Health
Vol.09 No.13(2017), Article ID:81219,9 pages
10.4236/health.2017.913133
Investigating the Association of Smoking with Thyroid Volume and Function
Saddig Jastaniah1,2*, Amer Abed Alhazmi1, Hassan Alabdullah1, Faisal Fawzi Selamee1, Waleed Khalid Barahim1, Mohammad Wazzan3, Mohamed Yousef2, Shyma M. Alkhateeb1
1Department of Diagnostic Radiology, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, KSA
2Batterjee Medical College for Science and Technology, Jeddah, KSA
3Department of Radiology, Faculty of Medicine, King Abdulaziz University, Jeddah, KSA
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 16, 2017; Accepted: December 18, 2017; Published: December 21, 2017
ABSTRACT
This study was conducted to explore possible correlation between smoking habit and thyroid volume and function in Jeddah. A total of 226 volunteers i.e. 128 male and 98 female were screened at Radiology Department King Abdul-Aziz University Hospital. They were categorized as smokers and non- smokers; the number of smokers was 99 cases i.e. 48 Cigarette smokers and 51 Shisha smokers, and the non-smokers were 127 cases. The information was gathered via a questionnaire distributed at the radiology department. Ultrasonography of thyroid and thyroid function test were performed for 166 participants; and data were analyzed Using SPSS version 22 and Microsoft excel. The study was carried out based on random selection and findings revealed that most of the cases were in the age group between 20 to 30 years old, high body mass index (BMI) was 33.1 kg/m2 in persons above 60 years old and the highest percentage smokers in the age group from 20 to 30 years (40.02%). Ultrasonography of thyroid showed 96 (58%) normal cases, 46 (28%) abnormal “solid” cases and 24 (14%) abnormal “cyst” cases. A lower proportion of cigarette and shisha smokers (15.4% and 5.6% respectively) had an enlarged thyroid gland compared to no cigarettes or shisha smokers (47.9% and 47.3% respectively). The difference between these frequencies was statistically significant (Chi-square = 9.446 and 11.424, p = 0.002 and p = 0.001 for cigarette and shisha smoking respectively). Consequently, it can be concluded from this research that there are no direct significant values correlating smoking habit to thyroid volume or function. However, it is always recommended not smoke due to other well-known threats.
Keywords:
Ultrasound, Thyroid Volume, Thyroid Function, Smoking

1. Introduction
The thyroid is a gland, a butterfly-shaped organ and it is producing the hormone. The organ is situated inferior to the Adam’s apple and anterior side of the neck. It is composed of two lobes or wings, right lobe (Dexter) and left lobe (sinister), where the isthmus is joining. The upper and lower border of gland’s with vertebral levels is difficult to demarcate them because it moves position with these during swallowing. There are two types of the thyroid production hormones T3, T4, and calcitonin [1] . Over 80% of the T4 is converted to T3 by organs such as the kidney, liver, and spleen [2] .
There are several different disorders that can arise when the thyroid produces too much hormone (hyperthyroidism) or not enough (hypothyroidism). Four common thyroid disorders include Hashimoto’s disease, Graves’ disease, goiter, and thyroid nodules. A simple physical exam can reveal an enlarged thyroid, enlarged bulging eyes, and signs of increased metabolism, including rapid pulse and high blood pressure. Doctors will also order blood tests to check for high levels of thyroxin (T4) and low levels of TSH, both of which are signs of Graves’ disease. A radioactive iodine uptake might also be administered to measure how quickly the thyroid takes up iodine, which it needs to function properly. A high uptake of iodine is consistent with Graves’ disease.
Tobacco smoke contains substances that affect the function of the thyroid. Studies show that smokers are more likely to have thyroid enlargement, and it is possible that mild thyroid enlargement in smokers could be a sign of subtle thyroid disturbance. According to a previous study [3] smokers are twice as likely as nonsmokers to develop Graves’ disease. Smoking also apparently worsens eye problems in people with Graves’ disease. Smoking may increase the risk of hypothyroidism in patients with Hashimoto’s thyroiditis. In a study [4] , the purpose is to evaluate the epidemiological evidence for a causal association between tobacco smoking and thyroid eye disease (TED). Method is Systematic review, including quality assessment, of published epidemiological studies and evaluation of the evidence using established causality criteria. Fourteen papers describing 15 studies were included. Smokers were more likely to experience disease progression or poorer outcome of treatment. Patients with Graves’ disease and the general public should be educated about the risk of smoking and TED. These findings suggest that patients with Graves’ disease or TED who are smokers should be given effective support to stop smoking.
The effects of smoking on the of endocrine glands function have been investigated. It is revealed that the most component of smoke, produced from tobacco, has effects on the endocrine system is nicotine [5] [6] [7] . The present study aimed to study a possible association between smoking habit and thyroid volume and function.
The relation between tobacco smoking and thyroid function disorder is not well understood. [8] [9] [10] [11] and smoking appears to increase the risk of Graves hyperthyroidism [12] [13] [14] [15] .
2. Subject and Methods
This prospective, randomized, and observational study was conducted at King Abdul-Aziz University Hospital, Radiology Department, in Jeddah city. The study groups involved random invitations of 226 volunteers; basically health sciences students, their friends and relatives. They were 128 male and 98 female, they were categorized as smokers 99 cases (48 Cigarette smokers and 51 Shisha smokers) and 127 non-smokers according to their responses to a questionnaire. Demographic characteristics (body weight and height were measured, and body mass index (BMI) was calculated using the formula: BMI = body weight (kg)/[height(m)]2. Ultrasonography of thyroid and thyroid function test was performed for 166 participants. The Rejection criteria of some cases that were excluded depends on two factors no show on scan day, although they have filled their questionnaire, and secondly, it was not possible to reach to some volunteer not answering the phone calls.
2.1. Ultrasound
After obtaining ethical approval and patients consent forms from volunteers, thyroid ultrasound (US) was performed for 166 of the participants by one radiologist who was part of the group. Volunteers were examined in the supine position with the head in mild hyperextension. The scan was performed with a linear array transducer, it was important to use high-frequency 5 - 7 MHz to provide a short wavelength for low penetration in thyroid scan. Commencing survey scan in transverse down the midline was done to assess for tracheal deviation and obvious pathology. After that, volunteers head was tilted slightly to the contrala- teral side and scan down in transverse. Then measure each lobe and isthmus of thyroid each lobe width. Anterior and posterior diameters are measured in the transverse scan rotating into longitudinal and scan from medial to lateral. This was repeated for the other lobe with the head tilted the opposite side. The isthmus in longitudinal and transverse were scanned. Finally, each side of the neck in transverse for alternative pathology was scanned down e.g. enlarged lymph nodes or thrombosis jugular vein. The Parathyroid Glands are not usually seen due to their small size. In the case of any abnormality like large, solid cyst, nodules and tumors measurements were recorded and color Doppler was conducted to inspect blood supply to this lesion.
2.2. Blood Test
Blood samples were obtained for the cases that showed abnormality in the screening US; these were analyzed on the same day for thyroid function test i.e. TSH examination. Patients with positive results were contacted and referred to the clinic for further investigations and treatments.
2.3. Statistical Analysis
Data were analyzed using Microsoft Excel and the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 22, Statistical significance for the difference in frequencies was determined by Pearson chi-square χ2 test. A p < 0.05 values (two-sided test) was accepted as statistically significant.
3. Results
The results of the study are represented in the following Tables 1-7 and Figures 1-4.
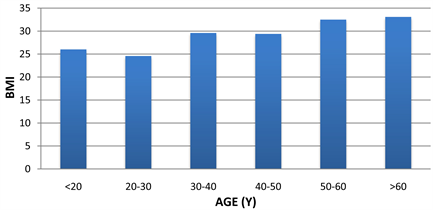
Figure 1. Histogram of age groups Vs. BMI in 226 patients.
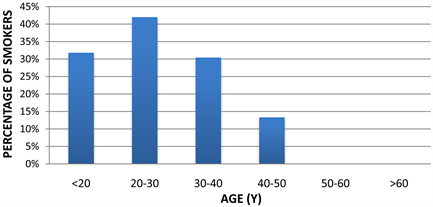
Figure 2. Histogram showing the percentage of smokers in different age groups.
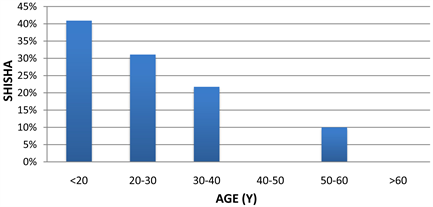
Figure 3. Histogram showing the percentage of “Shisha” smokers at different age groups.
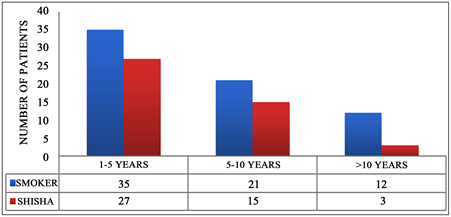
Figure 4. Multiple parameters histogram showing smokers numbers vs. smoking period.
Table 1. Total study sample age groups and gender.
Table 2. Patients age groups and ultrasound findings.
Data in n and N% columns are numbers and percentages of smokers and non-smokers. P-value column refers to results of Chi-square (χ2) test for the relation between smoking and thyroid function.
Table 3. Effect of smoking on thyroid function.
Data in n and N% columns are numbers and percentages of smokers and non-smokers. P-value column refers to results of Chi-square test for the relation between smoking and thyroid gland enlargement.
Table 4. Effect of smoking on thyroid gland enlargement.
Data in n and N% columns are numbers and percentages of smokers and non-smokers. P-value column refers to results of Chi-square test for the relation between smoking and thyroid gland enlargement.
Table 5. Effect of the smoking period on thyroid gland enlargement.
Data in n and N% columns are numbers and percentages of smokers and non-smokers. P-value column refers to results of Chi-square test for the relation between smoking and thyroid gland enlargement.
Table 6. Effect of cigarette smoking frequency on thyroid gland enlargement.
Data in n and N% columns are numbers and percentages of smokers and non-smokers. P-value column refers to results of Chi-square test for the relation between smoking and thyroid gland enlargement.
Table 7. Effect of Shisha smoking frequency on thyroid gland enlargement.
4. Discussion
The thyroid gland controls almost all of the metabolic processes in the body. The most common thyroid disorder involves abnormal production of thyroid hormones. Hyperthyroidism means too much of thyroid hormone, on the other hand, insufficient hormone production leads to hypothyroidism.
The results of this study revealed that the percentage of randomly selected cases was higher in the male than the female cases i.e. 128 (57%) and male 98 (43%) respectively. Most of the cases i.e. 119 cases were in the age group between 20 to 30 years old. Less frequency of cases was in the age group above 60 years old which was 12 cases. The other age groups come close in the number of cases were between 20 to 30 cases. The vast majority of cases scanned were males i.e. 88 cases in the age group of 20 to 30 years old, and the least number of cases were patients above 60 years old which was only 2 cases as demonstrated in Table 1.
Age groups versus BMI in 226 patients revealed that the highest registered BMI (33.1 kg/m2) for the age group above 60 years old. The age group from 50 to 60 years old was close to the age group of patient who are above 60 years old and it was 32.5 kg/m2. These patients in these groups suffer from obesity (Note that: the obesity in BMI is between 30 to 40 kg/m2). The patient who are in the age groups less than 20, 30 to 40 and 40 to 50 years old see their numbers are very close together and were 26.0, 29.4 and 29.6 kg/m2. The age group of patient who are between 20 to 30 years old has a normal weight as shown in Figure 1.
The highest percentage of smokers in different age groups found to be in the age group from 20 to 30 years (40.02%). The second category was the patients who are below 20 years of age (30.82%). The third and fourth categories were in the age group between 30 to 40 and 40 to 50 years old (30.43% and 13.33% respectively). There were no smokers above 50 years old in the study whole sample. The study sample was randomly chosen, but it was concentrating on 20 to 30 years old because many university students accepted the invitation and performed the scan Figure 2.
The characteristic of the figure showing an increased number of “Shisha” smokers in the category of patient less than 20 years old whereas 20 to 30 years old comes 31.09% compared to 40.02% of cigarette smokers. The third and fourth categories were the patient were 30 - 40 and 50 - 60 years old which represent 21.74% and 10% of the smoking cases respectively. There were no “Shisha” smokers in age groups from 40 to 50 and above 60 years old Figure 3.
According to the different parameters demonstrating the period of smoking it was found that many smokers recently started smoking from 1 to 5 years which is 35 cases, and we report 27 cases of Shisha from the whole sample. It was also found that 21 smokers who have been smoking from 5 to 10 years and another 15 cases reported to smoke Shisha for more than 5 to 10 years and more than ten years of smoking were 12 cases of cigarette smokers and 3 cases of shisha smokers. However, this indicates more than 50% of our samples are smoking 1 to 5 years. Accordingly, the effect of long period smoking has the least cases percentage in our study which creates a limitation which needs further investigation with expansion and focus on this category Figure 4.
Ultrasonography of thyroid was performed for 166 participants (73%) and revealed that normal cases 96 (58%) abnormal “solid” 46 (28%) and abnormal “cyst” 24 (14%) as demonstrated in Table 2.
A larger proportion of cigarette smokers had a normal thyroid function (66.7%) compared to non-smokers (70.8%); however, the difference between these frequencies was not statistically significant, and the number of subjects in the cigarette smoking group was too small to draw a firm conclusion as listed in Table 3. The number of shisha smokers was too small to perform statistical tests.
A lower proportion of cigarette and shisha smokers (15.4% and 5.6% respectively) had an enlarged thyroid gland compared to no cigarettes or shisha smokers (47.9% and 47.3% respectively). The difference between these frequencies was statistically significant (χ2 = 9.446 and 11.424, p = 0.002 and p = 0.001 for cigarette and shisha smoking respectively; Table 4). The effect of the period and frequency of smoking on thyroid gland enlargement could not be addressed due to small numbers of smokers in study subjects (Tables 5-7). The study findings agrees with the findings conducted by Asvold BO et al. [16] and Aydin et al. [17] .
5. Conclusion & Recommendation
This work revealed that there are no direct significant values correlating smoking habit to thyroid volume or function. However, it is always recommended not smoke due to other well-known threats. Authors recommend further studies to be conducted due to limitations of smokers sample size.
Cite this paper
Jastaniah, S., Alhazmi, A.A., Alabdullah, H., Selamee, F.F., Barahim, W.K., Wazzan, M., Yousef, M. and Alkhateeb, S.M. (2017) Investigating the Association of Smoking with Thyroid Volume and Function. Health, 9, 1843-1851. https://doi.org/10.4236/health.2017.913133
References
- 1. Ferrara, A.M., Liao, X.H., Gil-Ibanez, P., Marcinkowski, T., Bernal, J., Weiss, R.E., Dumitrescu, A.M. and Refetoff, S. (2013) Changes in Thyroid Status during Perinatal Development of MCT8-Deficient Male Mice. Endocrinology, 154, 2533-2541. https://doi.org/10.1210/en.2012-2031
- 2. Pohlenz, J., Maqueem, A., Cua, K., Weiss, R.E., Van Sande, J. and Refetoff, S. (1999) Improved Radioimmunoassay for Measurement of Mouse Thyrotropin in Serum: Strain Differences in Thyrotropin Concentration and Thyrotroph Sensitivity to Thyroid Hormone. Thyroid, 9, 1265-1271. https://doi.org/10.1089/thy.1999.9.1265
- 3. Prummel, M.F. and Wiersinga, W.M. (1993) Smoking and Risk of Graves’ Disease. JAMA, 269, 479-482. https://doi.org/10.1001/jama.1993.03500040045034
- 4. Thornton, J., Kelly, S.P., Harrison, R.A. and Edwards, R. (2007) Cigarette Smoking and Thyroid Eye Disease: A Systematic Review. Eye, 21, 1135-1145. https://doi.org/10.1038/sj.eye.6702603
- 5. Völzke, H., Schwahn, C., Kohlmann, T., Kramer, A., Robinson, D.M., John, U., et al. (2005) Risk Factors for Goiter in a Previously Iodine-Deficient Region. Experimental and Clinical Endocrinology & Diabetes, 113, 507-515. https://doi.org/10.1055/s-2005-865741
- 6. Warmuz-Stangierska, I., Czarnywojtek, A., Florek, E. and Sowinski, J. (2004) Smoking among Thyroid Patients. Przeglad Lekarski, 61, 1077-1079.
- 7. Czarnywojtek, A., Kurdybacha, P., Florek, E., Warmuz-Stangierska, I., Zdanowska, J., Zgorzlewicz, M., et al. (2010) Smoking and Thyroid Diseases—What Is New? Przeglad Lekarski, 67, 1056-1060.
- 8. Ericsson, U.-B. and Lindgãrde, F. (1991) Prevalence of Goitre, Thyrotoxicosis and Autoimmune Thyroiditis. Journal of Internal Medicine, 229, 67-71. https://doi.org/10.1111/j.1365-2796.1991.tb00308.x
- 9. Fisher, C.L., Mannino, D.M., Herman, W.H. and Frumkin, H. (1997) Cigarette Smoking and Thyroid Hormone Levels in Males. International Journal of Epidemiology, 26, 972-977. https://doi.org/10.1093/ije/26.5.972
- 10. Jorde, R. and Sundsfjord, J. (2006) Serum TSH Levels in Smokers and Non-Smokers: The 5th Tromso Study. Experimental and Clinical Endocrinology & Diabetes, 114, 343-347. https://doi.org/10.1055/s-2006-924264
- 11. Knudsen, N., Bulow, I., Laurberg, P., Perrild, H., Ovesen, L. and Jorgensen, T. (2002) High Occurrence of Thyroid Multinodularity and Low Occurrence of Subclinical Hypothyroidism among Tobacco Smokers in a Large Population Study. Journal of Endocrinology, 175, 571-576. https://doi.org/10.1677/joe.0.1750571
- 12. Holm, I.A., Manson, J.E., Michels, K.B., Alexander, E.K., Willett, W.C. and Utiger, R.D. (2005) Smoking and Other Lifestyle Factors and the Risk of Graves’ Hyperthyroidism. Archives of Internal Medicine, 165, 1606-1611. https://doi.org/10.1001/archinte.165.14.1606
- 13. Prummel, M.F. and Wiersinga, W.M. (1993) Smoking and Risk of Graves’ Disease. JAMA, 269, 479-482. https://doi.org/10.1001/jama.1993.03500040045034
- 14. Vestergaard, P. (2002) Smoking and Thyroid Disorders: A Meta-Analysis. European Journal of Endocrinology, 146, 153-161. https://doi.org/10.1530/eje.0.1460153
- 15. Vestergaard, P., Rejnmark, L., Weeke, J., et al. (2002) Smoking as a Risk Factor for Graves’ Disease, Toxic Nodular Goiter, and Autoimmune Hypothyroidism. Thyroid, 12, 69-75. https://doi.org/10.1089/105072502753451995
- 16. Asvold, B.O., Bjøro, T., Nilsen, T.I. and Vatten, L.J. (2007) Tobacco Smoking and Thyroid Function. Archives of Internal Medicine, 167, 1428-1432. https://doi.org/10.1001/archinte.167.13.1428
- 17. Aydin, L.Y., Aydin, Y., Besir, F.H., Demirin, H., Yildirim, H., Önder, E., Dumlu, T. and Celbek, G. (2011) Effect of Smoking Intensity on Thyroid Volume, Thyroid Nodularity and Thyroid Function: The Melen Study. Minerva Endocrinologica, 36, 273-280.