Engineering
Vol.5 No.2(2013), Article ID:27820,6 pages DOI:10.4236/eng.2013.52030
Decorating and Filling of Multi-Walled Carbon Nanotubes with TiO2 Nanoparticles via Wet Chemical Method
1Department of Chemical Engineering, Faculty of Engineering, Ferdowsi University of Mashhad, Mashhad, Iran
2Department of Material Science and Engineering, Faculty of Engineering, Ferdowsi University of Mashhad, Mashhad, Iran
Email: *zebarjad@um.ac.ir
Received October 14, 2012; revised November 20, 2012; accepted December 19, 2012
Keywords: MWCNT; TiO2 Nanoparticles; Modification; Decoration; Filling
ABSTRACT
Multi-walled carbon nanotubes (MWCNTs) have been successfully modified with TiO2 nanoparticles via wet chemical method. For this purpose tetra chloride titanium (TiCl4) was used as titanium source. MWCNTs were exposed at different amount of TiCl4 (0.25 and 0.1 ml) and different soaking times. The modified MWCNTs have been characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM). TEM results showed that the MWCNTs were fully decorated with TiO2 at short term immersion. Increasing soaking time caused to fill the MWCNTs with TiO2 nanoparticles. The results showed that the amount of precursor had a significant role on quantity of decoration. The decoration of outer surface of MWCNTs with TiO2 was more noticeable at large amount of TiCl4. XRD results revealed that the crystalline structure of TiO2 on the surface and inner of MWCNTs was rutile. The average size of TiO2 nanoparticles which modified MWCNTs were 20 nm.
1. Introduction
In the recent years, various publications have reported the outstanding physicochemical characteristics of Carbon Nanotubes (CNTs) [1]. The study of these nanostructures lead to diagnose individual properties such as high strength to weight ratio, excellent Young’s modulus which has been measured to reach about 1 TPa, exceptional mechanical and electrical properties and high flexibility [2]. The mentioned properties cause to appear many applications in different field of researches and new technologies [3]. For instance excellent elasticity and flexibility of CNTs plays an important role for choosing CNTs as alternative reinforcing filler in composite materials. Numerous authors have reported the incorporation of these tubular structures in diverse matrices, although the most reproducible and successful advances have been achieved when fabricating ceramic and polymer composites [4-7].
Modification of CNTs has been paid a great deal of interest as a fascinating model system for fundamental scientific research with the potential technological applications. In particular, fabrication of CNT-based devices which modified with metal oxides is more applicable than non modified devices. Up to now, various metal oxides such as TiO2, SnO2, ZnO, Fe2O3, MnO2 and RuO2 have been reported to modify CNTs [8-13]. Nowadays finding a simple and low-cost approach to modify CNTs with the metal oxides is still an active research. Most published work to date has focused on the coating and filling of carbon nanotubes with metals and metal oxides. However, the difficulty of obtaining a uniform coated nanotube imposes some limitations. Multi-walled carbon nanotube (MWNT) based metal oxide composites [14] were prepared by an impregnation method using organometallic compounds as precursor. Tasviri et al. [15] obtained TiO2-coated carbon nanotubes which were functionalized with amine groups. Bouazza et al. [6] have coated Multi-walled carbon nanotubes with TiO2 layer by sol-gel method. The CNTs-TiO2 composites fabricated by hydrolysis in which the formation of TiO2 and its compounding with CNTs happened almost simultaneously [16] have also been reported. Chun Oh et al. [7] synthesized CNT/TiO2 composites by an improved oxidation method.
CNTs and titanium dioxide (TiO2) composite materials have attracted attention of researchers in relation to the treatment of contaminated water and air by heterogeneous photo-catalysis, hydrogen evolution, CO2 photoreduction, and dye sensitized solar cells. These composite materials have been fabricated by a range of different methods, including mechanical mixing of TiO2 and CNTs [17], sol-gel synthesis of TiO2 in the presence of CNTs [18], electro-spinning methods [19], electrophoretic deposition [20], and chemical vapor deposition [8]. The uniformity of the decoration of CNTs with oxide and the physical properties of the composite materials depend on the procedure of modification. For example uniform decorating of CNTs with TiO2 nanoparticles have been reported by chemical vapor deposition and electro-spinning methods [21]. However these methods are complex and need special equipments. The modified CNTs with sol-gel methods lead to heterogeneous and nonuniform decorations and aggregation of TiO2 on the surface of CNTs [22].
Here we produced modified CNTs with TiO2 by a new and simple two-step wet chemical method.The preparation of modified CNTs by this technique is very simple and doesn’t need to special equipments.
2. Materials and Experimental
For preparation of the synthesized CNT-TiO2 the following materials were used:
Multi-walled carbon nanotubes (MWCNTs, 95.9% purity, diameter: ~40 - 60 nm, length: ~5 - 15 μm), Tetra chloride titanium (TiCl4, M = 189.79, 99%, Merck), Nitric Acid (HNO3, M = 63, 65%, Merck), Hydrochloride Acid (HCl 37 wt%, Merck).
A typical experimental procedure was followed. Firstly, the MWNTs were opened by oxidation with nitric acid solution (65%) at room temperature for 2 h in an ultrasound bath and then the mixture was stirred at high speed for 2 h, and the treated MWCNTs were washed with distilled water several times until the pH reached 7 and dried at 90˚C for an overnight. Secondly a certain amount of TiCl4 was added to 100 ml of distilled water, followed by adding a little HCl (37 wt%) to the distilled water before TiCl4 was dissolved in the water. The addition of a small amount of acid is only for reducing the hydrolysis of TiCl4. 75 mg of acid treated MWCNTs were dispersed in this solution using ultrasound bath for 2 h. The mixture was stirred for 22 h at room temperature and then raised the temperature up to 80˚C. The mixture was stirred for 3 h then filtered and dried at 80˚C for 1 h and calcinated at 370˚C for 3 h. A flow chart of the technique is shown in Figure 1.
X-ray powder diffraction was performed to characterize the phase composition and crystal structure of the samples, using a Philips Analytical X-Ray B.V equipment (40 kV/30 mA) with Cu Kα (1.542 Å) radiation. The scanning velocity was 0.02˚ s−1, and the 2θ range scanned ranged from 4˚ to 90˚.
The crystalline sizes of the produced phase were determined by the Scherrer formula, using a K factor of 0.9:
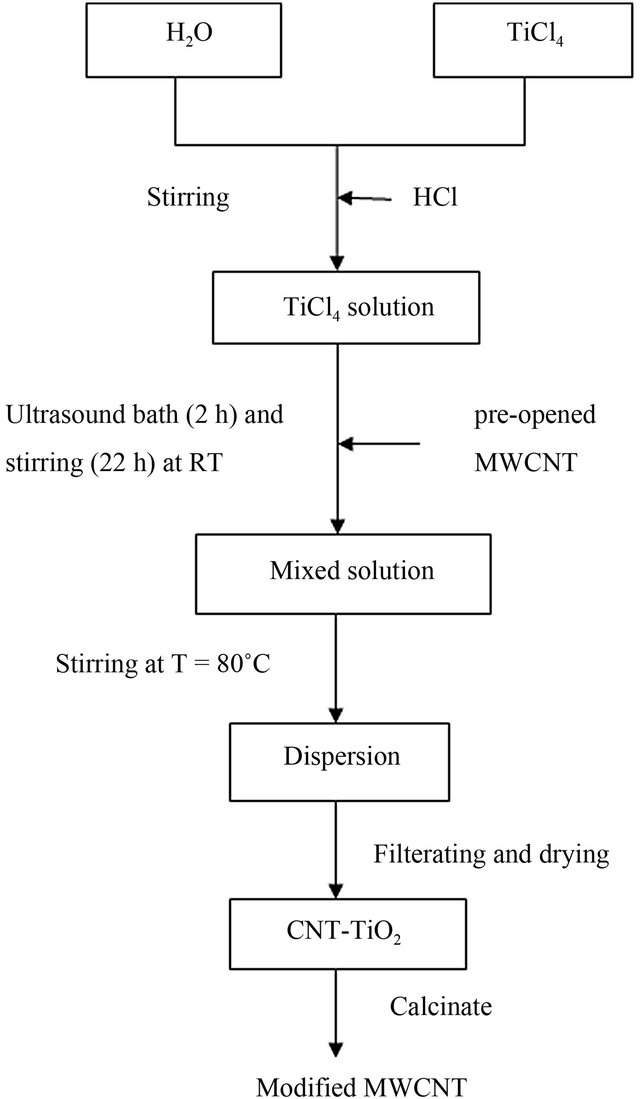
Figure 1. Flow chart of the steps involved in the preparation modified MWCNT.
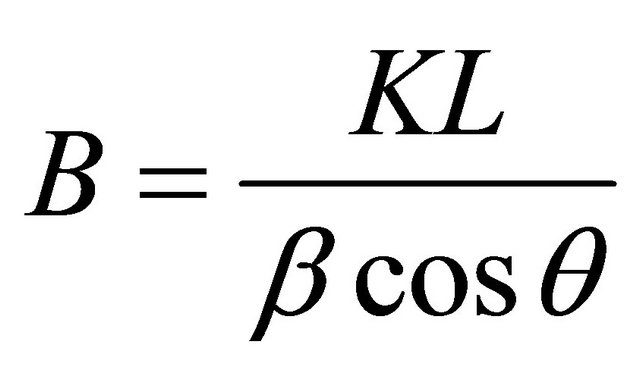 (1)
(1)
where B: crystalline size, in nm; L: wavelength for the radiation used, which is 1.5406˚A for Cu; β: full width at half maximum intensity (FWHM); θ: angle for the XRD maximum peak.
The weight fractions of the TiO2 nanoparticle and CNT in the modified MWCNT were calculated from the relative intensities of the strongest peaks corresponding to TiO2 and graphite as described by Spurr and Myers [23]:
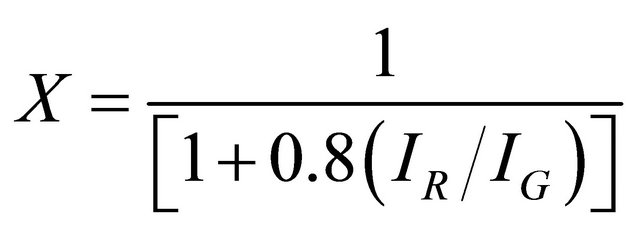 (2)
(2)
where X is the weight fraction of CNT in the product, while IR and IG are the X-ray integrated intensities of the (110) reflection of rutile and (002) reflection of graphite, respectively.
The transmission electron microscopy (TEM, LEO 912 AB.) was used to investigation the quality of modification and measurement of inner and outer diameter of CNTs and particle size of formed TiO2.
3. Results and Discussion
3.1. Oxidation of MWCNTs
Figure 2 illustrates the opening of MWCNTs by oxidative phenomenon. The TEM micrographs show opening of the MWCNTs at the end tips at different magnifications. It can be seen that the ends of some tubes were opened and would cut to short length. This is because the hexagon electrophilic destroyed by acid on the integrated graphene structures probably cut the nanotubes down to short tubes [24].
3.2. Modification of MWCNTs
Figure 3 shows one of the low-magnification TEM images of modified MWCNT, which reveals that the outer
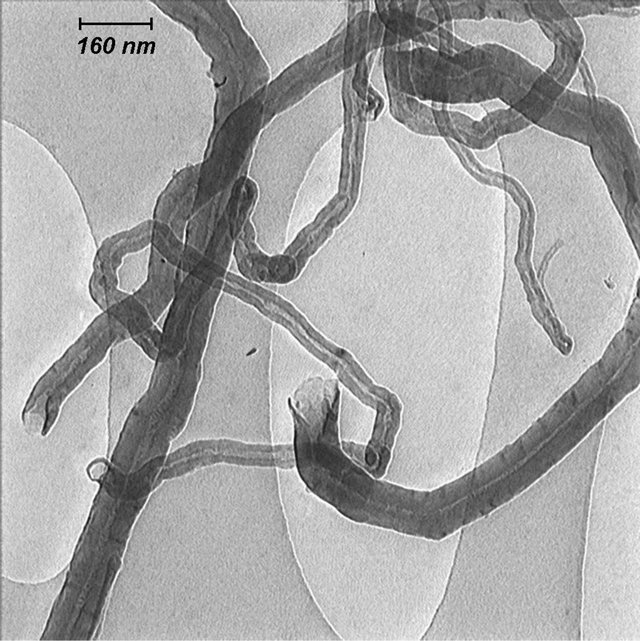
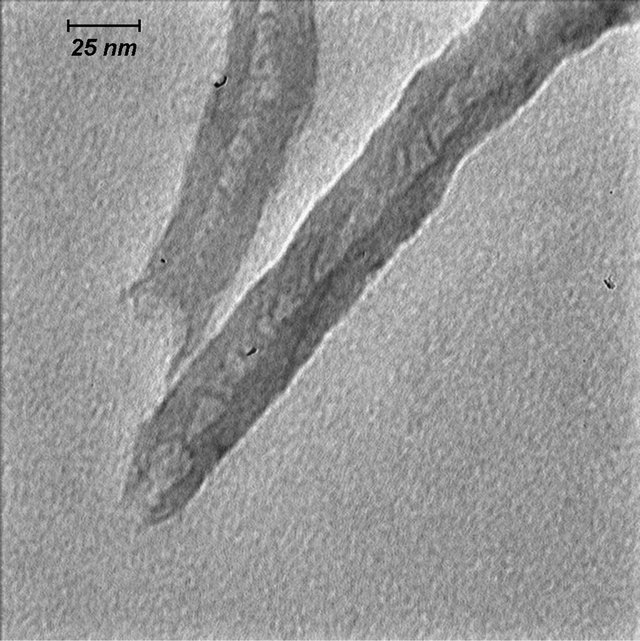
Figure 2. TEM images of opened multiwall carbon nanotube at different scales.
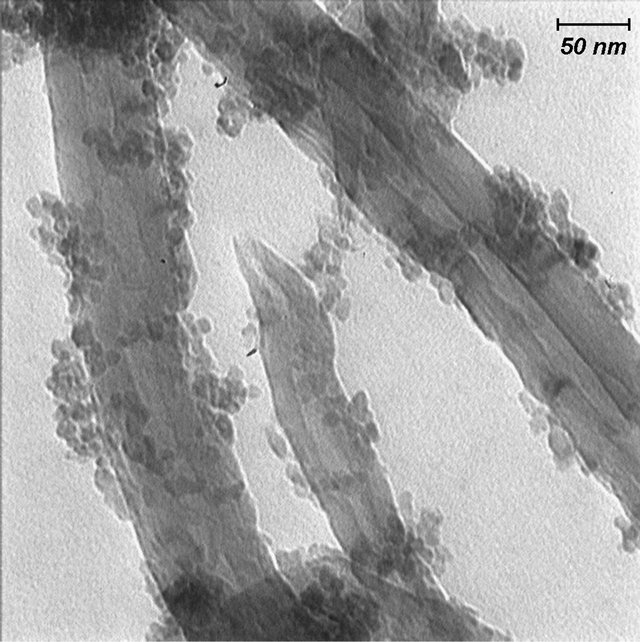
Figure 3. TEM image of outer surface decoration of MWNTs.
surface of MWCNTs is fully coated with TiO2 nanocrystals. The method which we used for modification of MWCNTs involves three steps. Firstly, negatively charged functionalities such as -COOH and -OH are introduced to the surface of MWCNTs after oxidation [25]. Then, the titanium ions in the solution which produced by hydrolyses of TiCl4 are adsorbed to the surfaces due to the electrostatic attraction. Finally, TiO2 nanocrystals are in-situ formed on the outer surface of MWCNTs. The mechanism of nano size formation of TiO2 is as below:
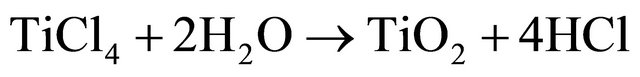 (3)
(3)
3.2.1. Effect of Time on Modification
The result of experiments revealed that soaking time has an effective influence on modification of samples. The dominant mechanism in short-term immersion (24 h) is decoration of outer surface with TiO2. At the same time the inner of MWCNTs are filled with TiO2 nanoparticles. They are most noticeable because of the striking contrast from the filling material to the empty cavity of MWCNTs, which is consistent with the presence of TiO2 nanoparticles (Figure 4). It can be clearly seen that the fine TiO2 nanoparticles attach to one another densely and continuously, and form an almost continuous dark line in the cavity of the MWCNTs. The average size of the TiO2 nanoparticle is about 20 nm.
Figure 5 shows the filling of MWCNTs with TiO2 due to its exposure under TiCl4 solution for 48 h. As seen there is obvious filled materials to the empty cavity of MWCNTs. With comparison of Figure 4 and Figure 1 may conclude that the amount of filled TiO2 depends strongly on the soaking time in TiCl4 solution.
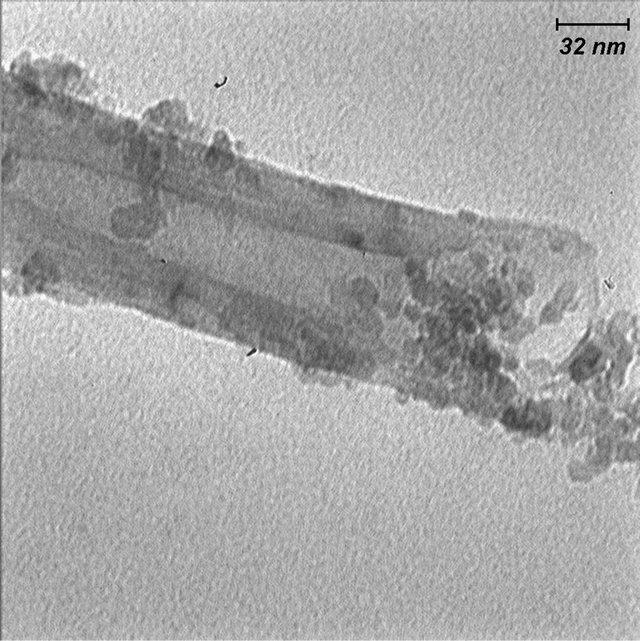
Figure 4. TEM image of decoration of outer surface and filling of MWNTs at short mixing duration (24 h).
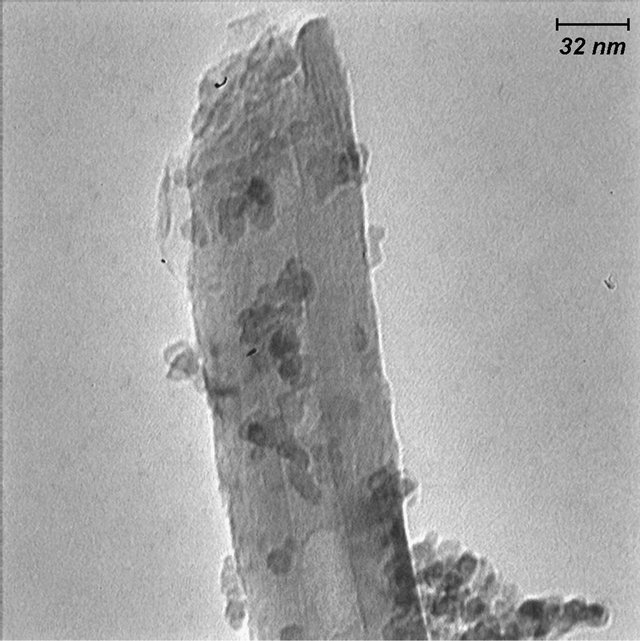
Figure 5. TEM image of decoration of outer surface and filling of MWNTs at long mixing duration (48 h).
3.2.2. Effect of TiCl4 Content on Modification
As mentioned before the amount of TiCl4 was added to 100 ml of distilled water at the same amount of HCl and MWCNT is an important factor for the decoration of MWCNTs with TiO2. For instance by increasing the amount of TiCl4, the probability of decoration of outer surface of MWCNTs will be increased. As shown in Figures 6(a) and (b) at large amount of TiCl4 decoration is more obvious. It can be concluded that at large amount of TiCl4, the titanium ions in the solution are more and further titanium ions are adsorbed to the surfaces due to the electrostatic attraction and hence decoration of outer surface of MWCNTs with TiO2 nanoparticles is large.
 (a)
(a) (b)
(b)
Figure 6. TEM micrographs of decoration of MWCNTs at different amount of TiCl4, (a) A little amount; (b) A large amount.
3.3. Characterization
Figure 7 shows the XRD pattern of the pristine MWCNTs (a) and MWCNTs-TiO2 (b) which reveals various crystal structures. The XRD pattern of MWCNT exhibits a sharp peak at around 2θ = 26˚ and a broad peak centered at 2θ = 43˚ corresponding to the (002) and (100) Bragg reflection planes having interlayer spacing of 3.348 and 2.027 A respectively. The observed diffractions in sample MWCNTs-TiO2 are displayed both the peaks assigned to the MWCNT and TiO2, respectively, and their broadness suggests the presence of very small crystals. The peak at a 2θ of 26.603˚ is typical for the (002) diffraction of
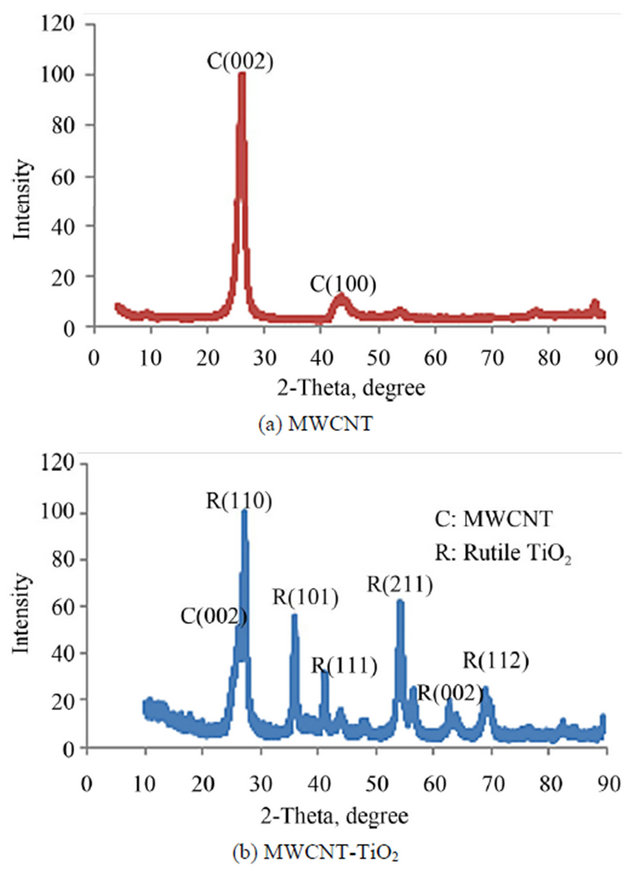
Figure 7. XRD patterns of (a) Pristine MWCNTs and (b) CNT-TiO2.
graphite [6] and confirms the presence of CNTs in the samples. Notice that there are no traces of anatase in this sample. The peaks at 27.387˚, 36.007˚, 41.158˚, and 54.213˚ belong to the diffraction peaks of (110), (101), (111), and (211) of rutile. Therefore, it can be concluded that the CNT/TiO2 had a structure like rutile crystals. As XRD analysis confirmed, the average size of the TiO2 crystallite is about 13.21 nm in accordance with the results calculated using the Scherrer equation which mentioned in Equation (1). Modified MWCNT consists of both TiO2 nanoparticle and graphite at the ratio X = 0.401 using the Spurr and Myers equation that means the weight fraction of MWCNT in the sample is 0.401.
4. Conclusion
In summary, we have demonstrated a simple and reproducible wet chemical method to modify MWCNTs with TiO2 nanoparticles using TiCl4 as precursor. The amount of TiCl4 and soaking time play critical roles in modification of MWCNTs. According to the TEM results, the average size of the TiO2 nanoparticle is about 20 nm. In the XRD pattern, the sample was observed to be a phase of rutile and their broadness suggests the presence of very small crystals. It is worth noting that by using this method one can obtain different amount of TiO2 nanocrystals modified MWCNTs only by changing the concentration of precursor.
REFERENCES
- M. Terrones, “Carbon Nanotubes: Synthesis and Properties, Electronic Devices and Other Emerging Applications,” International Materials Reviews, Vol. 49, No. 6, 2004, pp. 325-377.
- C. Li and T. Chou, “Elastic Moduli of Multi-Walled Carbon Nanotubes and the Effect of van der Waals Forces,” Composites Science and Technology, Vol. 63, No. 11, 2003, pp. 1517-1524. doi:10.1016/S0266-3538(03)00072-1
- M. S. Dreselhaus, G. Dresselhaus and P. Avouris, “Carbon Nanotubes: Synthesis, Structure, Properties and Applications,” Springer, Berlin, 2001. doi:10.1007/3-540-39947-X
- E. T. Thonstenson, Z. Ren and T. W Chou, “Advances in the Science and Technology of Carbon Nanotubes and Their Composites: A Review,” Composites Science and Technology, Vol. 61, No. 13, 2001, pp. 1899-1912. doi:10.1016/S0266-3538(01)00094-X
- W. A. Curtin and B. W. Sheldon, “Review: Ceramic and Metal Nanocomposites,” Materials Today, Vol. 7, No. 11, 2004, pp. 44-48. doi:10.1016/S1369-7021(04)00508-5
- N. Bouazza , M. Ouzzine , M. A. L. denas, D. Eder and A. L. Solano, “TiO2 Nanotubes and CNT-TiO2 Hybrid Materials for the Photocatalytic Oxidation of Propene at Low Concentration,” Applied Catalysis B: Environmental, Vol. 92, No. 3-4, 2009, pp. 377-383. doi:10.1016/j.apcatb.2009.08.017
- W. Chun Oh, M. L. Chen and B. K. Chem, “Synthesis and Characterization of CNT/TiO2 Composites Thermally Derived from MWCNT and Titanium(IV) n-Butoxide,” Bulletin of the Korean Chemical Society, Vol. 29, No. 1, 2008.
- C. S. Kuo, Y. H. Tseng, H. Y. Lin, C. H. Huang, C. Y. Shen, Y. Y. Li, S. I. Shah and C. P. Huang, “Synthesis of a CNT-Grafted TiO(2) Nanocatalyst and Its Activity Triggered by a DC Voltage,” Nanotechnology, Vol. 18, No. 46, 2007, Article ID: 465607. doi:10.1088/0957-4484/18/46/465607
- G. M. An, N. Na, X. R. Zhang, Z. J. Miao, S. D. Miao, K. L. Ding and Z. M. Liu, “SnO2/Carbon Nanotube Nanocomposites Synthesized in Supercritical Fluids: Highly Efficient Materials for Use as a Chemical Sensor and as the Anode of a Lithium-Ion Battery,” Nanotechnology, Vol. 18, No. 43, 2007, Article ID: 435707. doi:10.1088/0957-4484/18/43/435707
- X. Y. Wang, B. Y. Xia, X. F. Zhu, J. S. Chen, S. L. Qiu and J. X. Li, “Controlled Modification of Multiwalled Carbon Nanotubes with ZnO Nanostructures,” Solid State Chemistry, Vol. 181, No. 4, 2008, pp. 822-827. doi:10.1016/j.jssc.2008.01.005
- J. W. Liu, X. J. Li and L. M. Dai, “Water-Assisted Growth of Aligned Carbon Nanotube-ZnO Heterojunction Arrays,” Advanced Materials, Vol. 18, No. 3, 2006, pp. 1740-1744. doi:10.1002/adma.200502346
- X. B. Fan, F. Y. Tan, G. L. Zhang and F. B. Zhang, “A Novel Strategy to Fabricate γ-Fe2O3-MWCNTs Hybrids with Selectively Ferromagnetic or Superparamagnetic Properties,” Materials Science and Engineering A, Vol. 454-455, 2007, pp. 37-42. doi:10.1016/j.msea.2007.01.027
- Z. Y. Wang, G. Chen and D. G. Xia, “Coating of MultiWalled Carbon Nanotube with SnO2 Films of controlled Thickness and Its Application for Li-Ion Battery,” Power Sources, Vol. 184, No. 2, 2008, pp. 432-436. doi:10.1016/j.jpowsour.2008.03.028
- K. Hernadi, E. Ljubovic, J. W. Seo and L. Forro, “Synthesis of MWNT-Based Composite Materials with inorganic Coating,” Acta Materialia, Vol. 51, No. 5, 2003, pp. 1447-1452. doi:10.1016/S1359-6454(02)00539-6
- M. Tasviri, H. A. R. Pourb, H. Ghourchianb and M. R. Gholami, “Amine Functionalized TiO2 Coated on Carbon Nanotube as a Nanomaterial for Direct Electrochemistry of Glucose Oxidase and Glucose Biosensing,” Molecular Catalysis B: Enzymatic, Vol. 68, No. 2, 2011, pp. 206- 210. doi:10.1016/j.molcatb.2010.11.005
- L. Chen, B. L. Zhang, M. Z. Qu and Z. L. Yu, “Preparation and Characterization of CNTs-TiO2 Composites,” Powder Technology, Vol. 154, No. 1, 2005, pp. 70-72. doi:10.1016/j.powtec.2005.04.028
- C. Y. Kuo, “Prevenient Dye-Degradation Mechanisms Using UV/TiO2/Carbon Nanotubes Process,” Hazardous Mater, Vol. 163, No. 1, 2009, pp. 239-244. doi:10.1016/j.jhazmat.2008.06.083
- W. Wang, P. Serp and P. Kalck, “Photocatalytic Degradation of Phenol on MWNT and Titania Composite Catalysts Prepared by a Modified Sol-Gel Method,” Applied Catalysis B: Environmental, Vol. 56, No. 4, 2005, pp. 305-312. doi:10.1016/j.apcatb.2004.09.018
- S. Aryal, C. K. Kim and K. W. Kim, “Multi-Walled Carbon Nano-Tubes/TiO2 Composite Nanofiber by Electrospinning,” Materials Science and Engineering: C, Vol. 28, No. 1, 2008, pp. 75-79. doi:10.1016/j.msec.2007.10.002
- J. Cho, S. Schaab and J. A. Roether, “Nanostructured Carbon Nanotube/TiO2 Composite Coatings Using Electrophoretic Deposition (EPD),” Journal of Nanoparticle Research, Vol. 10, No. 1, 2008, pp. 99-105. doi:10.1007/s11051-007-9230-x
- H. Yu, X. Quan and S. Chen, “Aligned TiO2-Multiwalled Carbon Nanotube Heterojunction Arrays and Their Charge Separation Capability,” The Journal of Physical Chemistry C, Vol. 111, No. 35, 2007, pp. 12987-12991.
- J. Sun, L. Gao and M. Iwasa, “Noncovalent Attachment of Oxide Nanoparticles onto Carbon Nanotubes Using Water-in-Oil Microemulsions,” Chemical Communications, Vol. 7, 2004, pp. 832-833. doi:10.1039/b400817k
- Q. H. Zhang, L. Gao and J. K. Guo, “Preparation and Characterization of Nanosized TiO2 Powders from Aqueous TiCl4 Solution,” Nanostructured Materials, Vol. 11, No. 8, 1999, pp. 1293-1300. doi:10.1016/S0965-9773(99)00421-3
- J. Zhang, H. Zou, Q. Qing, Y. Yang, Q. Li, Z. Liu, X. Gou and Z. Du, “Effect of chEmical Oxidation on the Structure of Single-Walled Carbon Nanotubes,” The Journal of Physical Chemistry B, Vol. 107, No. 16, 2003, pp. 3712-3718. doi:10.1021/jp027500u
- L. Zhao and L. Gao, “Filling of Multi-Walled Carbon Nanotubes with tin(IV) Oxide,” Carbon, Vol. 42, No. 15, 2004, pp. 3251-3272.
NOTES
*Corresponding author.

