Materials Sciences and Applications
Vol.5 No.1(2014), Article ID:42562,5 pages DOI:10.4236/msa.2014.51008
Study on NH3 Plasma-Treated Polyimide/MWNT Composites on Electrical and Surface Properties
![]()
Institute of Organic and Polymeric Materials, National Taipei University of Technology, Taiwan.
Email: *ycheng@ntut.edu.tw
Copyright © 2014 Yao-Yi Cheng et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Yao-Yi Cheng et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received October 27th, 2013; revised December 5th, 2013; accepted December 28th, 2013
KEYWORDS
Polyimide; Carbon Nanotube; Plasma Treatment; Contact Angle
ABSTRACT
We studied the surface modification of polyimide/multi-walled carbon nanotube (MWNT) composites resulting from plasma treatment. After NH3 plasma treatment, the surface properties of polyimide containing MWNT (U-CNT) or acid modified MWNTs (M-CNTs) were investigated using X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), contact angle and electrical measurements. The surface of polyimide/MWNT composites became more hydrophilic after the NH3 plasma treatment, which improved the surface electrical conductivity of polyimide/MWNT composites. The XRD results indicate that the formation of a MWNT network structure in the polyimide matrix impeded the motion of polyimide molecules.
1. Introduction
Carbon nanotubes (CNTs) [1] have excellent strength, elastic modulus, electrical and thermal conductivity [2,3] because of their high aspect ratio (L/D). However, the application of CNT in composites is challenging because the intrinsic van der Waals interaction among CNTs causes poor dispersion in organic polymer matrix [4,5]. There are two main approaches to improve CNTs dispersibility in the polymer matrix: grafting functional groups [6,7] or non-convalently method [8,9]. Polyimide (PI), a well-known engineering plastic that demonstrates excellent chemical resistance and dielectric, mechanical, and thermal properties, has been extensively used in aerospace, microelectronic and optoelectronic industries. The reduction of PI electrical resistance reduces the build-up of electrostatic charges on a PI surface. By adding singlewalled carbon or multi-wall carbon nanotubes (MWNTs) to PI composites, researchers have reported a reduction in surface resistance to approximately 106 - 108 Ω/cm2. The network-like structure of dispersed CNT reduces the amount of CNT required to improve the properties of polymer/MWNT nanocomposites.
Plasma technology [10-12] has been widely used to activate the surface of films without changing their bulk structure. The plasma modification technology utilizes the ionized, molecules and radicals made by the electrical field to bombard and react with the surface of the substrate [8,13]. Difference of ionized gases can be used to produce various types of plasma [14,15]. Valentini et al. investigated the fluorinated single-wall CNTs treated by plasma [16] and Xu et al. investigated the surface modification of multi-walled CNTs under oxygen plasma treatment [9]. Chen et al. reported that Ar/H2O surfacewave plasma can be used to modify the surface characteristics of CNTs and thus improve their dispersion ability in water [17]. CNTs can be treated with plasma to enhance their compatibility with the polyimide matrix [18, 19]. In this study, we prepared PI/MWNT nanocomposites and applied various MWNT surface modifications to improve their dispersion in the PI matrix. The effect of plasma treatment on PI/MWNT nanocomposites [8,17, 20] was investigated.
2. Experimental
2.1. Materials
Pristine MWNT 10 - 30 nm in diameter and 5 - 15 μm in length were fabricated using chemical vapor deposition (CVD) with 95% purity. 1-methyl-2-pyrrolodinone (NMP, Acros, 99%), sulfuric acid (H2SO4, Acros, 98%), nitric acid (HNO3, Acros, 65%), 3,3’,4,4’-biphenyl tetracarboxylic dianhydride (BPDA, Chriskev, 98%), and p-phenylenediamine (p-PDA, Acros, 99%) were used as received without further purification.
2.2. Preparation of Oxidized and Acid-Modified CNTs
The pristine MWNTs were purified by oxidation in air, at 550˚C for 45 min, to remove amorphous carbon and residual metal catalysts to produce the unmodified MWNTs (U-CNTs). U-CNTs were mixed with sulfuric and nitric acids (3:1 according to volume) by using ultra-sonication for 2 h. The dispersed mixture was then washed and filtered with distilled water and methanol to produce acid-modified MWNTs (M-CNTs).
2.3. Preparation of PI/MWNT Composites
The U-CNTs or M-CNTs were added to NMP and ultrasonicated for 6 h to obtain a uniformly dispersed MWNT suspension. Diamine p-PDA was then added to the solution, and vigorously stirred in a flask. After 30 min, dianhydride BPDA was added and reacted at room temperature under nitrogen for 4 h. The resulting PAA solution had a 12% wt% solid content. The molar ratio of dianhydride and diamine was maintained at 1.02:1 to control the molecular weight. The PI/MWNT films were prepared by coating the PAA solution on glass for multistep thermal curing (80˚C, 100˚C, 150˚C, 200˚C, 250˚C, 300˚C, and 400˚C, each temperature maintained for 1 h).
2.4. Plasma Treatment
NH3 was the gas used for plasma treatment of PI/MWNT composites. The treatment powers were 30 W and 100 W at radio frequency of 13.56 MHz. The treatment time was 5 minutes for all samples. Figure 1 shows the process scheme.
2.5. Characterization
Fourier transform infrared spectroscopy (FTIR) analysis was performed using a Perkin-Elmer FTIR system, and transmission electron microscopy (TEM) was performed using a JEOL-JEM-1230 transmission electron microscope. Contact angle analysis was performed using an FTA-1000 contact angle meter. The X-ray photoelectron spectroscopy (XPS) analysis of powdered MWNT samples was performed using the ULVAC, PHI 5000 VersaProbe/scanning ESCA microprobe. The electrical properties of the samples were measured using an ULTRA Mesoh-meter SM-8200, and tests were conducted at room temperature, and a voltage of 100 V. Scanning electron microscopy (SEM) was performed using a JEOL-5610 scanning electron microscope. X-ray diffraction (XRD) analysis was performed using a RINT 2000 wide-angle goniometer.
3. Results and Discussion
As shown in Figure 2, the surfaces of modified MWNTs (M-CNTs) exhibited carboxyl acid and hydroxyl groups after the acid-treatment process. The FTIR spectra in this figure show absorption peaks at approximately 3400 cm−1 for the -OH group and 1700 cm−1 for C=O group. Figure 3 shows TEM image of MWNTs that were treated with mixed acid and MWNTs that were not. The mixed acidmodified MWNTs were thoroughly dispersed.
Table 1 shows the angles at which the surfaces of the PI/MWNT films contact water at various NH3 plasma treatment powers. The PI film surface became hydrophobic when U-CNT and M-CNT were added, increasing

Figure 1. Reaction scheme for preparing plasma-treated PI/ MWNT composites.

Figure 2. FTIR spectra of unmodified and modified MWNT.
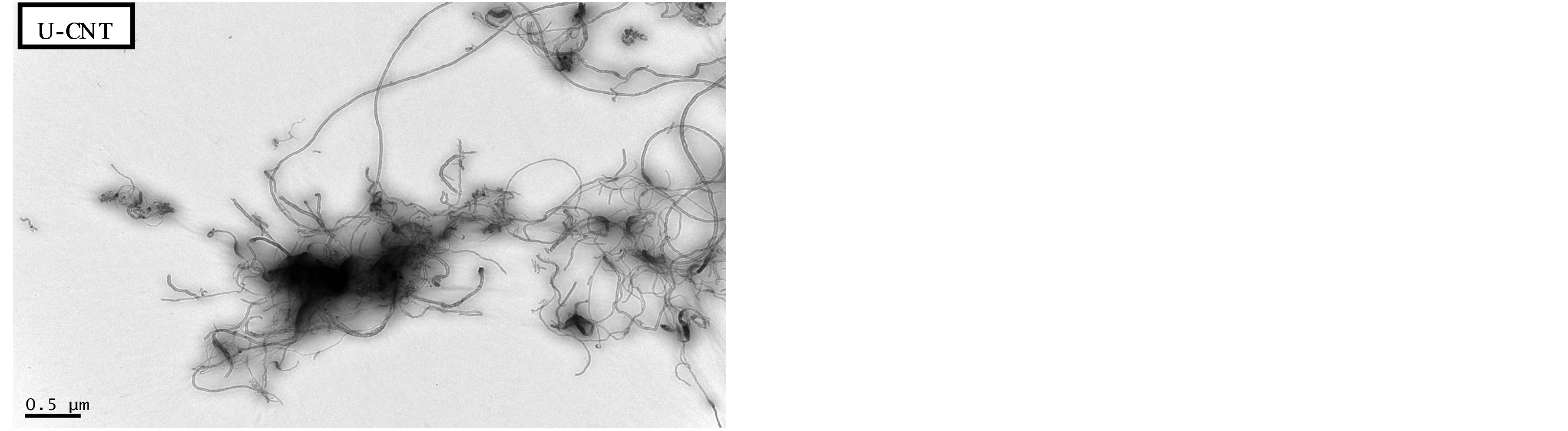
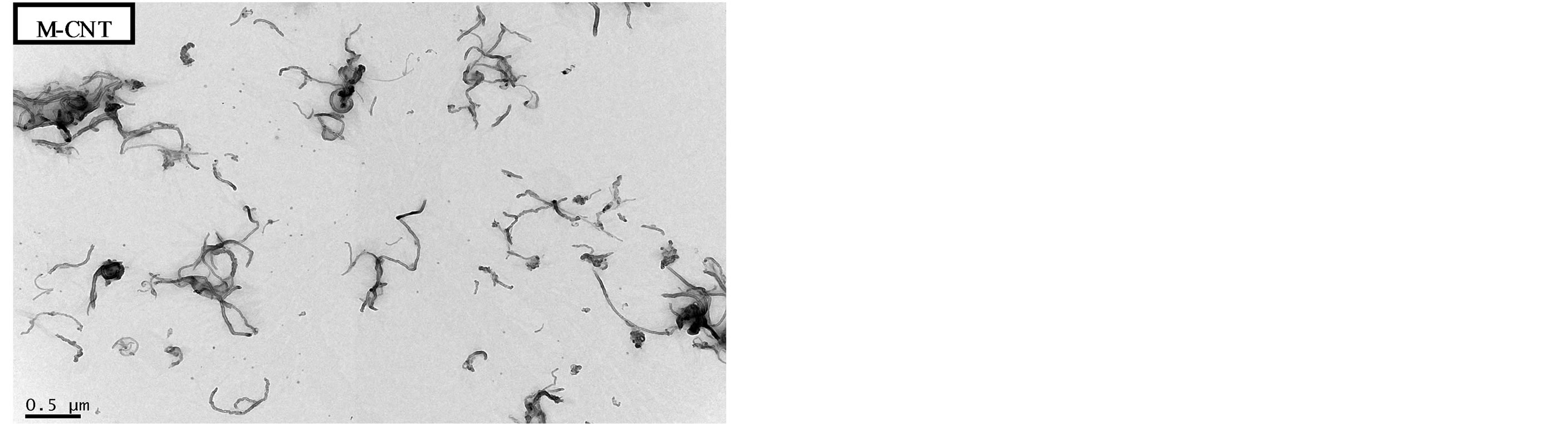
Figure 3. TEM image of unmodified and modified MWNT.
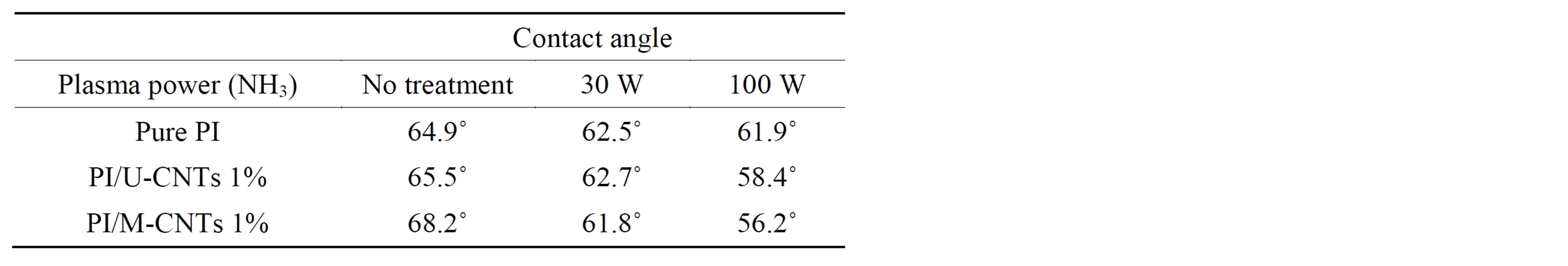
Table 1. Contact angle results of PI/MWNT composites.
the degree of the contact angle with water. As the NH3 plasma treatment power increased from 30 W to 100 W, the surfaces of PI or PI/MWNT film became increasingly hydrophilic. As a result, the degree of the contact angle with water decreased.
The XPS results suggest that the N and O atoms on the surface of PI/MWNT composites increased after NH3 plasma treatment, which could cause the hydrophilic characteristic of PI/MWNT composites (Table 2). Figure 4 shows the XPS O 1s spectra of the PI and PI/UCNT composite after the plasma treatment. The components at approximately 532 eV and 533 eV are attributed to the C=O and C-O groups. After the NH3 plasma treatment processes, the C=O intensity decreased and the C-O intensity increased, suggesting that the NH3 plasma treatment broke the C=O double bond.
The high aspect ratio of the MWNTs substantially improved the electrical conductivity of the polymer matrix, enabling a reduction in surface electrostatic charge (ESC) build-up. Table 3 shows that the surface resistance of PI/MWNT composites gradually decreased as the MWNT content increased. The criterion for electrostatic charge mitigation (106 - 108 Ω/cm2) was met when PI was loaded with 2 wt% of U-CNTs. However, 2 wt% of M-
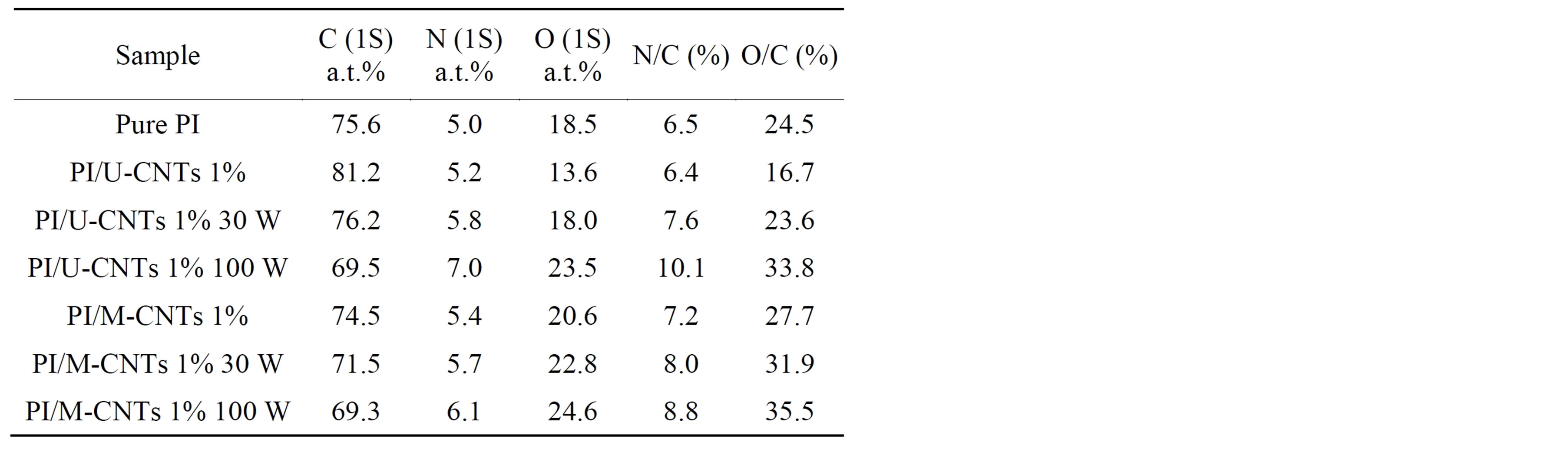
Table 2. XPS results of PI/MWNT composites.
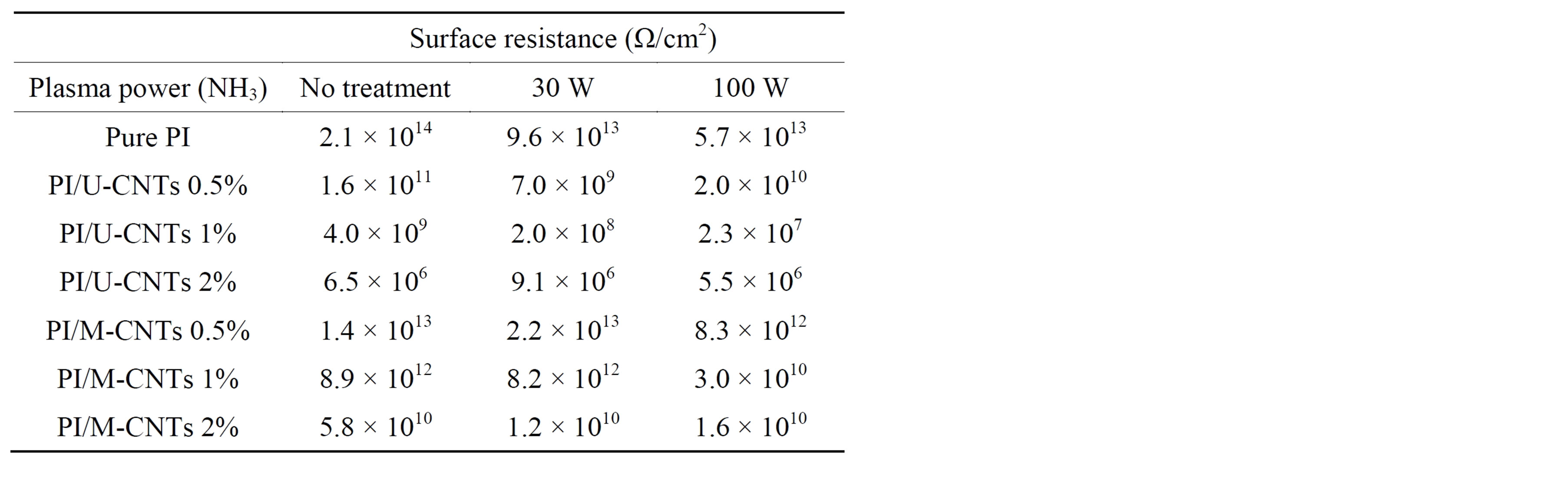
Table 3. Electrical properties of PI/MWNT composites.
CNT is not sufficient for PI composites. This is primarily because the acid treatment reduces the length of CNTs. On the other hand, the electrostatic charge mitigation criterion can be met when PI is loaded with only 1 wt% of U-CNT, with subsequent 100 W NH3 plasma treatment to make the surface of PI/MWNT composites more hydrophilic.
Figure 5 shows the surface morphology of the PI/ MWNT films after NH3 plasma treatment. The U-CNT and M-CNT were exposed outside the surface of PI film, which could form a conductive path that effectively reduces the surface resistance of the PI/MWNT composites.
The 1 wt% U-CNTs formed a network structure in the PI matrix. As shown in Figure 6, the XRD results indicate that the motion of PI molecules was impeded for the crystallization of PI because of the formation of a network structure of 1 wt% U-CNTs in PI matrix. Therefore, 100 W NH3 plasma treatment effectively reduced the surface resistance of 1 wt% PI/U-CNTs.
4. Conclusions
In conclusion, we fabricated a PI nanocomposite composed of homogeneously dispersed MWNTs. The results show that the conductive paths could be formed in PI/ MWNT nanocomposites to reduce the surface resistance. With 100 W NH3 plasma treatment on the PI loaded only 1 wt% of U-CNT, the surface resistance can be reduced to meet the electrostatic charge mitigation criterion.
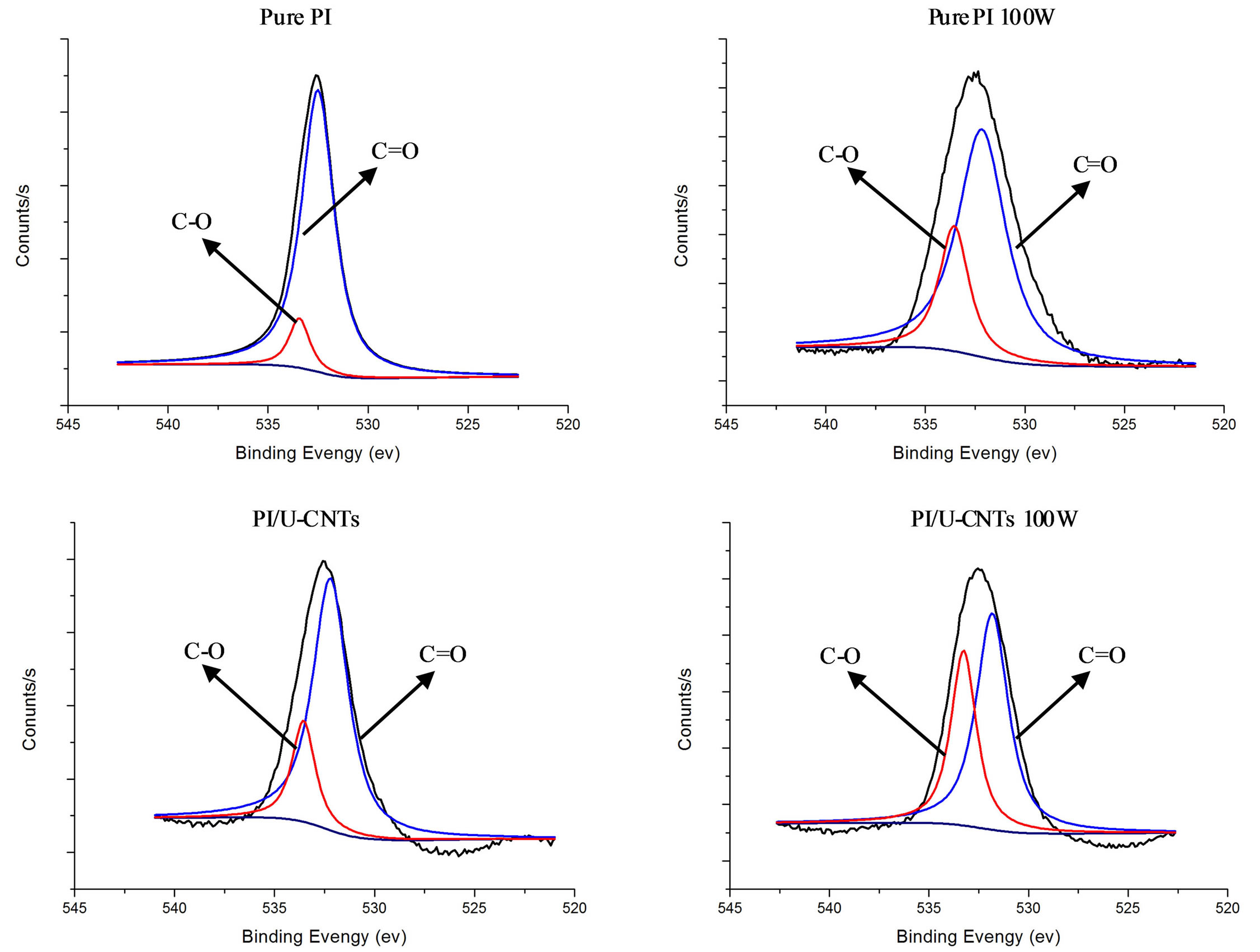
Figure 4. XPS O 1s spectrum of PI and PI/U-CNT composite after plasma treatment processes.
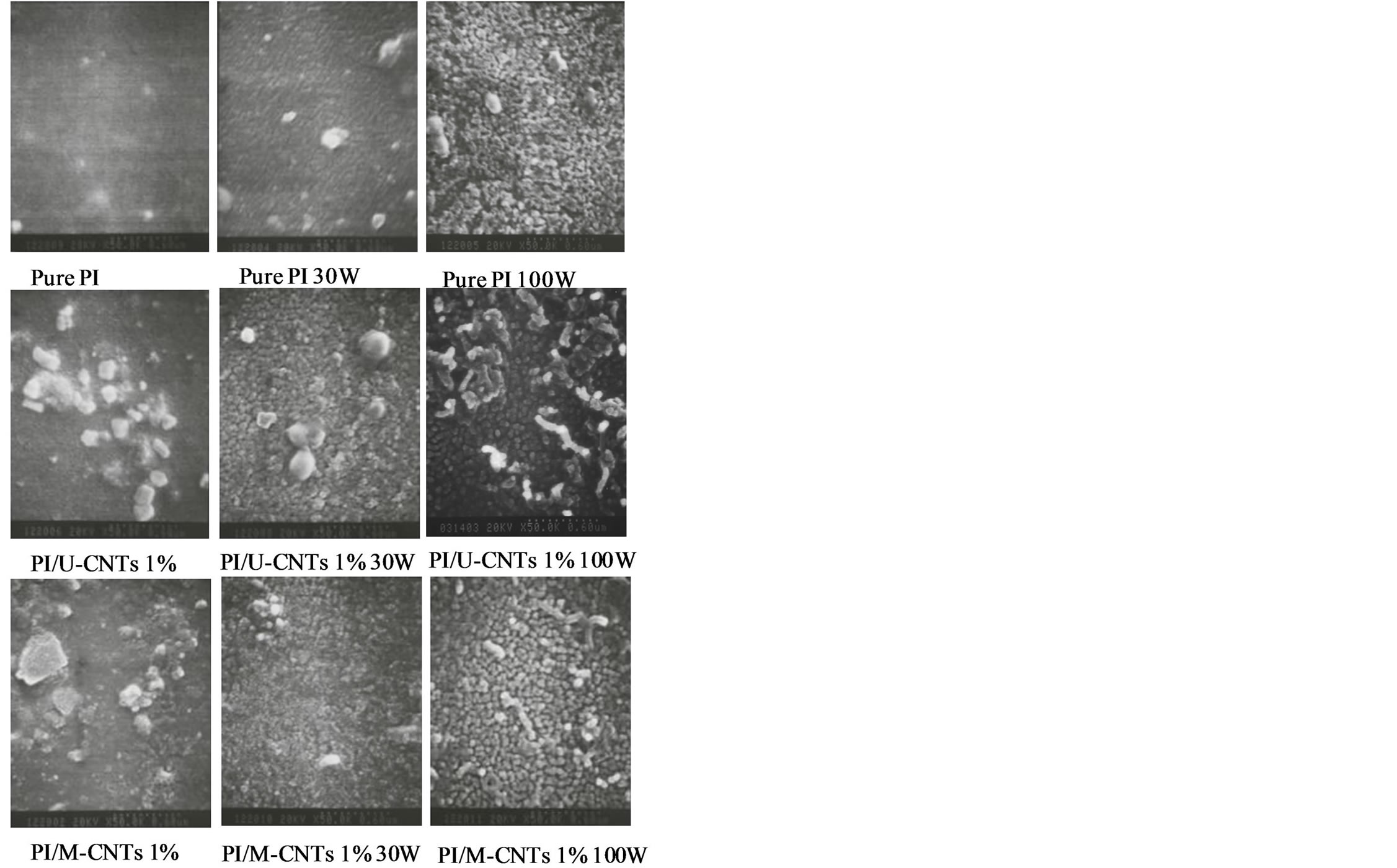
Figure 5. Top view SEM images (50,000×) of PI/MWNT composites after various plasma treatments.
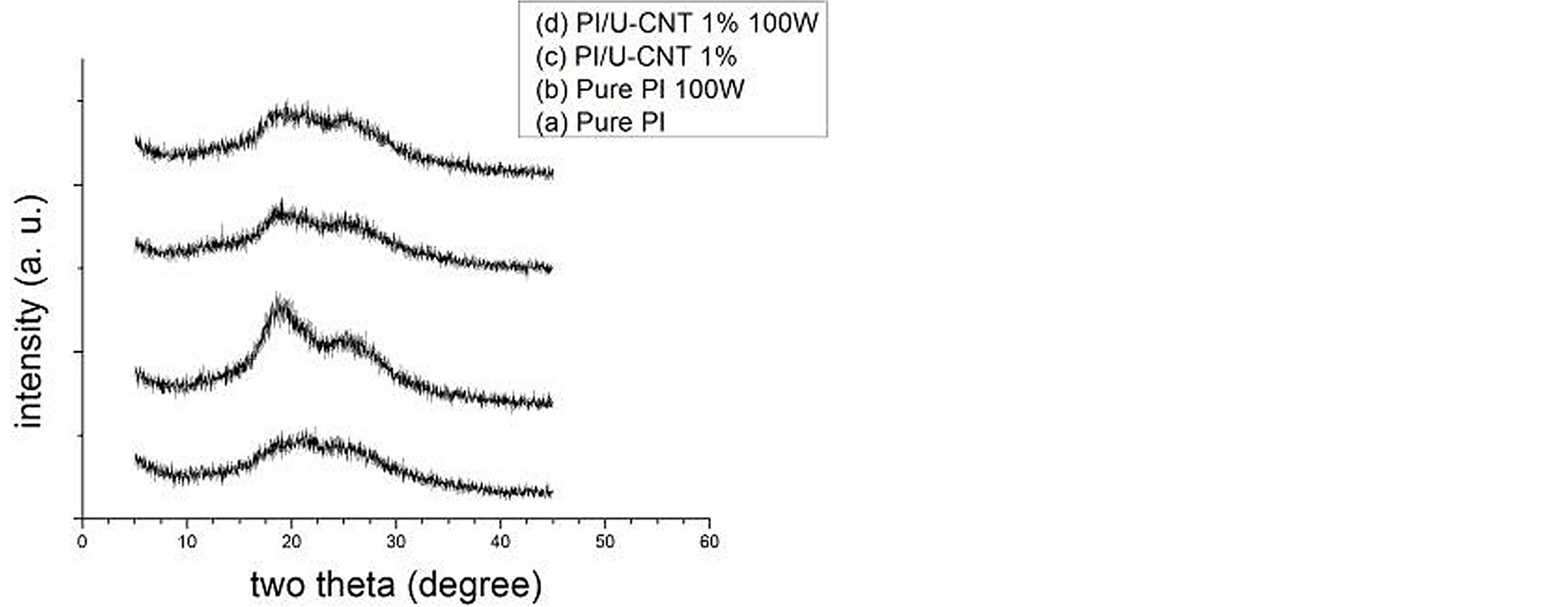
Figure 6. XRD spectrum of PI/MWNT composites after various plasma treatment processes.
Acknowledgements
We gratefully acknowledge the plasma treatment support the National Nano Device Laboratories in Taiwan.
REFERENCES
- S. Iijima, “Helical Microtubules of Graphitic Carbon,” Nature, Vol. 354, 1991, pp. 56-58. http://dx.doi.org/10.1038/354056a0
- S. Shenogin, A. Bodapati, L. Xue, R. Ozisik and P. Keblinski, “Effect of Chemical Functionalization on Thermal Transport of Carbon Nanotube Composites,” Applied Physics Letters, Vol. 85, No. 12, 2004, pp. 2229-2231. http://dx.doi.org/10.1063/1.1794370
- C.-H. Tseng, C.-C. Wang and C.-Y. Chen, “Functionalizing Carbon Nanotubes by Plasma Modification for the Preparation of Covalent-Integrated Epoxy Composites,” Chemistry of Materials, Vol. 19, No. 2, 2007, pp. 308- 315. http://dx.doi.org/10.1021/cm062277p
- I.-H. Chen, C.-C. Wang and C.-Y. Chen, “Preparation of Carbon Nanotube (CNT) Composites by Polymer Functionalized CNT under Plasma Treatment,” Plasma Processes Polymers, Vol. 7, No. 1, 2010, pp. 59-63. http://dx.doi.org/10.1002/ppap.200900067
- K. Yang, M. Gu, Y. Guo, X. Pan and G. Mu, “Effect of Carbon Nanotube Functionalization on the Mechanical and Thermal Properties of Epoxy,” Carbon, Vol. 47, No. 7, 2009, pp. 1723-1737. http://dx.doi.org/10.1016/j.carbon.2009.02.029
- S.-M. Yuen, C.-C. M. Ma, Y.-Y. Lin and H.-C. Kuan, “Preparation, Morphology and Properties of Acid and Amine Modified Multiwalled Carbon Nanotube/Polyimide Composite,” Composites Science and Technology, Vol. 67, No. 11-12, 2007, pp. 2564-2573. http://dx.doi.org/10.1016/j.compscitech.2006.12.006
- C. Velasco-Santos, A. L. Marìnez-Hernàndez, M. Lozada-Cassou, A. Alvarez-Castillo and V. M. Castaño, “Chemical Functionalization of Carbon Nanotubes through an Organosilane,” Nanotechnology, Vol. 13, 2002, pp. 495- 498. http://dx.doi.org/10.1088/0957-4484/13/4/311
- C. A. Ávila-Orta, V. J. Cruz-Delgado, M. G. Neira-Velázquez, E. Hernández-Hernández, M. G. Méndez-Padilla and F. J. Medellín-Rodríguez, “Surface Modification of Carbon Nanotubes with Ethylene Glycol Plasma,” Carbon, Vol. 47, No. 8, 2009, pp. 1916-1921. http://dx.doi.org/10.1016/j.carbon.2009.02.033
- T. Xu, J. Yang, J. Liu and Q. Fu, “Surface Modification of Multi-Walled Carbon Nanotubes by O2 Plasma,” Applied Surface Science, Vol. 253, No. 22, 2007, pp. 8945- 951. http://dx.doi.org/10.1016/j.apsusc.2007.05.028
- M. J. Chuang, “Carbon Tetrafluoride Plasma Modification of Polyimide: A Method of In-Situ Formed Hydrophilic and Hydrophobic Surfaces,” Surface & Coatings Technology, Vol. 203, No. 23, 2009, pp. 3527-3532. http://dx.doi.org/10.1016/j.surfcoat.2009.05.020
- S. J. Park, H. J. Sohn, S. K. Hong and G. S. Shin, “Influence of Atmospheric Fluorine Plasma Treatment on Thermal and Dielectric Properties of Polyimide Film,” Journal of Colloid and Interface Science, Vol. 332, No. 1, 2009, pp. 246-250. http://dx.doi.org/10.1016/j.jcis.2008.12.040
- C. H. Wen, M. A. Chuang and G. H. Hsiue, “Asymmetric Surface Modification of Poly(ethylene terephthalate) Film by CF4 Plasma Immersion,” Applied Surface Science, Vol. 252, No. 10, 2006, pp. 3799-3805. http://dx.doi.org/10.1016/j.apsusc.2005.05.059
- C. Chen, B. Liang, D. Lu, A. Ogino, X. Wang and M. Nagatsu, “Amino group Introduction onto Multiwall Carbon Nanotubes by NH3/Ar Plasma Treatment,” Carbon, Vol. 48, No. 4, 2010, pp. 939-948. http://dx.doi.org/10.1016/j.carbon.2009.10.033
- H. Bubert, S. Haiber, W. Brandlb, G. Margineanb, M. Heintzec and V. Brüser, “Characterization of the Uppermost Layer of Plasma-Treated Carbon Nanotubes,” Diamond and Related Materials, Vol. 12, No. 3-7, 2003, pp. 811-815. http://dx.doi.org/10.1016/S0925-9635(02)00353-9
- K. S. Ahn, J. S. Kim, C. O. Kim and J. P. Hong, “NonReactive rf Treatment of Multiwall Carbon Nanotube with Inert Argon Plasma for Enhanced Field Emission,” Carbon, Vol. 41, No. 13, 2003, 2481-2485. http://dx.doi.org/10.1016/S0008-6223(03)00294-X
- L. Valentini, J. Macan, I. Armentano, M. Francesco and J. M. Kenny, “Modification of Fluorinated Single-Walled Carbon Nanotubes with Aminosilane Molecules,” Carbon, Vol. 44, No. 11, 2006, pp. 2196-2201. http://dx.doi.org/10.1016/j.carbon.2006.03.007
- C. Chen, A. Ogino, X. Wang and M. Nagatsu, “Plasma Treatment of Multiwall Carbon Nanotubes for Dispersion Improvement in Water,” Applied Physics Letters, Vol. 96, 2010, Article ID: 131504. http://dx.doi.org/10.1063/1.3377007
- W.-J. Chou, C.-C. Wang and C.-Y. Chen, “Characteristics of Polyimide-Based Nanocomposites Containing PlasmaModified Multi-Walled Carbon Nanotubes,” Composites Science and Technology, Vol. 68, No. 10-11, 2008, pp. 2208-2213. http://dx.doi.org/10.1016/j.compscitech.2008.04.008
- L. Valentini, D. Puglia, F. Carniato, E. Boccaleri, L. Marchese and J. M. Kenny, “Use of Plasma Fluorinated Single-Walled Carbon Nanotubes for the Preparation of Nanocomposites with Epoxy Matrix,” Composites Science and Technology, Vol. 68, No. 3-4, 2008, pp. 1008-1014. http://dx.doi.org/10.1016/j.compscitech.2007.07.011
- P. C. Ma, J. K. Kim and B. Z. Tang, “Functionalization of Carbon Nanotubes Using a Silane Coupling Agent,” Carbon, Vol. 44, No. 6, 2009, pp. 3232-3238
NOTES
*Corresponding author.

