Journal of Cancer Therapy
Vol.3 No.1(2012), Article ID:17217,7 pages DOI:10.4236/jct.2012.31008
Organ Preservation in Bilharzial Bladder Cancer in Egypt: Single Institutional Experience
![]()
1Departments of Radiotherapy, Assiut University, Assiut, Egypt; 2Departments of Urology Surgery, Assiut University, Assiut, Egypt; 3Departments of Surgical Oncology, Assiut University, Assiut, Egypt; 4Departments of Radiology, Assiut University, Assiut, Egypt; 5Departments of Medical Oncology, Assiut University, Assiut, Egypt.
Email: maboziada70@yahoo.com
Received October 22nd, 2011; revised November 26th, 2011; accepted January 20th, 2012
Keywords: Bladder; Gemcitabine; Radiotherapy
ABSTRACT
Background: Phase II study was conducted to evaluate bladder preservation protocol in Bilharzial and non Bilharzial invasive transitional cell carcinoma (TCC) bladder cancer using gemcitabine and conformal radiotherapy (RT). Methods: 30 TCC patients with good performance status and renal function subjected to maximum trans-urethral resection of bladder tumor (TURBT). Patients received 66 Gy/33 fractions/6.5 weeks with weekly gemcitabine 125 mg/m2. Evaluation was done after one month with cystoscopy and CT/MRI pelvis. Patients who had complete remission (CR) subjected for follow up and patients who had invasive bladder tumor subjected to radical cystectomy. Results: 24 patients had CR after one month evaluation. Stage 2 tumor, low grade, non Bilharzial and maximum TUR were the only prognostic factors. The treatment schedule was tolerable and was associated with infrequent incidence of moderate toxicity that was easily controlled without interruption of RT. Cystectomy free survival was 88% at a median follow up for 2 years. Conclusions: Gemcitabine and conformal RT after TURBT treatment could be an effective way to achieve a high response rate in the treatment of invasive TCC of the bladder with good tolerance. Organ preservation in Bilharzial bladder is still possible.
1. Introduction
Bladder cancer in Egypt constitutes 30% of all cancer cases treated at Egyptian National Cancer Institute. Bilharziasis (schistosomiasis) is an endemic disease in Egypt and mostly associated with squamous cell carcinoma [1]. Our south Egypt cancer institute (SECI) reported a relative frequency of 18.5% from year 2004-2006.
Although Radical cystectomy with lymphadenectomy continues to be the standard of care and primary choice for patients with muscle-invasive [2] it carries significant physical, sexual, and psychological morbidity, and quality of life in some patients is impaired, even when neobladder reconstruction is used. In addition, patients with muscle-invasive bladder cancer (MIBC) frequently have significant co-morbidities that render them unsuitable for high-risk radical surgery. This group of patients would benefit from a non-surgical therapy that would improve the local control of the bladder cancer [3,4].
There are no randomized data to compare radiotherapy (RT) and surgery, but a study of patients recruited from one geographic area within the United Kingdom has shown similar outcomes, including cause-specific survival rates of approximately 50% at 5 years from either modality [5]. When transurethral resection of a bladder tumor (TURBT), radiation, and multi-agent chemotherapy are combined, complete response rates of 70% have been achieved. Most chemo-radiation regimens for MIBC employ concurrent cisplatinum in various doses and schedules [6-9].
In spite of the evidence of cisplatinum efficacy [6-9], cisplatinum is nephrotoxic. Therefore, it requires preand post-infusion hydration, and it often necessitates hospital admission. It is significantly myelopsuppressive and emetogenic. Gemcitabine is active in bladder cancer and used in combination with platinum as a standard of care in the neoadjuvant and metastatic settings [7,10,11]. Studies have also shown that gemcitabine is a potent radiosensetizer, and its efficacy has been demonstrated in various cancer cell lines, including lines derived from bladder tumors [12-14]. The maximal tolerated dose for gemcitabine given once weekly with concurrent conformal bladder radiotherapy was 150 mg/m2, and 125 mg/m2 proved to be safe when given as a weekly radiosensetizer [15].
Aim of the Work
The primary objectives are to determine the tumor response rate and prognostic factors affecting patients’ response to chemo-radiotherapy (CRT) using gemcitabine and conformal radiotherapy in the treatment of patients with muscle-invasive transitional cell carcinoma (TCC) of bilharziasis and non bilharziasis bladder, as assessed by cystoscopy one month after completion of treatment. Secondary objectives are to measure the severity of toxicity, cystectomy free survival, rate of local recurrence and distant metastasis.
2. Patients and Methods
This study recruited 30 patients between February 2008 and November 2010 and approved by our institute ethics committee and informed consent.
Eligible patients had histologically confirmed muscleinvasive TCC that were clinical stage T2 or T3, N0, M0 and after safe maximum trans-urethral resection of bladder tumor (TURBT). All patients had absolute neutrophil count (ANC) ≥ 1500/µL, platelets ≥ 100,000/µL; creatinine ≤ 2.0, bilirubin ≤ 3 times the institutional upper limit of normal, AST ≤ 4 institutional upper limit of normal, Eastern Cooperative Oncology Group performance status ≤ 1, and no prior radiotherapy or systemic therapy for bladder transitional cell carcinoma. Patients were ineligibile if they have multicentric tumors and received intra-vasical BCG, chemotherapy or pelvic irradiation.
2.1. Treatment Protocol
Treatment started within 6 weeks following maximum safe transurethral resection and all patients were treated with combined radio-chemotherapy. Chemotherapy protocol was gemcitabine 125 mg/m2 weekly on Saturday as a 30-minute infusion for 7 weeks, within 2 hours before radiation therapy. We delivered radiotherapy in 2 phases: Phase I, 46 Gy/23 fractions/4.5 weeks to the whole pelvis and Phase II, 20 Gy/10 fractions/2 weeks to the bladder.
• Radiation therapy:
We used 3D conformal radiotherapy. The patient must void to empty the bladder immediately prior to simulation. The rectum should be as empty as possible before simulation (may use enema). On CT simulator, we positioned the patient supine, with leg support. Multiple CT cuts at 0.5 cm interval were obtained throughout the pelvis. CT data transferred to the computer treatment planning system (XiO 4.2). At each CT slice, we defined the clinical target volume (CTV) bladder, prostate and prostatic urethra (in men), and the lymph nodal pelvic regions of the internal iliac, the external iliac, and the obturator lymph nodes and organs at risk; rectum, small intestine, bilateral femoral head. Radiotherapy delivered in 2 phases.
Phase I (Pelvic Fields): CTV-pelvis encompassed the entire bladder, prostate and prostatic urethra (in men), and the lymph nodal pelvic regions of the internal iliac, the external iliac, and the obturator lymph nodes. The field margins of planning target volume (PTV) in the inferior and superior dimensions extended 1 cm below the lower pole of the obturator foramen to the mid-sacrum (the anterior aspect of the S1-S2 junction). Laterally, the anterior field extended at least 1.5 cm beyond the widest point of the bony margin of the pelvis. For the parallel opposed lateral fields, the field edges extended 3.0 cm posterior to the CTV-bladder and 1.5 cm anterior to the anterior tip of the bladder, whichever is the most anterior. We used blocks on the anterior field to shield the medial border of the femoral heads and on the lateral opposed fields inferiorly to shield the soft tissue anterior to the pubic symphysis and to block the anal canal posteriorly. Superiorly, the lateral fields might include blocks anteriorly to exclude the small bowel and the anterior rectus fascia, which lay anterior to the external iliac lymph node chain.
Phase II (Whole Bladder Field): The CTV-bladder included any gross tumor volume (GTV), the entire bladder volume, and the entire bladder wall thickness. The PTV bladder consisted of a margin 1 cm around the CTVbladder edges except superiorly where the extension was 1.5 cm.
Dose Limiting Structures: The maximum dose to the femoral heads should be less than 45 Gy. Fifty percent of the rectum volume should receive less than 55 Gy. The rectum volume was defined on CT from the anus (at the level of the ischial tuberosities) for a length of 15 cm.
Technical Factors: We used a high energy linear accelerator with photon energies 6 and 15 MV using a three field for the initial small pelvic fields and four field box for the boost for the whole bladder volume. We chose the field weighting and beam modifiers (wedges and blocks) to keep the maximum dose of OAR below their tolerance. Dose prescribed at isocenter and a complete 3D plan was done taken in consideration the ICRU 50 (International Commission on Radiation Units and Measurements) recommendations (A certain degree of heterogeneity should be kept within +7% and –5% of prescribed dose).
• Evaluation, Toxicity, and Response Criteria:
Pre-treatment evaluation included complete history and physical examination; performance status assessment; complete blood count; electrolytes; blood urea nitrogen; serum creatinine; liver function tests; total protein, albumin; and appropriate imaging studies; chest x-ray, and abdominal pelvic CT and/or MRI to assess extent of disease.
During treatment, we evaluated patients weekly and monitor toxicity. Every week, we performed complete blood counts, including differential and platelets before each administration of gemcitabine. Additional laboratory investigations every 3 weeks included electrolytes, liver function, and kidney function.
One month after completion of chemo-radiation, patients underwent repeat transurethral resection of the site of the original tumor, as well as CT and/or MRI scan of the pelvis. Complete remission (CR) defined as complete disappearance of all measurable and evaluable disease, confirmed by cystoscopy and biopsy. Local failure defined as presence of superficial recurrence or persistent invasive cancer. In case of persistent invasive cancer at initial evaluation after CRT, we recommended salvage cystectomy.
Patients underwent a follow-up visit every month on the first 6 months, every 2 months during the rest of the first year and every 3 months thereafter. At each visit, we performed history and physical examination. Chest x-ray, and abdominal pelvic CT and/or MRI every three months or when indicated, and cystoscopy every 6 months or when indicated. CT and/or MRI scans of the abdomen and pelvis performed either before or at least 4 weeks after any biopsy.
2.2. Statistical Methods
The statistical analysis included chi-square test for comparing percentages. Disease free survival (DFS) and overall survival (OS) rates were calculated according to Kaplan-Meier [16] actuarial method from the time of diagnosis. Log rank test was used to compare survival rates. Cox regression test was used for multivariate analysis. The p-values were double-sided and ≤0.05 was the level of significance. Acute and chronic toxicities from radiotherapy and chemotherapy were reported according to Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0 [17].
3. Results
We recruited 30 patients between February 2008 and November 2010. Patients characteristics are listed in Table 1.
Treatment response:
We assessed the response to chemo-radiation one month after completion of treatment and we found that 24 patients (80%) had CR, two patients had non-MIBC, and 4 patients (13%) had residual invasive disease and were subjected to salvage cystectomy. Patients who had nonMIBC were managed by TURBT and intra-vasical BCG, and became free of tumor. Cystoscopy after 3 months revealed 26 patients (86.7%) had CR.
Prognostic factors affecting early response after one month (Table 2): We studied the following known risk factors tumor size (T), grade, bilharzias, hydronephrosis

Table 1. Patient characteristics.

Table 2. Early response to treatment according to prognostic factors.
and TUR. Early stage (T2), high grade, absence of bilharziasis and complete TUR were associated with high CR rate. However, hydronephrosis did not have significant effect on rate of complete remission.
Survival:
The median follow up was 2-year (range: 8 - 38 months). Two years cystectomy free survival for completely studied (30) patients was 76.7% and DFS was 70% (Figure 1). Distant metastasis (DM) rate was 6.7%. During follow up two patients died from causes not related to bladder cancer. The first patient died from cardiac cause after 3 months and the second patient died after 5 month from postoperative complication after fixation for traumatic fracture neck of femur. Overall survival was 93.3% (Figure 1) however disease specific survival was 100%. Studying the different prognostic factors affecting disease free survival at 2 years revealed

Figure 1. OAS, DFS and Cystectomy free survival for all patients.
significant effects for early stage, high grade, and absence of bilharziasis and complete TUR (Figures 2-5). However, hydronephrosis (Figure 6) did not have significant effect on disease free survival at 2 years. Patients who had CR after chemo-radiation (26 patients), 2 years DFS was 80.7% and cystectomy free survival was 88.4% (Figure 7).
Toxicity:
All patients completed radical radiotherapy. However, five patients could not complete the concurrent gemcitabine regime; two patients had three weeks, two patients had four weeks and one patient had 5 weeks. All five patients stopped concurrent gemcitabine early because of grade 3 gastrointestinal toxicity during radiotherapy.
Adverse events (Table 3) of grade 3 or higher were anemia (5/30) and diarrhea (5/30). Other minor complications (grade 1/2) were cystitis in 17/30 cases, proctitis in 16/30 cases, nausea and vomiting in 11/30 cases, diarrhea in 15/30 cases and anemia in 10/30 cases. Mild chronic toxicity only was observed in this study (grade I only); 13 patients (43%) had grade I frequency and 10 patients (33%) had grade I dysuria which was mild requiring no intervention.
4. Discussion
The most important points of this study were the higher incidence of CR and the tolerability to treatment. Eighty seven percent (26 of 30) patients achieved CR after chemo-radiation. This is higher than previous results from our department (CR = 72%) [18] and also from other Egyptian result [19]. In the Massachusetts General Hospital series of 190 patients treated by tri-modality therapy between 1986 and 1998, 63% exhibited a CR [20]. In another series from Germany and Spain, CR was 80% and 89% respectively [21,22]. The radiotherapy oncology group (RTOG) trials demonstrated a CR rate after induction in 75% and 59% of the patients [8,23]. RTOG twice daily protocol revealed 81% CR after induction phase [24]. Cisplatinum was the cornerstone of chemotherapy in most of these trials. Bladder preservation protocols depend on two phases of treatment with break to evaluate the efficacy of management. Our study was one phase without interruption of chemo-radiation. This may improve the biological effect of radiotherapy.
The high response rate was achieved more in patients having early stage (T2), high grade, and absence of bilharziasis and complete resection of the tumor. Obstructive uropathy did not have significant effect. There is agreement that early stage and maximum TURBT are significantly affecting the response [8,18-24]. Grade of tumor is reflecting the behavior of disease. It was significance
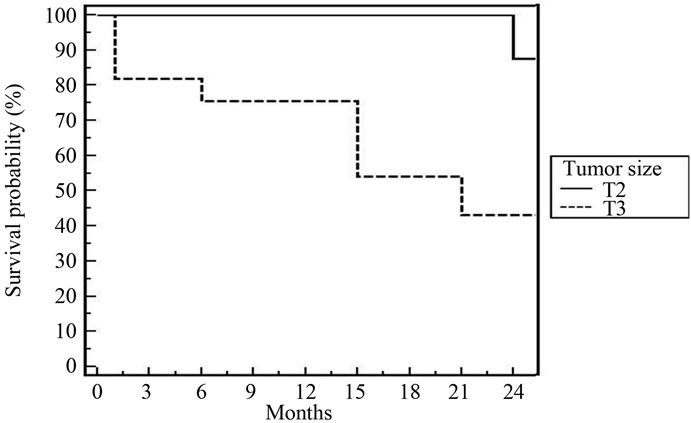
Figure 2. 2-year DFS for all patients according to tumor size [p = 0.04].
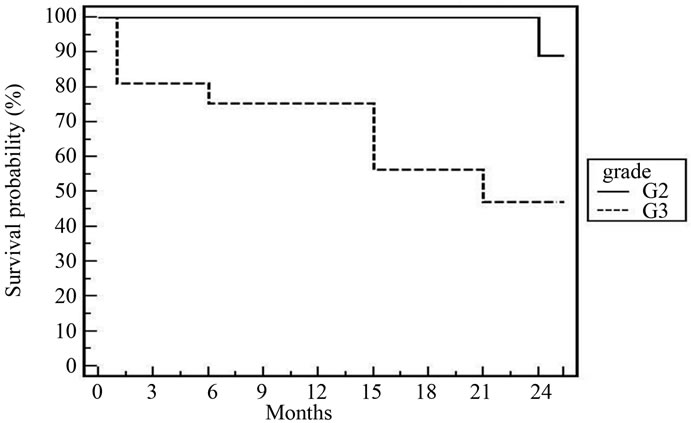
Figure 3. 2-year DFS for all patients according to grade [p = 0.037].
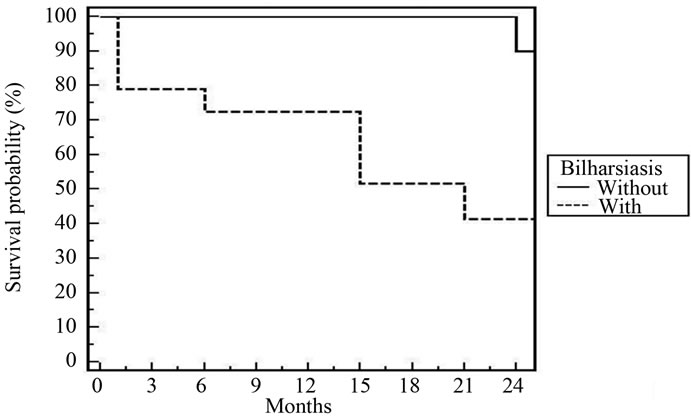
Figure 4. 2-year DFS for all patients according to bilharziasis [p = 0.017].
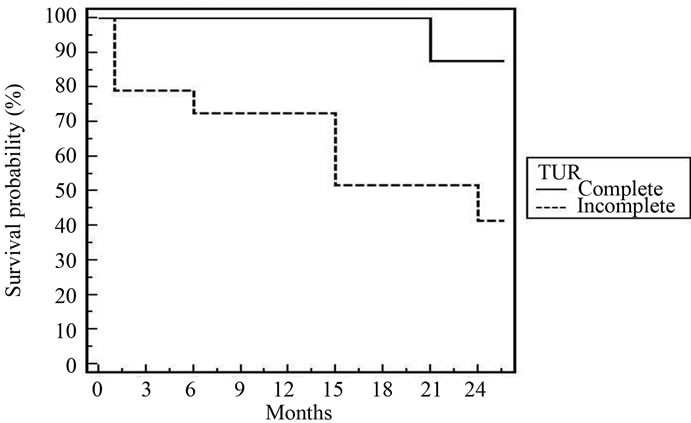
Figure 5. 3-year fig DFS for all patients according to TUR [p = 0.026].
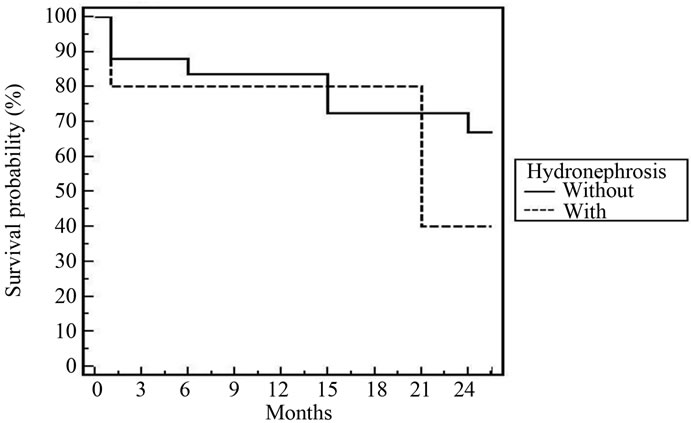
Figure 6. 3-year DFS for all patients according to hydronephrosis [p = 0.44].
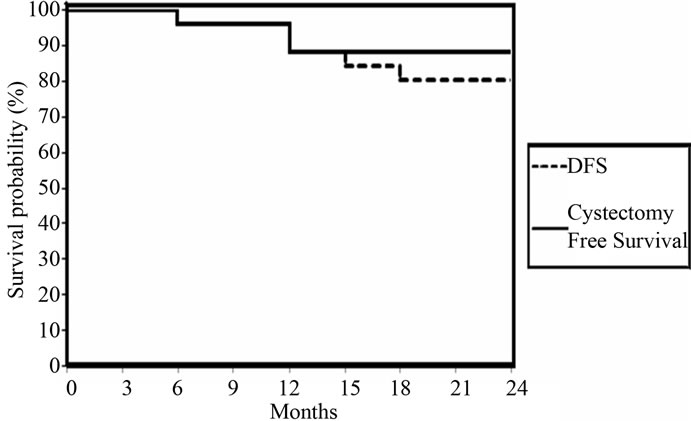
Figure 7. DFS and Cystectomy Free Survival for 26 patients who had CR at 3 months.

Table 3. Acute toxicities.
in some trials [18,21]. The presence of hydronephrosis at the time of diagnosis did not impact our treatment success. In the MGH series [20], 27 of 190 patients had hydronephrosis initially, and their complete response rate was 37%, compared to 68% in patients without hydronephrosis (p = 0.002). The impact of ostructive uropathy depends on the cause of obstruction and tolerability to cisplatinum chemotheapy. Bilharzial (schistosomiasis) bladder cases are the most common due to endemic disease in Egypt. Nineteen patients (63%) of our patients were bilharzial and 79% of bilharzial bladder (15/19) had CR. Still bilharziasis has a positive impact in the response of treatment compared with absence [18].
The profile of toxicity is tolerable in the majority of cases. Five patients (16%) did not complete the planned doses of chemotherapy. Gastrointestinal toxicity G3 reported in 4/30 and G3 anemia in 5/30. Shieply et al. [23] reported during induction phase that 26% of the 80 patients had acute toxicity of either grade 3 (25%) or grade 4 (1%). Among the grade 3 acute induction of toxicity, 15% were gastrointestinal and 4% were urologic. During the consolidation phase, 8% of the patients had grade 3 or grade 4 toxicity of gastrointestinal and urinary events respectively. Arias et al., [25] found that grade III diarrhea in 4% and Sauer et al., [21] who reported grade IV gastrointestinal toxicity in 0.6% of cases. Late toxicity reported in the present study was G1 frequency and dysuria. Grade III to IV frequency and dysuria were not encountered. Rodel et al., [26] reported that 10% only developed grade II dysuria. Shieply et al., [23] reported 4% G3 bladder toxicity at 5-years and no G3 of gastrointestinal toxicity.
In spite of the relative short follow up period (2 years), cystectomy free survival was 88% and DFS was 78.7%. These figures are comparable to that reported by most clinical trials [20,21,23,25,26]. According to multivariate and univariate analyses of tumor factors influencing DES after our treatment; T2, low grade, absence of bilharziasis with complete resection were the only prognostic factors. In the Boston experience [20], the 5-year overall survival for T2 tumours was 62%, and for T3-4 tumors, it was 47%. Rodel et al. [26] found a statistically significant as sociation between the completeness of the TURBT and both higher complete response rate and improved overall survival. Hydronephrosis was significant in some trials [20,25].
5. Conclusion
The use of gemcitabine is making that agent another potential drug for use in bladder preservation protocols. Organ preservation in bilharzial bladder is still possible. The toxicity was acceptable, and most of the patients were disease-free with a native bladder at a median follow-up of 24 months. Kent et al. [27] from the University of Michigan reported on a phase I trial using twiceweekly gemcitabine (27 mg/m2) concomitantly with conventionally fractionated RT. The use of intensity modulated radiotherapy in bladder cancer is still preliminary, and further experience is obviously needed. However, the prospect of delivering a higher biologically equivalent dose (concomitant boost technique) or of dose escalation under conventional fractionation is an attractive alternative that is now being explored in prospective trials. These preliminary but encouraging results will now be tested in a randomized phase II study by the RTOG.
REFERENCES
- G. M. Nazli, M. M. El Bolking and H. M. Khaled, “Bladder Cancer in Africa: Update,” Seminars in Oncology, Vol. 28, No. 2, 2001, pp. 174-178. doi:/10.1016/S0093-7754(01)90089-2
- A. Faysal, M. D. Yafi and M. D. Wassim Kassouf, “Radical Cystectomy Is the Treatment of Choice for Invasive Bladder Cancer,” Canadian Urological Association Journal, Vol. 3, No. 5, 2009, pp. 409-412.
- D. Raghavan and R. Huben, “Management of Bladder Cancer,” Current Problems in Cancer, Vol. 19, No. 1, 1995, pp. 3-63. doi:/10.1016/S0147-0272(07)80002-X
- A. Choudhury, R. Swindell, J. P. Logue, P. Anthony Elliott, J. E. Livsey, M. Wise, P. Symonds, J. P. Wylie, V. Ramani, V. Sangar, et al., “Phase II Study of Conformal Hypofractionated Radiotherapy with Concurrent Gemcitabine in Muscle-Invasive Bladder Cancer,” Journal of Clinical Oncology, Vol. 29, No. 6, 2011, pp. 733-738. doi:/10.1200/JCO.2010.31.5721
- S. Kotwal, A. Choudhury, C. Johnston, et al., “Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center,” International Journal of Radiation Oncology Biology Physics, Vol. 70, No. 2, 2008, pp. 456-463. doi:/10.1016/j.ijrobp.2007.06.030
- J. Dunst, R. Saur, K. M. Schrott, et al., “Organ-Sparing Treatment of Advanced Bladder Cancer: A 10-Year Experience,” International Journal of Radiation Oncology Biology Physics, Vol. 30, No. 2, 1994, pp. 261-266.
- D. S. Kaufman, W. U. Shipley, P. P. Griffin, N. M. Heney, A. F. Althausen and J. T. Efird, “Selective preservation by combination treatment of invasive bladder cancer,” The New England Journal of Medicine, Vol. 329, No. 19, 1993, pp. 1377-1382. doi:/10.1056/NEJM199311043291903
- M. Housset, C. Maulard, Y. C. Chretien, et al., “Combined Radiation and Chemotherapy for Invasive Transitional-Cell Carcinoma of the Bladder: A Prospective Study,” Journal of Clinical Oncology, Vol. 11, No. 11, 1993, pp. 2150-2157.
- L. Eapen, D. Stewart, C. Danjoux, P. Genest, N. Futter, et al., “Intraarterial Cisplatin and Concurrent Radiation for Locally Advanced Bladder Cancer,” Journal of Clinical Oncology, Vol. 7, No. 2, 1989, pp. 230-235.
- doi:/10.1016/j.eururo.2009.01.002
- A. Stenzl, N. Cowan, M. De Santis, et al., “The Updated EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer,” European Urology, Vol. 55, No. 4, 2009, pp. 815-825.
- H. Von der Maase, L. Sengelov, J. Roberts, et al., “LongTerm Survival Results of a Randomized Trial Comparing Gemcitabine plus Cisplatin, with Methotrexate, Vinblastine, Doxorubicin, plus Cisplatin in Patients with Bladder Cancer,” Journal of Clinical Oncology, Vol. 23, No. 2, 2005, pp. 4602-4608. doi:/10.1200/JCO.2005.07.757
- V. K. Sangar, C. A. McBain, J. Lyons, et al., “Phase I study of conformal radiotherapy with concurrent gemcitabine in locally advanced bladder cancer,” International Journal of Radiation Oncology Biology Physics, Vol. 61, No. 2, 2005, pp. 420-425. doi:/10.1016/j.ijrobp.2004.05.074
- V. K. Sangar, R. Cowan, G. P. Margison, et al., “An evaluation of gemcitabines differential radiosensitising effect in related bladder cancer cell lines,” British Journal of Cancer, Vol. 90, No. 2, 2004, pp. 542-548. doi:/10.1038/sj.bjc.6601538
- B. Pauwels, A. E. C. Korst, F. Lardon and J. B. Vermorken, “Combined Modality Therapy of Gemcitabine and Radiation,” The Oncologist, Vol. 10, No. 1, 2005, pp. 34- 51. doi:/10.1634/theoncologist.10-1-34
- V. K. Sangar, C. A. McBain, J. Lyons, V. A. Ramani, J. P. Logue, J. P. Wylie, N. W. Clarke and R. A. Cowan, “Phase I Study of Conformal Radiotherapy with Concurrent Gemcitabine in Locally Advanced Bladder Cancer,” International Journal of Radiation Oncology Biology Physics, Vol. 62, No. 2, 2005, pp. 420-425. doi:/10.1016/j.ijrobp.2004.05.074
- E. L. Kaplan and P. Meier, “Nonparametric estimation from incomplete observations,” Journal of American Statistical Association, Vol. 53, No. 282, 1958, pp. 457-481. doi:/10.2307/2281868
- Common Toxicity Criteria, Version 3. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- M. a. Aboziada, H. m. Hamza and A. m. Abdlrahem, “Initial Results of Bladder Preserving Approach by ChemoRadiotherapy in Patients with Muscle Invading Transitional Cell Carcinoma,” Journal of the Egyptian National Cancer Institute, Vol. 21, No. 2, 2009, pp. 167-174.
- H. Shawky and G. El-Deen, “Initial Results of Retrospective Study: Preoperative Transurethral Excision plus Chemotherapy and Radiation Therapy and Trial of Bladder Preservation,” Journal of the Egyptian National Cancer Institute, Vol. 19, No. 2, 2007, pp. 133-146.
- W. U. Shipley, D. S. Kaufman, E. Zehr, et al., “Selective Bladder Preservation by Combined Modality Protocol Treatment: Long-Term Outcomes of 190 Patients with Invasive Bladder Cancer,” Urology, Vol. 60, No. 1, 2002, pp. 62-67. doi:/10.1016/S0090-4295(02)01650-3
- R. Sauer, S. Birkenhake, R. Kuhn, C. Wittekind, K. M. Schrott and P. Martus, “Efficacy of radiochemotherapy with platin derivatives compared to radiotherapy alone in organ-Sparing treatment of bladder cancer,” International Journal of Radiation Oncology Biology Physics, Vol. 40, No. 1, 1998, pp. 121-127. doi:/10.1016/S0360-3016(97)00579-8
- A. Zapatero, C. Martin de Vidales, R. Arellano, et al., “Updated results of bladder sparing trimodality Approach for invasive bladder cancer,” Urologic Oncology: Seminars and Original Investigations, Vol. 28, No. 4, 2009, pp. 368-374.
- W. U. Shipley, K. A. Winter, D. S. Kaufman, et al., “Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89-03,” Journal of Clinical Oncology, Vol. 16, No. 11, 1998, pp. 3576-3583.
- D. S. Kaufman, K. A. Winter, W. U. Shipley, et al., “Phase I-II RTOG Study (99-06) of Patients with Muscle-Invasive Bladder Cancer Undergoing Transurethral Surgery, Paclitaxel, Cisplatin, and Twice-Daily Radiotherapy Followed by Selective Bladder Preservation or Radical Cystectomy and Adjuvant Chemotherapy,” Urology, Vol. 73, No. 4, 2009, pp. 833-837. doi:/10.1016/j.urology.2008.09.036
- F. Arias, M. A. Dominguez, E. Martinez, et al., “Chemoradiotherapy for Muscle Invading Bladder Carcinoma. Final Report of a Single Institutional Organ-Sparing Program,” International Journal of Radiation Oncology Biology Physics, Vol. 47, No. 2, 2000, pp. 373-378. doi:/10.1016/S0360-3016(00)00444-2
- C. Rodel, G. G. Grabenbauer, R. Kuhn, et al., “Organ preservation in patients with invasive bladder cancer: initial results of an intensified protocol of transurethral surgery and radiation therapy plus concurrent cisplatin and 5-fluorouracil,” International Journal of Radiation Oncology Biology Physics, Vol. 52, No. 2, 2002, pp. 1303- 1309. doi:/10.1016/S0360-3016(01)02771-7
- E. Kent, H. Sandler, J. Montie, et al., “Combined-Modality therapy with gemcitabine and radiotherapy as a bladder preservation strategy: results of a phase I trial,” Journal of Clinical Oncology, Vol. 22, No. 13, 2004, pp. 2540-2545. doi:/10.1200/JCO.2004.10.070

