Journal of Cancer Therapy
Vol.2 No.5(2011), Article ID:16595,5 pages DOI:10.4236/jct.2011.25087
Study of the Peripheral Blood CD44 Expression in the Patients with Non-Small Cell Lung Cancer
![]()
1College of Pharmacy, Xinxiang Medical University, Xinxiang, China; 2Hospital of People’s Library Army, Huludao, China; 3Hospital of People’s Library Army, Hangzhou, China.
E-mail: *bcd2009@126.com
Received October 2nd, 2011; revised November 3rd, 2011; accepted November 12th, 2011.
Keywords: CD44 expression, Non-small cell lung cancer, Peripheral blood, Treatment
ABSTRACT
Previous study has demonstrated that the peripheral blood CD44 expression level is related with the clinical stage and lymph node metastasis of lung cancer. The present comment was to investigate the relationship between the peripheral blood CD44 expression level and clinic pathological change in 50 patients with non-small cell lung cancer (NSCLC) by flow cytometry method. The results showed that 1) the peripheral blood CD44 expression level in the NSCLC group was higher than that in the benign group (467 ± 15) or the normal group (448 ± 15); 2) operation decreased the peripheral blood CD44 expression level in the NSCLC group (533 ± 10 vs 324 ± 11); 3) it also showed same results in NSCLC patients with and without lymph node metastasis (559 ± 12 vs 477 ± 15) or before and after chemotherapy (550 ± 13 vs 372 ± 10); 4) there were significant differences in the peripheral blood CD44 expression level in non-small cell lung cancer patients of the clinical stage I, II, III and IV (474 ± 14, 526 ± 12, 528 ± 16 and 599 ± 20); And the peripheral blood CD44 expression level was not associated with the clinical pathology parameter including the patient age, gender and tumor size. The data suggested that the peripheral blood CD44 expression level was related with the NSCLC progress, lymphatic metastasis and clinical treatment, and the peripheral blood CD44 expression level as the clinical regular examination should evaluate the progress, lymphatic metastasis and clinical treatment for the patients with NSCLC.
1. Introduction
The hematogenous metastasis is very complicated pathological progress, and the integrins and other adhesion molecules play roles in tumor progression and metastasis [1]. CD44 is a polymorphic family of immunologically related cell surface proteoglycans and glycoproteins, which normally take part in cell-cell and cell-matrix adhesion interactions, lymphocyte activation and homing, and cell migration. The serum CD44 expression level is a mark relating with clinic pathological feature in the patients with non-small cell lung cancer (NSCLC)[2,3]. Some studies have pointed that the peripheral blood CD44 expression levels are correlated with the invasion and metastasis of neoplasm [4]. For understanding the role of the peripheral blood CD44 expression level in clinical treatment of NSCLC, the present comment was to investigate the role of the peripheral blood CD44 expression level in the progress, clinic pathological stage, lymphatic metastasis and clinical treatment for the patients with NSCLC.
2. Material and Methods
2.1. Patient Selection
For the NSCLC cancer group, 50 cases including male 34 and female 16, aged 30 - 74 years, average aged 38.1 ± 3.5 years with NSCLC received physical examination, X ray and CT. The patients were diagnosed by the operation and needle biopsies, in which 36 patients had lymphatic metastasis and 14 patients have not; 28 patients were diagnosed of squamous cancer, 18 of adenocarcinoma, and 4 of large cell undifferentiated carcinoma. For histological classification, 10 patients were in high differentiation development (HDD), 18 in middle differentiation development (MDD) and 22 in low differentiation development (LDD). According UICC classification [5], 10 patients were in stage I, 10 in stage II, 16 in stage Ⅲ, and 14 in stage IV.
In the benign group, 25 cases including male 15 and female 10, aged 24 - 56 years, average aged 30.1 ± 3.5 years, suffered pneumonia or pulmonary tuberculosis. The normal group included 30 healthy donors, male 19 and female 11, aged 18 - 25 years, average aged 22.9 ± 2.5 years.
2.2. Therapeutic Methods
In the NSCLC group, 32 patients were treated with the operation and 18 with chemotherapy.
2.3. Sample Collection
Two ml peripheral venous blood was collected using the EDTA.Na2 treated tube.
2.4. CD44 Expression Assay
Mouse anti-human monoclonal antibody CD44-FITC (The immunogen was purified human T-Cells) and sheep anti mouse IgG FITC as negative control provided by French International Immunity Company. We treated the blood samples and detected CD44 expression level by flow cytometry according to the literature [6]. Cells suspension labeled fluorescent antibody were examined by laser scanning co-focal microscope, then positive expression level of green phosphor was elevated.
2.5. Statistical Analysis
All data were expressed as mean ± standard error of the mean (SEM) and were analyzed between groups by analysis of variance (ANOVA), with two-way ANOVA followed by the Bonferroni test and one-way ANOVA followed by the Dunnett test and the Newmann-Keuls test. P < 0.05 was considered statistically significant.
3. Results
3.1. Correlation with Non-Small Cell Lung Cancer
The CD44 positive spots were observed in the cell membrane in the NSCLC group, benign group and normal group, and CD44 expressed relatively in lymphocyte cells. The peripheral blood CD44 expression level in the NSCLC group (533 ± 10) was higher than that in the benign group (467 ± 15) and normal group (448 ± 15), which statistical analysis showed significant difference (F = 7.21, 7.42, P < 0.01) and there was no significant difference between the benign group and normal group (F = 2.17, P > 0.05) (Table 1).
However, there were not significant difference (all P > 0.05) for the peripheral blood CD44 expression levels in the gender (male and female), age (≤50 years and >50 years), lump (≤3 cm and >3 cm) and pathologic type (squamous cancer and adenocarcinoma) (Table 2).
3.2. Operation Treatment
The peripheral blood CD44 expression level before the operation (497 ± 15) was higher than that after the operation (324 ± 11) in the NSCLC group, which statistical analysis showed significant difference (F = 7.85, P < 0.01) (Table 3).

Table 1. Peripheral blood CD44 expression levels in the patients with non-small cell lung cancer.
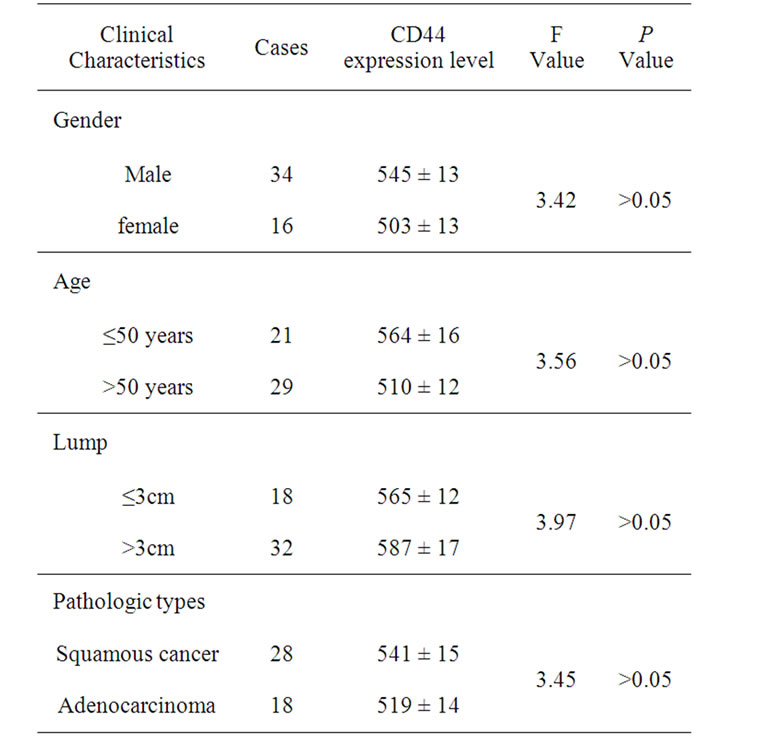
Table 2. Peripheral blood CD44 expression levels in the clinical lung cancer cases.
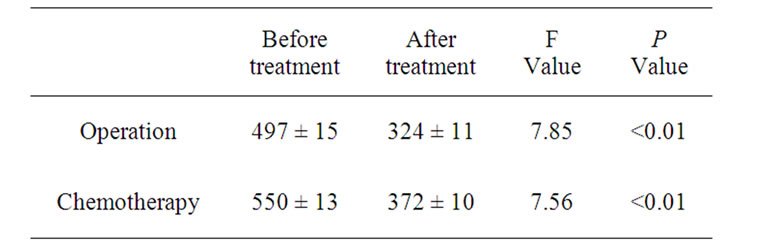
Table 3. Peripheral blood CD44 expression level in the patients with non-small cell lung cancer before and after treatment.
3.3. Chemotherapy Treatment
Chemotherapy treatment decreased the peripheral blood CD44 expression level in the NSCLC group from 550 ± 13 to 372 ± 10, which statistical analysis showed significant difference (F = 7.56, P < 0.01) (Table 3).
3.4. Histological Types
There was the negative relationship between the peripheral blood CD44 expression level and the histological types in the NSCLC group (Table 4).
3.5. Non-Small Cell Lung Cancer Progress
The peripheral blood CD44 expression level in the NSCLC group was 474 ± 14, 526 ± 12, 528 ± 16 and 599 ± 20 respectively in clinical stage I, II, III and IV. Although there were no significant difference between stage I and II (F = 3.24, P > 0.05) or between stage III and IV (F = 3.11, P > 0.05), it showed significant differences between stage III + IV and stage I + II (F = 7.32, P < 0.01).
3.6. Lymph Node Metastasis
The peripheral blood CD44 expression with lymph node metastasis (559 ± 12) was higher than that without lymph node metastasis (477 ± 15), which statistical analysis showed significant difference (F = 7.35, P < 0.01).
4. Discussion
Many studies have demonstrated that the peripheral blood CD44 expression level relates with the neoplasm progress and lymph node metastasis [4,7]. It has also been proven that the peripheral blood CD44 expression level is an important mark of the lung cancer stage and lymph node metastasis by ELISA method [8]. By the flow cytomety method, the present study showed that the CD44 expression level in peripheral lymphocytes increased significantly in the patients with NSCLC, although there was no difference between the benign group
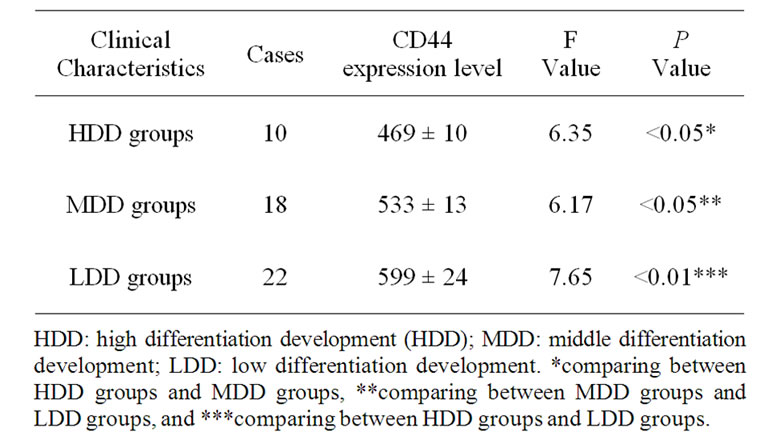
Table 4. Peripheral blood CD44 expression level in different histological types.
and normal group, there were significant differences in the peripheral blood CD44 expression level in non-small cell lung cancer patients of the clinical stage I, II, III and IV. The data suggested that the peripheral blood CD44 expression level could reflect the change of NSCLC progress. Lesley et al. believed that CD44 participated in the activation, multiplication and recirculation of peripheral lymphocytes, which indicated that tumor cells expressing CD44 related with the activation transportation and multiplication of lymphocytes after the departure from the primary lesion to enter the blood circulation [8]. Expression of CD44 and its variants has been shown to be relevant to tumor progress in various human malignancies. CD44 might be correlated with histogenesis of NSCLC, and its decreased expression may be an adverse prognostic indicator for the patients with stage I NSCLC, especially for those with stage IB diseases [9]. The present study showed that the peripheral blood CD44 expression level in the NSCLC patients with lymph node metastasis was higher than that in the patients without lymph node metastasis. Our data supported the point that the activation, transportation and multiplication of lymphocytes related with the cell transfer of lung cancer through the peripheral blood.
The present study showed that the operation decreased the peripheral blood CD44 expression in the patients with NSCLC. We believed that the reason, the CD44 expression declined, was induced by the overload, intrinsic metastasis of lung tumor before the operation, and the shrinkage of tumor burden and reduction of the tumor cell entering into blood after the operation. One of several splice variants of CD44 expressed in metastasizing cell lines of rat tumors has been shown to confer metastatic potential to the non-metastatic variant of a rat pancreatic carcinoma line. The distribution of CD44 splice variants is consistent with the speculation that they fulfill functions in only a few restricted differentiation pathways and that in tumor cells these pathways have been reactivated [10].
Chemotherapy not only retards cancer cells but also kills lymphocytes [11]. The present study showed that the peripheral blood CD44 expression level in the NSCLC group before the operation was higher than that in the NSCLC group after the operation. CD44 designates a large family of proteins with a considerable structural and functional diversity, which are generated from one gene by alternative splicing. As such, the over expression of CD44 variant isoform (CD44v) has been causally related to the metastatic spread of cancer cells. CD44v-mediated matrix formation is crucial for the settlement and growth at a secondary site, whereas apoptosis resistance supports the efficacy of metastasis formation [12].
Expression of CD44, a transmembrane glycoprotein involved in cell-cell and cell-matrix interactions, has been associated with growth and metastatic behavior in several malignant tumors. In contrast to most other malignancies, in which up-regulation of CD44 is related to tumor progression, the absence of CD44 expression characterizes the aggressiveness of neuroblastomas in clinical studies. The role of CD44 in the formation of metastasis can be evaluated [13]. The progress of NSCLC patients is largely determined by the occurrence of distant metastases. In patients with primary tumors, this relapse is mainly due to clinically occult micrometastasis present in secondary organs at primary diagnosis but not detectable even with high resolution imaging procedures. Sensitive and specific immunocytochemical and molecular assays enable the detection and characterization of disseminated tumor cells (DTC) at the single cell level in bone marrow (BM) as the common homing site of DTC and circulating tumor cells (CTC) in the peripheral blood. Because of the high variability of results in DTC and CTC detection, there is an urgent need for standardized methods. In this review, we will focus on BM and present currently available methods for the detection and characterization of DTC. Furthermore, we will discuss data on the biology of DTC and the clinical relevance of DTC detection. While the prognostic impact of DTC in BM has clearly been shown for primary breast cancer patients, less is known about the clinical relevance of DTC in patients with other carcinomas. Current findings suggest that DTC are capable to survive chemotherapy and persist in a dormant nonproliferating state over years. To what extent these DTC have stem cell properties is subject of ongoing investigations. Further characterization is required to understand the biology of DTC and to identify new targets for improved risk prevention and tailoring of therapy [14]. The present study reported the positive correlations of sensitivity by chemotherapy to peripheral lymphocyte in patients with NSCLC, in which the activity and transfer ability of peripheral lymphocyte cells could cause the decline of CD44 expression of lung cancers after chemotherapy. The data suggested that the peripheral blood CD44 expression level might be the indicator of the clinical efficacy for NSCLC treatment.
The present study also investigated the interaction of the peripheral blood CD44 expression level, the lymphatic metastasis and clinical stages in patients with NSCLC. The data demonstrated that the peripheral blood CD44 expression level was related with the progress and lymphatic metastasis in patients with NSCLC, and also related with the histopathological grading of primary lung neoplasm. But the data did not demonstrate that the peripheral blood CD44 expression level was not associated with the clinical pathology parameter including the patient age, gender and tumor size. We believed that the peripheral blood CD44 expression level as the clinical regular examination should evaluate the progress, clinic pathological stage, lymphatic metastasis and clinical treatment for the patients with NSCLC.
5. Conflict of Interest Statement
All authors declare no conflict of interest including financial and personal relationship with other people or organization that could inappropriately influence (bias) the work.
6. Acknowledgements
This work was supported by Xinxiang Medical University and the grants from National Basic Research Program of China (2007CB936104) and the 863 National High Technology Research Development Program of China (2007AA021905).
REFERENCES
- S. M. Albelda, “Role of Integrins and Other Cell Adhesion Molecules in Tumor Progression and Metastasis,” Laboratory Investigation, Vol. 68, No. 1, 1993, pp. 4-17.
- N. Takigawa, Y. Segawa, K. Manadai, I. Takata and N. Fujimoto, “Serum CD44 Levels in Patients with NonSmall Cell Lung Cancer and Their Relationship with Clinicophathological Features,” Lung Cancer, Vol. 18, No. 2, 1997, pp. 147-157. doi:10.1016/S0169-5002(97)00060-3
- H. A. Kargi, M. F. Kuyucuoğlu, M. Alakavuklar, O. Akpinar and S. Erk, “CD44 Expression in Metastatic and Non-Metastatic Non-Small Cell Lung Cancers,” Cancer Letters, Vol. 119, No. 1, 1997, pp. 27-30. doi:10.1016/S0304-3835(97)00254-1
- T. Miyoshi, K. Knodo, N. Hino, T. Uyama and Y. Monden, “The Expression of the CD44 Variant Exon 6 Is Associated with Lymph Node Metastatis in Non-Small Cell Lung Cancer,” Clinic Cancer Research, Vol. 3, 1997, pp. 1289-1297.
- C. F. Mountain, “A New International Staging System for Lung Cancer,” Chest, Vol. 89, No. 4, 1986, pp. 5225- 5228.
- X. M. Sun and G. P. Yi, “The Establishment of Researching Adhesive Molecules in Solid Tumors by Flow Cytometric Method,” Chinese Journal of Medical Laboratory Sciences, Vol. 20, 1997, pp. 9-11.
- M. B. Penno, J. T. August, S. B. Baylin, M. Mabry, R. L. Linnoila, V. S. Lee, D. Croteau, X. L. Yang and C. Rosada, “Expression of CD44 in Human Lung Tumors,” Cancer Research, Vol. 54, 1994, pp. 1381-1387.
- J. Lesley, R. Hyman and P. W. Kincade, “CD44 and Its Interactions with the Extracellular Matrix,” Advances in Immunology, Vol. 54, 1993, pp. 271-335. doi:10.1016/S0065-2776(08)60537-4
- D. Situ, H. Long, P. Lin, Z. Zhu, J. Wang, X. Zhang, Z. Xie and T. Rong, “Expression and Prognostic Relevance of CD44v6 in Stage I Non-Small Cell Lung Carcinoma,” Journal of Cancer Research and Clinic Oncology, Vol. 136, No. 8, 2010, pp. 1213-1219. doi:10.1007/s00432-010-0771-5
- A. S. Adhikari, N. Agarwal and T. Iwakuma, “Metastatic Potential of Tumor-Initiating Cells in Solid Tumors,” Front Biosciences, Vol. 16, 2011, pp. 1927-1938. doi:10.2741/3831
- M. Hofmann, W. Rudy, M. Zöller, C. Tölg, H. Ponta, P. Herrlich and U. Günthert, “CD44 Splice Variants Confer Metastatic Behavior in Rats: Homologous Sequences Are Expressed in Human Tumor Cell Lines,” Cancer Research, Vol. 51, 1991, pp. 5292-5297.
- P. Klingbeil, R. Marhaba, T. Jung, R. Kirmse, T. Ludwig and M. Zöller, “CD44 Variant Isoforms Promote Metastasis Formation by a Tumor Cell-Matrix Cross-Talk That Supports Adhesion and Apoptosis Resistance,” Molecular Cancer Research, Vol. 7, 2009, pp. 168-179. doi:10.1158/1541-7786.MCR-08-0207
- U. Valentiner, F. U. Valentiner and U. Schumacher, “Expression of CD44 Is Associated with a Metastatic Pattern of Human Neuroblastoma Cells in a SCID Mouse Xenograft Model,” Tumour Biolology, Vol. 29, No. 3, 2008, pp. 152-160. doi:10.1159/000143401
- S. Riethdorf, H. Wikman and K. Pantel, “Review: Biological Relevance of Disseminated Tumor Cells in Cancer Patients,” International Journal of Cancer, Vol. 123, No. 9, 2008, pp. 1991-2006. doi:10.1002/ijc.23825

