Natural Science
Vol.5 No.10(2013), Article ID:37952,6 pages DOI:10.4236/ns.2013.510137
Developmental stages and quality traits of giant African land snails [Archachatina marginata (swainson)] eggs
![]()
1Department of Animal Science, University of Calabar, Calabar, Nigeria;
*Corresponding Author: blessedbassey4destiny@yahoo.com, basokon@unical.edu.ng
2Departrment of Animal Science, University of Uyo, Uyo, Nigeria
Copyright © 2013 B. Okon, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 22 May 2013; revised 22 June 2013; accepted 29 June 2013
Keywords: Development; Quality; Traits; Eggs; Snail; Stages
ABSTRACT
Sixty sexually mature purebred black-skinned Archachatina marginata snails with weight ranging from 120.50 g to 135.70 g were used to generate eggs in this study. The eggs collected were incubated in a chamber filled with loamy soil for appropriate observation and assessment (cracking at four days intervals to check snailet’s development). Results obtained from the study showed that at laying snail egg weights ranged from 1.54 - 2.45 g (mean of 2.00 g). The egg lengths and widths ranged from 13.50 - 16.90 mm (mean of 15.20 mm) and 10.00 - 12.70 mm (mean of 11.40 mm) respectively. The results also revealed that at laying (day one) the egg content was translucent or clear (blank) when observed with light from a powered microscope. Observation of eggs on day four showed the formation of embryo with a semi-transparent cup attached to a long string body. On day 12, some specific organs of the snailet had developed, and there was a reduction in the liquid content. On day 28, the snailet was fully formed, but the shell still contained very small volume of liquid. The snailet hatched on day 29.
1. INTRODUCTION
Archachatina marginata is the second largest gastropod among the giant African land snails [1,2], but the most popular breed of snail is kept and reared in Nigeria. Snails have high rate of prolific fecundity compared with other livestock and are capable of continuous egg laying several times over a period after a single mating. Reference [3] opined that snails can start to lay eggs when they are sexually mature at 8 - 12 months or when their body weight is between 110 g and 125 g. On the other hand, [4] opined that the body weight of mature snails A. marginata (S) ranges from 120 g to 140 g, while [5] noted that body weight of mature A. marginata ranges from 226.22 g to 237 g for black-skinned ectotype and from 57.54 g to 67.07 g for white-skinned ectotype.
Although, snails are hermaphrodites, they practice sexual reproduction [6,7]. Moreso, [7-9] opined that snails are very choosy in their mating partners and sometimes are uninterested in mating with other snails of the same species originating from a considerable distance away. Snails lay 4 - 18 eggs in 1 - 2 minutes [8,10] unlike hens that lay one egg per day. Reference [11] observed that A. marginata lays 5 - 11 eggs within the same period (1 - 2 minutes). Reference [12] described the egg as being spherical and cream yellow in color, while [13] described the eggs as being chalky white. On the other hand, [7] opined that the eggs of A. marginata are spherical, translucent and yellowish in color.
Reference [10] gave the average egg weights of the black and white skinned ectotypes of A. marginata to be 1.80 g and 1.05 g respectively. The mean egg lengths for black-skinned and white-skinned ectotypes of A. marginata reported by [10] were 1.61 mm and 1.43 mm respectively. Besides, the same authors had earlier given very low mean width values of 1.29 mm and 1.05 mm for the black-skinned and white-skinned ectotypes of A. marginata respectively.
Reference [14] opined that embryonic development is a ceaseless process till eclosion, which involves several aspects such as zygote metabolism, cleavage, blastulation, gastrulation and organogenesis. The morphological and behavioral aspects of development of fresh water snail have been well documented [15]. Besides, [16-18] have also described the reproduction and embryology of the different species of Lymnaea.
While there is the paucity of information on the developmental stages of the land snails, this research seeks to find out the different important stages of embryonic development of A. marginata snails. The knowledge of optimal conditions for successful embryonic development will be useful for biochemical and cytological studies of early development in A. marginata for achieving maximal productivity in snail hatcheries, as well as for genetic manipulation.
2. MATERIALS AND METHODS
The research was carried out at the Snailery Unit of Animal Science Department, University of Uyo, Uyo, Nigeria. The snailery provided micro-environment similar to the natural habitat of snails as it is planted with mango trees and tall grasses which provided shades and protected the hutches from direct sunlight and heavy rainfall. Uyo, the capital of AkwaIbom State, is situated between Latitude 4˚31′ N and 4˚45′ N, Longitude 7˚31′ E and 4˚45′ E with mean temperature of 30˚C and rainfall of 2000 - 3000 mm per annum [19,20].
Sixty sexually mature purebred black-skinned A. marginata snails (Figure 1) of mean fresh body weight range from 120.50 g to 135.70 g purchased from a local market (Itam) in Uyo metropolis were used for the study. The description of the selection, management of the snails and breeding (natural mating) pattern were as prescribed in [9,21].
The fertile, fresh eggs were collected at lay, on the first day usually in the morning after carefully searching through the soil and under the potato leaves in the hutches. The eggs (Figure 2) were taken to the laboratory and were carefully cleansed of adhering soil, sorted

Figure 1. Black skinned Archachatina marginata (S) snail.
against cracks and morphological abnormalities, weighed and external parameters (length and width) measured. The eggs were then placed in an incubation chamber filled with loamy soil and maintained at moderate soil temperature range from 25˚C to 29˚C, relative humidity range from 70% - 80% for proper development of the snail eggs. The eggs were turned during measurements. The eggs were later removed from the soil and cracked at four (4) days interval to study their developmental stages. Photographs of the developmental stages were taken (Figures 3-10).
Data collected were number of eggs laid as counted, egg weight (g), egg length (mm) and egg width (mm). An electronic balance, ScoutTM pro scale with 0.01 g to 1000 g sensitivity was used to measure egg weight, while Vernier Caliper was used to measure length and width. The egg traits were analyzed using simple statistics.
3. RESULTS AND DISCUSSION
The results of egg traits at lay are presented in Table 1.

Figure 2. Translucent (clear) eggs of A. marginata at lay (1st day).

Figure 3. Embryo formation (4th day).
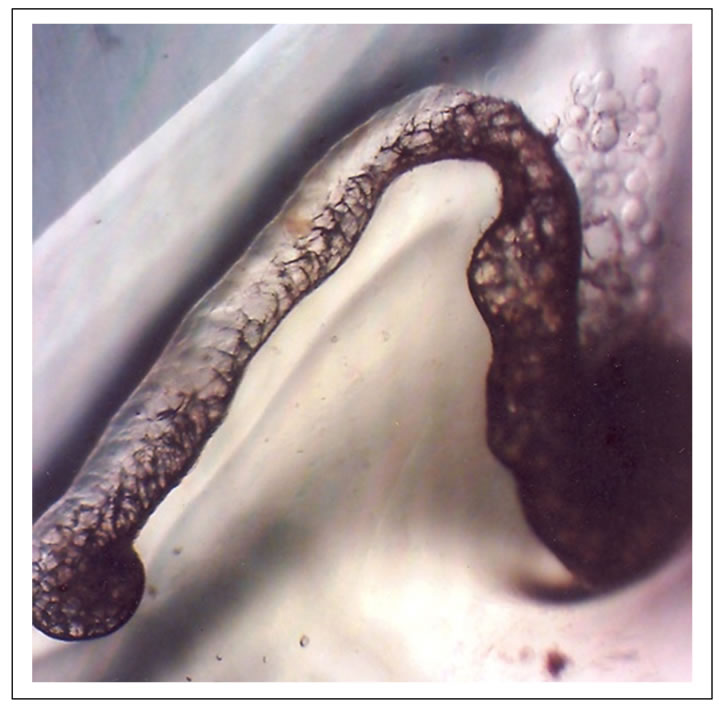
Figure 4. Full cell formation without specialized organs (8th day).
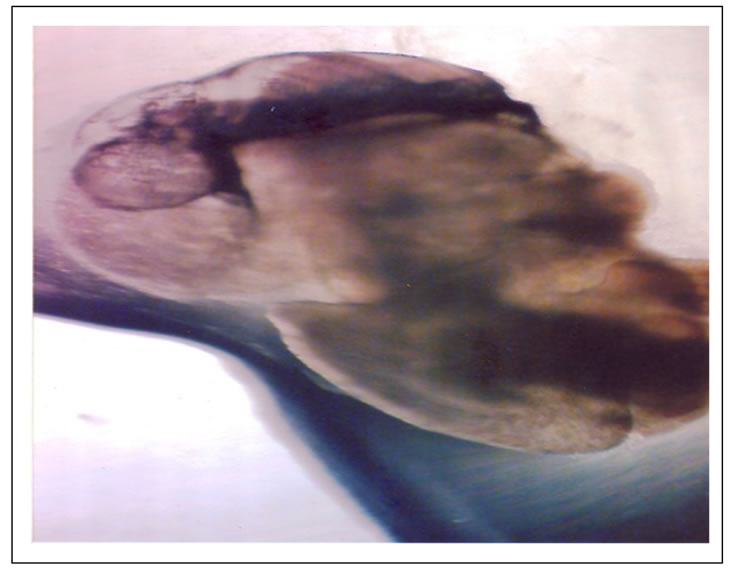
Figure 5. Organs were seen as intestinal body moved into the shell and the foot hanged out (12th day).
Figure 6. Complete condensation of the body parts into the shell (16th day).
The mean egg weight at lay of 2.00 g, range (1.54 - 2.45 g) obtained in the study is quite higher than the mean egg weight values reported in [10,21] for A. marginata. However, the mean egg weight decreased with age as the egg
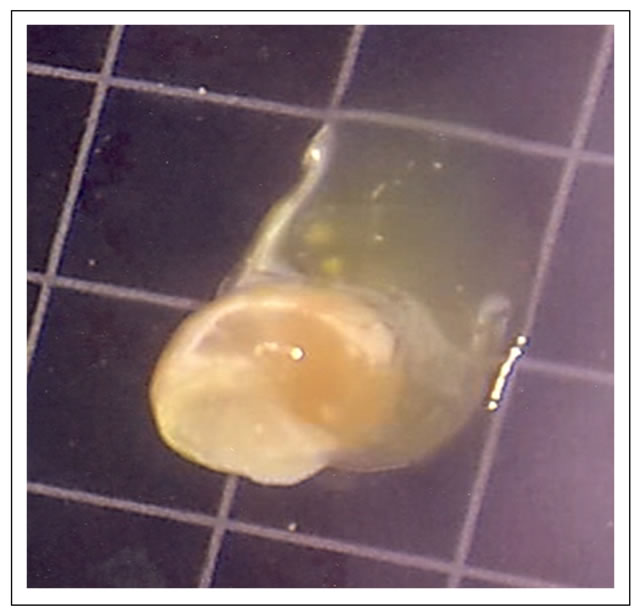
Figure 7. Edible part (foot) is completely buried in a large volume of egg fluid (20th day).

Figure 8. Volume of egg liquid became relatively reduced while shell colour became dark brown (24th day).

Figure 9. Snailet was fully developed but with a very little volume of egg fluid (28th day).
approached hatchling date. This, [9] opined are due to changes in the egg liquid mass to baby snail during the embryonic development as well as the baby snail feeding

Figure 10. Actual hatching of eggs assnailets crawled out of their shells (29th day).
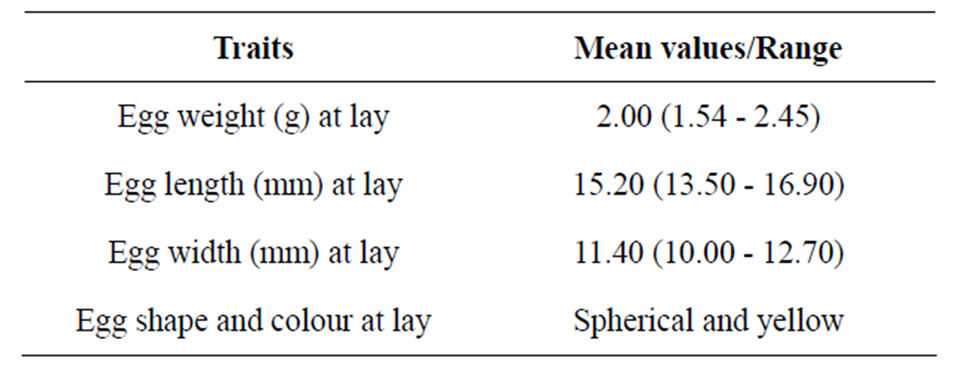
Table 1. Egg quality traits of black-skinned Archachatina marginata (S) snails.
on the liquid mass, thus making the egg lighter.
The shape and colour of the eggs obtained in this study was spherical and yellow. These agreed with the observations of [7,12].
The developmental stages of snail eggs are shown on Figures 3-10. The egg content on the first day of observation at lay was clear (blank) or translucent. No structure was observed with the light powered microscope. This result agreed with the views of [7] of the snail egg being translucent (clear) at lay.
On the 4th day of incubation, embryo formation was observed as a semi-transparent cup attached to a long string body (Figure 3). This structure was quite similar to the embryo observed by [22] for L. acuminate (fresh water) snail. These authors reported that the embryo at this stage took an elongated shape, with the development of mouth, velum or prototroch and head vesicle. On the 8th day, the embryonic development continued with full cell formation but with no specialized organ formed. There was also additional condensation of the intestine as the embryo separated from the yolk as observed in the shell (Figure 4).
There were dramatic changes observed on the 12th day. The intestinal body moved into the shell and the edible part (foot) was seen hanging out of the shell. A dispatch part was also seen in the snail fluid with mantle and other developed organs of the body shown (Figure 5). The heart was physically well observed, with 43 heart beat counted per minute. This observation was quite similar to that reported by [22] at the sixth (6th) day of incubation. The authors noted that at veliger state, the L. acuminate head, foot region sharply became demarcated from the visceral mass with a pair of tentacles bearing darkly pigmented eyes at their bases. On the 16th day, there was complete condensation of the body part into the shell bearing the mantle. The shell colour which was transparent now took up a yellowish colour. It was also observed that the egg still contained a large volume of liquid outside the snailet of the snail’s egg (Figure 6). This agreed with [23] the observations of large fluid volume.
On the 20th day, the edible part of the snail was clearly buried in a large volume of egg fluid as on day 16th. The colour of the shell became dark yellow, while the edible part had a milky colour (Figure 7). On the 24th day, the colour of the shell became dark brown and the volume of egg liquid was relatively reduced (Figure 8).
On the 28th day, the young snailet was fully developed but with a very small volume of egg liquid upon which the snailet was observed crawling (Figure 9). The actual hatching took place on 29th day when the egg shell broke naturally and the snailet crawled out of the shell (Figure 10).
The results of the developmental stages and timing of 29 days obtained in this study confirmed mean incubation period of 29 days, (ranged 28 - 30 days). This result agreed with [21] incubation period report of 29.54 ± 1.48 days for black-skinned purebred A. marginata, but higher than [24] reported value of 14 - 21 days. Stievenant [25] reported values of 14 - 28 days for A. marginata snail. Reference [26] and [12] reported that incubation period for black-skinned A. marginata ranged between 29 and 32 days. Whereas [8] gave a slightly higher incubation period range of 25 - 32 days. The disparity in incubation period may be attributed to variation in genetic factors like breed, strain, age and size of the snail, egg size and environmental factors like temperature and relative humidity. Besides, [21] opined that exposure of eggs to fluctuating environmental conditions which differed from their near constant uterine environment may influence or increase incubation period. In addition, [21,27] noted that incubation conditions such as uptake and loss of moisture and increased transpirational water loss resulting from increased heat produced by the developing embryo can also cause this variation.
4. CONCLUSION
The mean egg weight (2.00 g), egg length (15.20 mm) and egg width (11.40 mm) for giant African land snail, Archachatina marginata (S) and the 29 days incubation period obtained depended on genetic and environmental factors governing the snail and the environment of the study. The shape and color of the eggs are spherical and yellow. The embryonic developmental stages on four (4) days interval for Archachatina marginata snail confirmed that the snail hatched between 28 and 30 days with mean incubation period of 29 days. The observations made in this study have opened a new avenue for future workers to establish developmental mechanism and snail culture farms.
5. RECOMMENDATION
It is however highly recommended that in-depth studies on developmental stages of giant African land snail eggs probably using scanning method with two (2) days interval should be carried out. This will give detailed structures and organs of each developmental stage which will help the snail breeders in morphological, biochemical, cytological and behavioral studies as well as genetic manipulation of embryonic development in Archachatina marginata snails.
REFERENCES
- CAB (2003) Crop protection compendium: Global module. Commonwealth Agricultural Bureau International, Wallingford.
- Venette, R.C. and Larson, M. (2004) Mini risk assessment giant Africant snail, Achatinafulica (Bowdich) [Gastropoda: Achatinidae]. Department of Entomology, University of Minnesita, St. Paul, 1-30.
- Omole, A.J., Twaiwo, A.A. and Amusan, J.A. (2007) Technical guide/bulletin. Practical snail farming. Ibadan, Nigeria. Institute of Agricultural Research and Training, Moor Plantation, 26.
- Ebenso, I.E. (2003) Nutritive potential of white snail (Archachatinamarginata) in Nigeria. Discovery Innovation, 15, 156-158.
- Okon, B., Ibom, L.A., Etuk, N.E. and Akpan, E.W. (2008) Variations in growth patterns and conformation of snail. Influence of strain and location on isometry of growth in Cross River State, Nigeria. Journal of Agriculture, Forestry and the Social Sciences (JOAFSS), 6, 218-226.
- Akinnusi, O. (2004) Introduction to snails and snail farming. 2nd Edition, Abeokuta, Nigeria, Triolas Exquisite Ventures, 90.
- Okon, B. and Ibom, L.A. (2012) Snail breeding and snailery management. Freshdew Productions, Calabar, 1-70.
- Omole, A.J. and Kehinde, A.S. (2005) Backyard snail farming at a glance. Back to Agricultural Series (1) Ibadan Technovisor Agricultural Publications.
- Ibom, L.A. (2009) Variations in reproductive and growth performance trait of white-skinned x black-skinned African giant snail hatchlings [Archachatinamarginata (Swainson)] in Obubra, Nigeria. Ph.D. Thesis, Department of Animal Science, University of Calabar, Calabar, 166.
- Ibom, L.A., Okon, B. and Essien, A. (2008) Morphometric analysis of eggs laid by two ectotypes of snails Archachatinamarganata (Swainson) raised in captivity. Global Journal of Agricultural Sciences, 7, 119-121.
- Akinnusi, O. (1999) Introduction to snails and snail farming. Triolas Exquisite Ventures, Abeokuta.
- Ogogo, A.U. (2004) Wildlife management in Nigeria. Objectives, principles and procedures. Median Communications, Calabar, 134-154.
- Raut, S.K. and Barker, G.M. (2002) Achatina fulica Bondich and other Achatinidae as pests in tropical agriculture. In: Barker G.M., Ed., Molluscs as Crop Pests, CABI Publishing, Hamilton, 55-114. http://dx.doi.org/10.1079/9780851993201.0055
- Bhramachery, A. (1992) Embryonic development of snail. www.rirdcgov.au.
- Morril, J.B. (1982) Development of Pulmonate Gastropod, Lymnaea. In: Harrison, F.W. and Cowden, R.R., Eds., Developmental Biology of Fresh Water Invertebratesliss, Mc Grad Hill, New York, 399-483.
- Pressing, M. (1993) Influence of an insecticide K-othrine on the reproduction and mortality of the pond snail (Lymnaeastagnalis L.). Archives of Environmental Contamination and Toxicology, 25, 387-393.
- Herman, P.M., Meat, A. and Jansen, R.F. (1994) The neutral control of egg laying in the pond snail. Lymnaeastagnalis, mito control of shell turning. Journal of Experimental Biology, 197, 79-99.
- Roudeland, D. and Dreyfuss, G. (1996) The development of tissue lesions in the snail Lymnaea glabra exposed to a sublethal dose of molluscide. Verterinary Research, 27, 79-86.
- Udosen, C. (2000) Application of remote sensing and GIS techniques in terrain mapping and watershed management. In: Yang, S., Ed., South-Eastern Nigeria: It’s Environment, Abaam Publishing, Uyo, 23-28.
- Ebenso, I., Ekwere, A., Akpan, B., Okon, B., Inyang, U. and Ebenso, G. (2012) Occurrence of Salmonella, Vibro and E. coli in edible land snail in Niger Delta, Nigeria. Journal of Microbiology, Biotechnology and Food Sciences, 2, 610-618.
- Ibom, L.A., Okon, B. and Bassey, B.E.E. (2012) Egg traits, hatchability and survivability of black-skinned, white skinned and crossbred Archachatina marginata snails. International Journal of Agricultural Science and Bioresource Engineering Research, 1, 10-18.
- Md. Moniruzzanam, S., Badrun, N. and Md. Sarwar, J. (2007) Embryonic development ecology of fresh water snail Lymnaeae acuminate (Lymnaidae: Gastropoda) Pakistan Journal of Biological Sciences, 10, 23-31. http://dx.doi.org/10.3923/pjbs.2007.23.31
- Demian, E.S. and Yousif, F. (1975) Embryonic development and organogenesis in the snail Marisa cornuaristic (Mesogastropoda anpullaridae). Malacologia, 15, 29-42.
- Cobbinah, J.R. (1993) Snail farming in West Africa. A practical guide. Sayee Publication, CTA Publication.
- Stievenant, C. (1996) Shell morphology, growth reproduction and aestivation by African snails. Laboratory observations on Archachatina marginata Saturalis, Achatinaachatina and Achatinafulica. Ph.D. Thesis, University of Ibadan, Ibadan, 206.
- Awesu, M.O. (1980) The biology and management of the African giant land snail (A. marginata). M. Phil. Thesis, University of Ibadan, Ibadan.
- Tracy, C.R., Packard, G.C. and Packard, M.J. (1978) Water relations of chelonian eggs. Physiological Zoology, 51, 378-387.

