Materials Sciences and Applications
Vol.05 No.12(2014), Article ID:50684,8 pages
10.4236/msa.2014.512091
Silver Nanoparticles Supported on TiO2 and Their Antibacterial Properties: Effect of Surface Confinement and Nonexistence of Plasmon Resonance
Zoe Vineth Quiñones-Jurado1*, Miguel Ángel Waldo-Mendoza2, Hugo Marcelo Aguilera-Bandin1, Edgar Giovanny Villabona-Leal3, Elsa Cervantes-González4, Elías Pérez3
1Innovation and Development in Advanced Materials, POLYnnova Group, San Luis Potosí, México
2A Schulman of México, San Luis Potosí, México
3Institute of Physic, Autonomous University of San Luis Potosí, San Luis Potosí, México
4Academic Coordination, Altiplano Region, Autonomous University of San Luis Potosí, San Luis Potosí, México
Email: *zoevineth@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 29 July 2014; revised 10 September 2014; accepted 26 September 2014
ABSTRACT
Ag/TiO2 nanocomposites are usually regarded as an effective synergy for high antimicrobial performance under ultraviolet-visible light conditions. This study confirmed that the surface plasmon resonance of Ag NPs plays an important role in relation to the NPs size and consequently with the antibacterial effect of the nanocomposite. We observed that under visible light the reactivity of TiO2 cannot be amplified when it is supporting Ag NPs that have an inactive photocatalytically surface. The results confirmed that the antimicrobial effectiveness of nanocomposite based on Ag NPs supported-TiO2 is closely associated to the contact surface area and to the electronic performance of the noble metal.
Keywords:
Ag-TiO2, Antibacterial, Silver Nanoparticles, Surface Confinement

1. Introduction
The use of metallic silver of nanometric dimension (Ag NPs) has demonstrated excellent capability against many pathogen species, it is even considered better antibacterial that other silver compounds or silver ions embedded in a ceramic host [1] -[5] . The major publications on Ag NPs generalize that the antibacterial property increases with the reduction of particle size [6] -[9] , other studies suggest that the Ag NPs particle shape affects the reactivity, for example; Yang P. et al. reported that larger particles and particles with asymmetric shapes, give rise to more complex surface plasmon responses due to their higher degree of polarizability [10] . Recently, new interpretations about of the Ag NPs reactivity are emerging, Xiu et al. consider that the Ag NPs size do not cause by themselves toxicity to bacteria at less that occurs release of silver ions [11] .
Numerous studies about of nanoscaled metal oxides like TiO2, ZnO and CuO have demonstrated promising results for antibacterial activity. Additionally, materials produced by combinations of Ag NPs and those agents above mentioned exhibit much stronger antibacterial abilities [12] [13] .
Anatase TiO2 is a common agent used for the elimination of microbiological and toxic contaminants, for air, water and for self-cleaning surface applications [14] . In principle, TiO2 has proven strong catalytic activity via UV-light photons absorption, where electronic delocalization in the TiO2 generates electron/hole pairs, which, when exposed to water molecules, produce hydroxyl radicals (•OH) and superoxide anions (•O2). These radicals have the ability to oxide organic and microbial matter [15] .
Nowadays nanocomposites of Ag NPs supported on TiO2 are promoted as some of the most powerful antibacterial agents; the reason is that by linking these two components the photocatalytic process could take place even under visible light [16] -[21] . However, it is still required to learn the nanocomposites performance beyond the photocatalytic contribution, since in the practice the use of antibacterial is limited to applications where they are not exposed to light radiation. Also, it is interesting to study how, by the use of a TiO2 substrate, is it possible to enhance the contact of Ag NPs with the bacteria and understand if an Ag NPs smaller size increases the bactericidal effect of Ag/TiO2 nanocomposite.
In this work, the Ag NPs were synthesized via reduction reaction using a TiO2 aqueous suspension. The dimension and content of Ag NPs deposited on TiO2 NPs attained on the different pH conditions were evaluated and correlated with the activity of Ag/TiO2 nanocomposites against several representative bacteria; Escherichia coli and Salmonella sp and Staphylococcus aureus, which are some of the bacteria species that could easily cause skin diseases, intestinal infections and other illnesses.
2. Experimental
2.1. Materials
TiO2 ≥ 99.5% 25 nm (Evonik Degussa) was used as substrate material to prepare Ag/TiO2 nanocomposite. Silver nitrate 99.4% (Fermont) and Sodium hydroxide 99% (Caledon Laboratories LTD) were used to synthesize metallic silver NPs. Luria Bertani media (1.0% Tryptone 0.5% yeast extract and 1.0% NaCl) was used to Broth media. Microorganisms Control strains Escherichia coli ATCC 25922 (American Type Culture Collection, Rockville, Md.), Staphylococcus aureus ATCC 25923, and Salmonella sp.
2.2. Synthesis
Preparation of metallic silver on TiO2 surface was based on chemical reduction route of AgNO3. Aqueous suspension of TiO2 NPs was prepared by adding 5 g of TiO2 to 250 ml of deionized water and followed by sonication for 10 min. The slurry was heated at 80˚C with vigorous mixing. The required amount of AgNO3 solution was added to TiO2 suspension, maintaining heating and stirring. Silver concentration was of 0.5, 1.5, 5.0, 25, 35 and 45% w/w versus TiO2. The samples were named 0.5Ag/TiO2, 1.5Ag/TiO2, 5Ag/TiO2, 25Ag/TiO2, 35Ag/ TiO2 and 45Ag/TiO2. The pH of the suspension was adjusted to 4, 9 and 12 by the required addition of a 0.5 M NaOH solution. The slurry at 80˚C was mixed for two hours, after that, the solution was centrifuged and rinsed with water, the solid was dried in an oven at 80˚C for 12 hours.
2.3. Characterization
The deposited amount of Ag on TiO2 was measured by atomic absorption spectroscopy. Morphology, size and dispersion of Ag NPs supported on TiO2 nanoparticles were characterized by a TEM (Transmission Electron Microscope) TECNAI-F30 at 300 kV. The interaction between TiO2 and Silver NPs was characterized by Raman Spectroscopy, using a Horiba JobinYvo N, model M.F.O with laser 532 nm and 100 mW. Ultraviolet visible (UV-vis) spectroscopy showed the excitation energy to induce photocatalysis reactions, spectra were recorded in the range of 200 to 800 nm, using a spectrometer UV-Vis Thermoscientific model Genesys 10 UV, the sample consisted of 0.005 g of nanocomposite in 2 mL of deionized water.
2.4. Antibacterial Test
The Minimum Bactericidal Concentration (MBC) for each Ag/TiO2 sample was determined in duplicate. Tubes containing 3 ml of Luria Bertani media were prepared and Ag/TiO2 nanocomposites were added at different concentrations (0.5, 1.0, 1.5, 2.0, 3.0, 5.0, 7.0, 8.0, 9.0 and 11.0 mg/mL). A reference sample supplemented with only TiO2 and at the same concentrations was also evaluated. The solutions were sterilized at 121˚C and 15 lb/in2 for 15 minutes, and then suspensions of bacterial strains containing between 107 to 106 CFU/mL were suspended in the liquid media. Upon overnight growth at 37˚C and 150 rpm, the MBC for each strain was determined by subculturing 100 µL from each tube followed by massive striation onto agent-free Luria Bertani agar. MBC endpoints were read as the lowest concentrations of agent with no growth after overnight incubation at 35˚C. Samples were properly covered to ensure absence of visible light.
3. Results
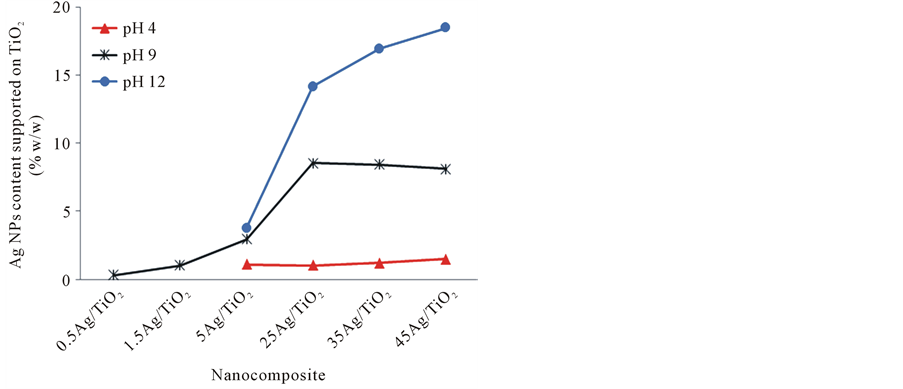
Figure 1 shows the yield of the reduction reaction of Ag(+) to Ag(0) during the synthesis of Ag/TiO2 nanocomposite was affected by pH conditions. Literature indicates that the zero charge point of TiO2 Degussa occurs at pH = 6.85 [22] . The studied pH values conveyed a different charge condition on TiO2 surface. The Ag NPs content supported on TiO2 at 4, 9 and 12 pH was analyzed by atomic absorption spectroscopy and the results shown that alkaline condition played an important role during the Ag NPs deposition on TiO2 (Figure 1). Such behavior was more evident at high silver precursor concentrations, which can be observed from the samples 5Ag/TiO2 to 45Ag/TiO2 (Figure 1). For instance, Ag NPs content supported on TiO2 for 5Ag/TiO2 samples, at 4, 9 and 12 pH was of 1%, 3% and 4% w/w, respectively. Thus, it was proven, that in absence of the precipitate agent (pH 4) and without UV-light the TiO2 surface could induce to a partial reduction of the AgNO3 amount through oxygen vacancies (O−). In alkaline conditions the TiO2 tended to be a better electro-attractive surface for nucleation Ag NPs, this was originated by deprotonation of the hydroxyl groups on the TiO2 surface. It was observed that the Ag NPs obtained by reduction-deposition depended on the density of O− sites in the TiO2, which was optimized under the most alkaline condition (pH 12).
Figure 2 shows the Raman vibrational spectra for pure TiO2 and those of 5Ag/TiO2 nanocomposite samples
Figure 1. Comparison of Ag concentration supported to TiO2 by the reduction reaction at different pH.
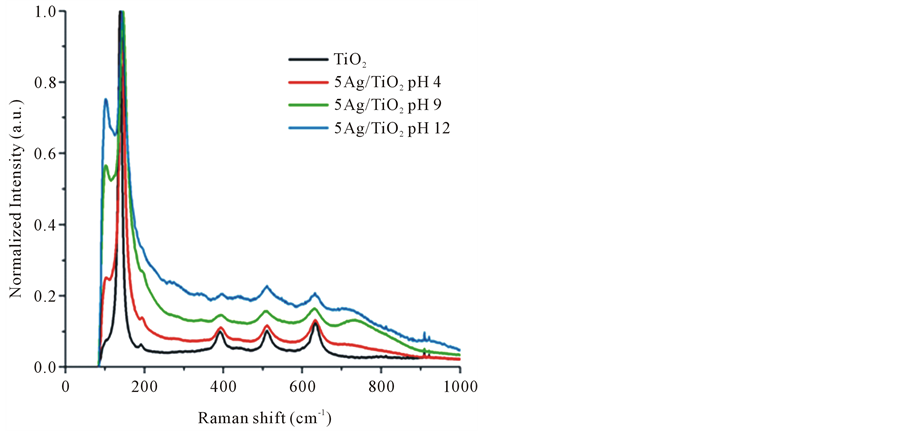
Figure 2. Raman spectra of neat TiO2 and 5Ag/TiO2 nanocomposites synthetized at different pH condition: 4, 9 and 12.
synthetized at different pH conditions. The Raman spectrum of TiO2 substrate presented an intense signal at 144 cm−1 which corresponds to the Eg mode and other low intense modes at 197, 399, 519 and 639 cm−1 [23] . The Ag NPs deposition on TiO2 particles was confirmed by the detection of others Raman peaks at 102, 259 and 735 cm−1. Additionally, these peaks seem to increase for the 5Ag/TiO2 synthesis when pH was raised from 4 to 12. This scenario suggests the formation of Ag NPs on TiO2 surface which is enhanced by basic values of pH, where both silver ions reduction and Ag NPs deposition mechanisms are apparently favored.
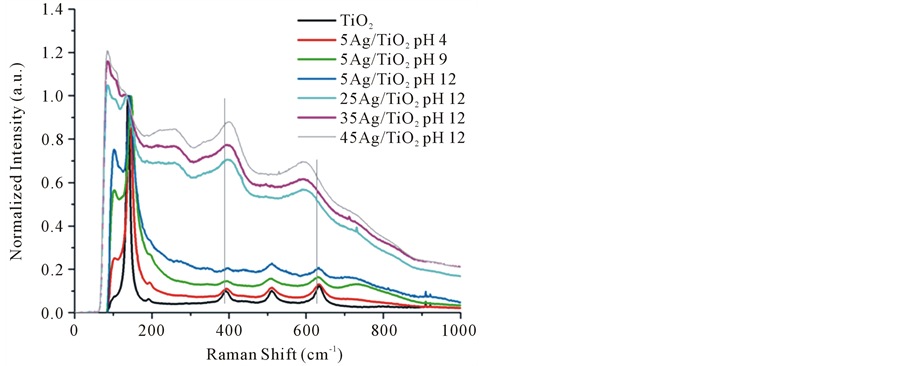
Comparatively we observed of the Figure 3 exist a relation between Ag NPs amount and the intensity of peak at 102, 144, 259 and 519 cm−1. To higher Ag NPs concentrations was consistent a higher intensity at 102 and 259 cm−1 peaks. On the other hand was observed that the peaks at 144 and 519 cm−1 belongs to TiO2, tended to overlap or even to disappear as result of the deposited Ag NPs amount increasing on surface TiO2. Additionally was observed a shift and broadening of TiO2 peaks at 399 cm−1 (B1g) and 639 cm−1 (Eg). Researchers have referred to that Raman peaks become broader when decreases the size of nanoparticle. This effect has been attributed to a volume contraction and radial pressure increasing, which consequently changes the force constant and vibrational amplitude of the nearest neighbor bond [24] . Referring to this phenomenon of Raman vibrational restriction, we demonstrate that also could take place alterations in the TiO2 interatomic distances with a surface pressure increasing to a more proportion Ag NPs deposited on TiO2 surface.
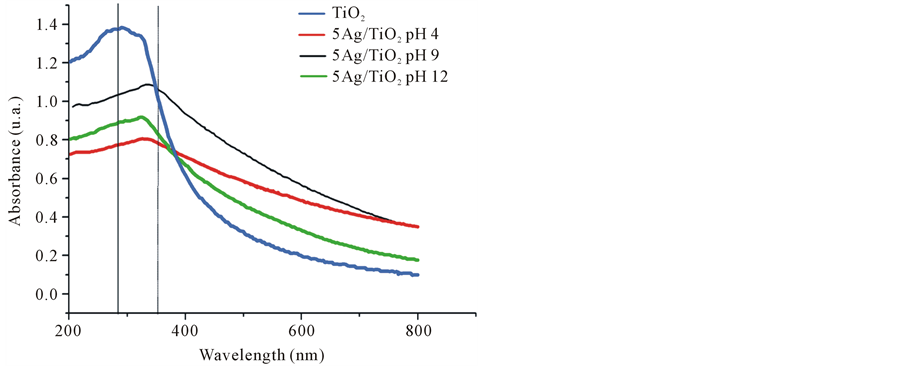
Figure 4 shows the UV-vis absorption spectrum for 5Ag/TiO2 samples obtained by silver deposition-reduc- tion reaction at fixed pH values of 4, 9 and 12, respectively. UV-Vis Spectrometry was employed to characterize the photocatalytic property and predict antimicrobial activity under light conditions of Ag/TiO2.
The optical properties and excitation frequency of the Ag/TiO2 samples suspended in deionized water were obtained via diffuse reflectance analysis. The interaction of photon energies with the Ag/TiO2 nanocomposites mainly occurred under UV wavelength. This means that the antimicrobial activity can be enhanced under UV condition by the reactivity of TiO2 substrate. The position of absorption peaks for TiO2 defines the excitation frequency of the semiconductor band gap, where only a slight wavelength change was observed from 283.5 nm to a maximum value of 334 nm, the narrowing of the energy band-gap confirmed the interface formation between Ag NPs and TiO2. Surprisingly, the UV-Vis spectra revealed that the Ag NPs did not enhanced the catalytic response of TiO2 at visible light and also the Plasmon resonance of Ag NPs was no showed. Based on this interpretation, the Ag/TiO2 antimicrobial cannot generate reactive species by means of surface plasmon under visible light. Therefore, it is assumed that the polarizability at the surface of Ag NPs could be restricted. The peak for the Ag/TiO2 samples obtained at different pH conditions suffered a modification on the full width of the Uv-vis spectrum which depends on the extent of the size or aggregation of Ag/TiO2 particles, this shift towards longer wavelengths can be due to larger particles aggregates.
Figure 3. Raman spectra of neat TiO2 and Ag/TiO2 Nanocomposites with different loading amount of Ag NPs.
Figure 4. UV-vis spectra of neat TiO2 and 5Ag/TiO2 nanocomposites synthetized at different pH: 4, 9 and 12.
According to TEM micrographs it is evident that the Ag deposits on TiO2 have nodular or spherical shape (Figure 5). These nanocomposites are composed by Ag NPs with narrow size distribution. In reference to the reaction yield, it can be observed a slight growth of Ag NPs size, which resulted of increasing the pH conditions and AgNO3 concentrations. A moderated deposition of Ag atom clusters on TiO2 was observed at pH 4 while the growth of Ag NPs was promoted at higher pH values and at a silver ion amount in excess. The particle size was predominantly smaller than 5 nm. The reduced size of the clusters may be related to the effect of confined electrons, which hinders the interaction of surface Plasmon resonance at visible light, such as was noted by UV-vis spectroscopy. This phenomenon can be associated with electronic barrier sets for Ag NPs with very small sizes, e.g. diameters < 20 nm, such as was explained by Tsivadze A, et al., 2013 [25] . Moreover, we expect this condition could help on the control of the Ag ion releasing. TEM images showed that TiO2 substrate attracts higher concentration of Ag NPs at the reduction reaction under the most basic condition, such as happened in the 45Ag/TiO2 compound synthesized at pH 12. The Ag NPs distribution was improved at high silver concentration, which is one of the principal challenges to achieve efficiency to kill bacteria by the contact of Ag NPs.
The bactericide activity of Ag/TiO2 nanocomposites was evaluated in absence of light, through detection of lowest concentration of Ag/TiO2 nanocomposites where no growth is visually observed for Gram-negative bacteria E. coli and Salmonella sp., and Gram-positive bacteria S. aureus. A summary of positive results is given in Table 1. Only the Ag/TiO2 nanocomposites exhibited biocide effect at Ag NPs contents greater than 8.5% w.t.,
Figure 5. TEM micrographs showing AgNPs deposited on TiO2 at different pH conditions: (a) 5Ag/TiO2 pH 4, (b) 25Ag/TiO2 pH 9 and (c) 45Ag/TiO2 pH 12.
Table 1. Minimun bactericidal concentrations for Ag/TiO2 nanocomposite against S. aureus, E. coli and Salmonella.
which corresponded to the subsequent nanocomposite from of the 25Ag/TiO2 composition synthetized at pH 9 (Figure 1), the other formulations with less Ag contents did not achieve the MBC on the tested amount of additive. In order to estimate the influence of pure TiO2 on the bacteria growth inhibition, control tests were parallely performed at these same concentrations of that tested for the nanocomposite; however antibacterial activity was not observed and is considered to be negligible mainly due to the test being performed under dark conditions. Thus hydroxyl groups or oxygen vacancies (O−) on TiO2 surface have not enough antibacterial power.
E coli bacteria was the most sensitive strain (Table 1), which showed to be highly affected in direct relation to the Ag NPs contents in the nanocomposite, it are related to the fact that they are gram negative and mainly could be susceptible to ionic interactions with Ag NPs. The second strain sensitive was Salmonella sp. which is also gram negative, but the MBC using the different formulation of nanocomposites was constant at 7 mg/mL; this means that the increase of Ag NPs loaded on TiO2 at contents above 8.5% w.t. did not change the antibacterial effectiveness. S. aureus also presented a constant MBC with different formulations, when using a constant dose at 10 mg/mL.
4. Discussion
Ag NPs supported on TiO2 can certainly increase the antibacterial performance more effectively than TiO2 by itself. We obtained an adequate attachment of a high Ag NPs number on the TiO2 surface, with a homogeneous dispersion and high available superficial area in strong alkaline suspension of TiO2.
It was verified by UV-vis spectroscopy that TiO2 photoactivity is enhanced by the adhesion of Ag NPs, however, the originated electronic overlap seems to be too weak to efficiently act under visible light. The explanation of the absence of reactivity in visible light was that; the Ag atoms not caused defects in the TiO2 structural network and additionally by the deficiency of surface Plasmon resonance of Ag NPs.
The nonexistence of surface Plasmon resonance of Ag NPs and the vibrational restriction of TiO2 bonding near to Ag NPs were unexpected. In fact, it actually reduced its catalytic activity due to electronic confinement barriers. In spite of the above mentioned, the antibacterial performance of silver nanocomposite was different of the bulk metallic silver properties, because even though the Ag NPs under this size (<5 nm) do not present the best catalytic property (plasmon resonance), the surface-to-volume ratio of Ag NPs ensured high surface area for the bacteria destruction by contact.
The limited antibacterial activity of Ag/TiO2, under visible light or dark conditions could be overcome by reaching a minimum Ag NPs content of 8.5% w.t. This indicates that the Ag/TiO2 toxicity was efficiently controlled by the activity of Ag NPs over any other possible oxidizing species released by TiO2.
The effect of bacterial death was more easily achieved for E. Coli and Salmonella sp. (gram negative) presumably due to the electrostatic factor. For S. aureus (gram positive) the bacterial decline was more limited, which we could suppose that the gram-positive strain caused different permeability and structural resistance due to the content of peptidoglycan in cell wall [26] . These results indicate that, although the Ag NPs reactivity become size dependent, when it is ensured an extensive contact of Ag NPs with the bacteria population in consequently is achieved the bacteria targeted death and decline. An antibacterial activity increase at higher concentrations of Ag NPs against E. Coli was observed. Therefore, we could assume that the Ag NPs with electronic confinement barriers can destroy the bacteria membrane by the penetration of Ag NPs due to reduced particle size, similar as reported Wen, et al., 2010 where Ag NPs produced pits or gaps in bacterial membrane of E. coli [27] . Thus, the interpretation of Xiu et al. [11] about of that the Ag NPs size do not cause by themselves toxicity to bacteria at less that occurs release of silver ions is considered incorrect, because it was demonstrated that even on confined surface Ag NPs if can exist destruction of the bacteria.
It is possible to obtain a satisfactory antibacterial effect at a Ag NPs reduced concentration which could be preferred to efficiently act on common applications for packaging, medical or hygienic industries, and as opposed to require exposition of electromagnetic radiation to activate Ag/TiO2.
5. Conclusions
Synthesis of Ag NPs on TiO2 was possible in acid or basic reaction conditions. More number of oxygen vacancies on TiO2 surface increased the nucleation of metallic Ag NPs. The limited antibacterial activity of Ag/TiO2 nanocomposite under visible light or dark conditions could be overcome by reaching a minimum Ag NPs content.
The results confirmed that the antimicrobial effectiveness of Ag-supported TiO2 is achieved principally due to a favored exposure and amount Ag NPs, without a photocatalytic effect of TiO2 and a surface plasmon resonance effect of Ag NPs that can be considered.
An extended Ag NPs surface reduced the catalytic activity due to electronic confinement barriers. However, higher surface-to-volume ratio of Ag NPs ensured more surface area to act by direct contact against bacteria, changed the permeability of the cellular membrane.
Acknowledgements
Authors thank to M. Lourdes González, Elisa Guerrero, Andrea K. Hernández and Erika J. Segura for obtaining the first Ag/TiO2 synthesis and Octavio Meza for instructive discussions.
References
- Marambio, C. and Hoek, E. (2010) A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. Journal of Nanoparticle Research, 12, 1531-1551. http://dx.doi.org/10.1007/s11051-010-9900-y
- Baker, C, Pradhan, A., Pakstis, L., Pochan, D. and Shah, I. (2005) Synthesis and Antibacterial Properties of Silver Nanoparticles. Journal of Nanoparticle Research, 5, 244-249.
- Rai, M., Yadav, A. and Gade, A. (2009) Silver Nanoparticles as a New Generation of Antibacterials. Biotechnology Advances, 27, 76-83. http://dx.doi.org/10.1016/j.biotechadv.2008.09.002
- Yoon, K., Byeon, J., Park, J., Hi, J., Bae, G and Hwang, J. (2008) Antibacterial Characteristics of Silver Aerosol Nanoparticles against Bacillus subtilis Bioaerosols. Environmental Engineering Science, 25, 289-294. http://dx.doi.org/10.1089/ees.2007.0003
- Lok, C., Ho, C., Chen, R., He, Q., Wing, Y., Sun, H., Tam, P., Chiu, J. and Che, C. (2006) Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. Journal of Proteome Research, 5, 916-924. http://dx.doi.org/10.1021/pr0504079
- Pal, S., Tak, Y. and Song, J. (2007) Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gramnegative Bacterium Escherichia coli. Applied and Environmental Microbiology, 73, 1712-1720. http://dx.doi.org/10.1128/AEM.02218-06
- Ayala, N., Lara, H., Ixtepan, L. and Rodríguez, C. (2009) Silver Nanoparticles Toxicity and Bactericidal Effect against Methicillin-Resistant. Staphylococcus aureus: Nanoscale Does Matter. Nanobiotechnology, 5, 2-9. http://dx.doi.org/10.1007/s12030-009-9029-1
- Dror, A., Mamane, H., Belenkova, T., Markovich, G. and Adin, A. (2009) Silver Nanoparticle—E. coli Colloidal Interaction in Water and Effect on E. coli Survival. Journal of Colloid and Interface Science, 339, 521-526. http://dx.doi.org/10.1016/j.jcis.2009.07.052
- Reidy, B., Haase, A., Luch, A., Dawson, K. and Lynch, I. (2013) Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications. Materials, 6, 2295-2350. http://dx.doi.org/10.3390/ma6062295
- Tao, A., Habas, S. and Yang, P. (2008) Shape Control of Colloidal Metal Nanocrystals. Small, 4, 310-325. http://dx.doi.org/10.1002/smll.200701295
- Xiu, Z., Zhang, Q., Puppala, H., Colvin, V. and Alvarez, P. (2012) Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Letters, 12, 4271-4275. http://dx.doi.org/10.1021/nl301934w
- Sunada, K., Watanabe, T. and Hashimoto, K. (2003) Bactericidal Activity of Copper-Deposited TiO2 Thin Film under Weak UV Light Illumination. Environmental Science & Technology, 37, 4785-4789. http://dx.doi.org/10.1021/es034106g
- Xiaoyang, P. and Yi-Jun, X. (2013) Defect-Mediated Growth of Noble-Metal (Ag, Pt, and Pd) Nanoparticles on TiO2 with Oxygen Vacancies for Photocatalytic Redox Reactions under Visible Light. The Journal of Physical Chemistry C, 117, 17996-18005. http://dx.doi.org/10.1021/jp4064802
- Yao, K., Wang, D., Ho, W., Yan, J. and Tzeng, K. (2007) Photocatalytic Bactericidal Effect of TiO2 Thin Film on Plant Pathogens. Surface and Coatings Technology, 201, 6886-6888. http://dx.doi.org/10.1016/j.surfcoat.2006.09.068
- Cai, Y.L., Strømme, M. and Welch, K. (2014) Disinfection Kinetics and Contribution of Reactive Oxygen Species When Eliminating Bacteria with TiO2 Induced Photocatalysis. Journal of Biomaterials and Nanobiotechnology, 5, 200-209. http://dx.doi.org/10.4236/jbnb.2014.53024
- Loganathan, K., Bommusamy, P., Muthaiahpillai, P. and Velayutham, M. (2011) The Syntheses, Characterizations, and Photocatalytic Activities of Silver, Platinum, and Gold Doped TiO2 Nanoparticles. Environmental Engineering Research, 16, 81-90. http://dx.doi.org/10.4491/eer.2011.16.2.81
- Huanjun, Zh. and Guohua, Ch. (2009) Potent Antibacterial Activities of Ag/TiO2 Nanocomposite Powders Synthesized by a One-Pot Sol−Gel Method. Environmental Science & Technology, 43, 2905-2910. http://dx.doi.org/10.1021/es803450f
- Keleher, J., Bashant, J., Heldt, N., Johnson, L. and Li, Y. (2002) Photo-Catalytic Preparation of Silver-Coated TiO2 Particles for Antibacterial Applications. Journal of Microbiology and Biotechnology, 18, 133-139. http://dx.doi.org/10.1023/A:1014455310342
- Ashkarran, A., Aghigh, S., Kavianipour, M. and Farahani, N. (2011) Visible Light Photo-and Bioactivity of Ag/TiO2 Nanocomposite with Various Silver Contents. Current Applied Physics, 11, 1048-1055. http://dx.doi.org/10.1016/j.cap.2011.01.042
- Zou, X., Silva, R., Huang, X., Jafar, F., Al-Sharab, J. and Asefa, T. (2013) A Self-Cleaning Porous TiO2-Ag Core-Shell Nanocomposite Material for Surface-Enhanced Raman Scattering. Chemical Communications, 49, 382-384. http://dx.doi.org/10.1039/c2cc35917k
- Pan, X., Medina, I., Mernaugh, R. and Liu, J. (2010) Characterization and Bactericidal Performance of Silver Modified Titaniaphotocatalyst. Colloids and Surfaces B: Biointerfaces, 77, 82-89. http://dx.doi.org/10.1016/j.colsurfb.2010.01.010
- Lin, Y.-C., Bai, H., Lin, C.-H. and Wu, J.-F. (2013) Applying Surface Charge Attraction to Synthesizing TiO2/Ag Composition for VOCs Photodegradation. Aerosol and Air Quality Research, 13, 1512-1520.
- Choi, H., Jung Y. and Kim, S. (2004) Characterization of Raman Spectra of Size-Selected TiO2 Nanoparticles by Two-Dimensional Correlation Spectroscopy. Bulletin of the Korean Chemical Society, 25, 426-428. http://dx.doi.org/10.5012/bkcs.2004.25.3.426
- Chen, X. and Mao, S. (2007) Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chemical Reviews, 107, 2891-2959. http://dx.doi.org/10.1021/cr0500535
- Tsivadze, A., Ionova, G., Mikhalko, V., Ionova, I. and Gerasimova, G. (2013) Plasmon Properties of Silver Spherical Nanoparticles and Films. Protection of Metals and Chemistry of Surfaces, 49, 169-172. http://dx.doi.org/10.1134/S207020511302010X
- Madigan, M., Martinko, J. and Parker, J. (2003) Brock Biology of Microorganisms. 10th Edition, Pearson, Prentice Hall, New York.
- Wen, L., Xiao, X., Qing, S., Hai, Z., You, O. and Yi, C. (2010) Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia coli. Applied Microbiology and Biotechnology, 85, 1115-1122. http://dx.doi.org/10.1007/s00253-009-2159-5
NOTES

*Corresponding author.