276-304.
- G. A. Higgins, J. H. Conn, P. H. Jordan, E. W. Humphrey, B. Roswit and R. J. Keehn, “Preoperative Radiotherapy for Colorectal Cancer,” Annals of Surgery, Vol. 181, No. 5, 1975, pp. 624-631. doi:10.1097/00000658-197505000-00017
- I. Kodner and R. J. Myerson, “Preoperative Radiation Therapy for Rectal Cancer,” In: J. A. Reis Neto, Ed., New Trends in Coloproctology, Revinter, 2000, pp. 385-399.
- M. M. Kligerman, “Radiotherapy and Rectal Cancer,” Cancer, Vol. 39, Suppl. S2, 1977, pp. 986-990. doi:10.1002/1097-0142(197702)39:2+<896::AID-CNCR2820390728>3.0.CO;2-E
- M. M. Kligerman, N. Urdaneta, A. Lnowlton, R. Vidone, P. V. Hartman and R. Vera, “Preoperative Irradiation of Rectosigmoid Carcinoma including Its Regional Lymph Nodes,” American Journalism Review, Vol. 114, No. 6, 1972, pp. 498-503.
- B. C. Morson, “Factors Influencing the Prognosis of Early Cancer of the Rectum,” Proceedings of The Royal Society of Medicine, Vol. 59, No. 4, 1966, pp. 607-611.
- I. Pählman and B. Glimelius, “Pre or Postoperative Radiotherapy in Rectal and Rectosigmoid Carcinoma,” Annals of Surgery, Vol. 211, No. 2, 1990, pp. 187-195. doi:10.1097/00000658-199002000-00011
- I. Pählman and B. Glimelius, “The Value of Adjuvant Radiotherapy for Rectal Cancer,” European Journal of Cancer, Vol. 31A, No. 7-8, 1995, pp. 1347-1350. doi:10.1016/0959-8049(95)91268-H
- J. Papillon, “New Prospects in the Conservative Treatment of Rectal Cancer (Abstr),” Diseases of the Colon & Rectum, Vol. 27, No. 11, 1984, pp. 566-567. doi:10.1007/BF02554589
- J. Papillon, “Rectal and Anal Cancer,” Springer-Verlag, Berlin, 1980, pp. 24-32.
- J. A. Reis Neto, F. A. Quilici and F. Cordeiro, “Radioterapia Pré-Operatória em Câncer do Reto,” XXIX Cong Bras Proctologia, Belo Horizonte, 1979.
- J. A. Reis Neto, F. A. Quilici and F. Cordeiro, “Radioterapia + Cirurgia: Novos Conceitos em Câncer do Reto,” XXX Cong Bras Proctologia, Rio de Janeiro, 1980.
- J. A. Reis Neto, F. A. Quilici and F. Cordeiro, “Pre-Operative Radiotherapy for Cancer of the Rectum. ColoRectal Mass Screening & Management,” 4th International Symposium Prevention and Detect of Cancer, London, 1980.
- J. A. Reis Neto, F. A. Quilici, F. Cordeiro and J. A. Reis, “Radiotherapy and Survival-Rate,” XIVth Biennial Congress ISUCRS, Crete, 1992.
- J. A. Reis Neto, F. A. Quilici and J. A. Reis Junior, “A Comparison of Nonoperative vs. Preoperative radiotherapy in Rectal Carcinoma. A 10-Year Randomized Trial,” Diseases of the Colon & Rectum, Vol. 32, No. 8, 1989, pp. 702-710. doi:10.1007/BF02555778
- J. A. Reis Neto, F. A. Quilici, F. Cordeiro, S. Ciquini, J. A. Reis Jr., O. Kagohara and J. Simões Neto, “LongTerm Results of Preoperative Radiotherapy for Cancer of the Lower Rectum,” In: J. A. Reis Neto, Ed., New Trends in Coloproctology, Revinter, 2000, pp. 401-410.
- B. Roswit, G. A. Higgins and R. J. Keehn, “Preoperative Irradiation for Carcinoma of the Rectum and Rectosigmoid Colon: Report of a National Veterans Administration Randomized Study,” Cancer, Vol. 35, No. 6, 1975, pp. 1597-1602. doi:10.1002/1097-0142(197506)35:6<1597::AID-CNCR2820350618>3.0.CO;2-S
- L. Sofo, C. Ratto, G. B. Doglietto, V. Valentini, et al., “Intraoperative Radiation Therapy in Integrated Treatment of Rectal Cancers: Results of Phase II Study,” Diseases of the Colon & Rectum, Vol. 39, No. 12, 1996, pp. 1396-1403. doi:10.1007/BF02054528
- M. W. Stearn Jr., M. R. Deddish and S. H. Quan, “Preoperative Roentgen Therapy for Cancer of the Rectum,” Surgery Gynecology & Obstetrics, Vol. 109, 1959, pp. 225-229.
- M. W. Stearn Jr., “Preor Post-Operative Radiation in Resectable Tumors,” In: K. Welwaart, L. H. Blumgart and J. Breuning, Eds., Colorectal Cancer, Leiden University Press, The Hague, 1980, pp. 153-159. doi:10.1007/978-94-009-9158-3_18
- K. R. Stevens, C. V. Allen and W. S. Fletcher, “Peroperative Radiotherapy for Adenocarcinoma of the Rectosigmoid,” Cancer, Vol. 37, No. 6, 1976, pp. 2866-2868. doi:10.1002/1097-0142(197606)37:6<2866::AID-CNCR2820370644>3.0.CO;2-M
- Swedish Rectal Cancer Trial, “Improved Survival with Preoperation Radiotherapy in Respectable Rectal Cancers,” New England Journal Medicine, Vol. 336, No. 3, 1997, pp. 423-430.
- B. J. Walz and J. W. Fleshman Jr., “Adjunctive Use of Radiation Therapy in Rectal Adenocarcinoma,” In: I. J. Kodner, R. D. Fry and J. P. Roe, Eds., Colon, Rectal and Anal Surgery, Current Techniques and Controversies, CV Mosby Co., Saint Louis, 1985, pp. 204-217.
- S. B. Wassif, B. L. Langenhorst and W. C. Hop, “The Contribution of Preoperative Radiotherapy in the Management of Borderline Operability Rectal Cancer,” In: S. E. Salmon and S. E. Jones, Eds., Adjuvant Therapy of Cancer, Grune & Stratton, New York, 1979, pp. 612-621.
- S. B. Wassif, “The Role of Preoperative Adjuvant Therapy in the Management of Borderline Operability Rectal Cancer,” Clinical Radiology, Vol. 33, No. 3, 1982, pp. pp. 353-358. doi:10.1016/S0009-9260(82)80289-4
- R. Zucali, G. Gardani and F. Volterrani, “Adjuvant Post-Operative Radiotherapy in Locally Advanced Rectal and Rectosigmoidal Cancer,” Tumori, Vol. 31, No. 4, 1980, pp. 592-600.
NOTES
*Corresponding author.

