Applied Mathematics
Vol.07 No.18(2016), Article ID:72929,12 pages
10.4236/am.2016.718187
A Within-Host Model of Dengue Infection with a Non-Constant Monocyte Production Rate
Jeremy J. Thibodeaux1, Michael Hennessey2
1Department of Mathematical Sciences, Loyola University New Orleans, New Orleans, LA, USA
2Department of Mathematical Sciences, Rensselaer Polytechnic Institute, Troy, NY, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 20, 2016; Accepted: December 19, 2016; Published: December 22, 2016
ABSTRACT
In this paper we modify previous models to develop a new model of within-host dengue infection without the assumption that monocyte production is constant. We show that this new model exhibits behavior not seen in previous models. We then proceed by obtaining an expression for the net reproductive rate of the virus and thus establish a stability result. We also perform a sensitivity analysis to test various treatment strategies and find that two strategies might be fruitful. One is the reduction of the infection rate of monocytes by viruses and the other, more effective, theoretical approach is to reduce the number of new viruses per infected monocyte.
Keywords:
Dengue, Within-Host Model, Net Reproductive Rate, Treatment Scenarios

1. Introduction
Dengue is a virus belonging to the Flavivirus genus. The Flavivirus genus includes mostly mosquito-borne viruses such as the West Nile virus and the yellow fever virus. The dengue virus exists in four different serotypes. A serotype is a distinct variation within a species of viruses that may present a different configuration or slightly different kind of antigen. All serotypes of the dengue virus can cause the full spectrum of disease symptoms [1] .
The World Health Organization estimates that nearly 50 million infections occur annually in over 100 countries [2] . As there are no specific anti-viral treatments for dengue infection, supportive care is the usual treatment. This may include bed rest, antipyretics and analgesics. A small subset of infections result in dengue hemorrhagic fever which can be fatal.
The incubation period of the virus in an infected host ranges from 5 to 10 days [3] . At the end of the incubation period, viral particles enter the bloodstream and cause the onset of symptomatic fever. Viremia, the presence of virus in the blood stream, occurs roughly two days before the onset of symptoms and lasts 5 to 6 days [4] . Viremia tends to peak at the time of or shortly after the onset of illness. The clearance of virus is performed by the immune system.
There have been many mathematical studies of dengue infection. Of those, relatively few [5] [6] [7] [8] [9] are concerned with within-host dynamics. In these, it is assumed that the production of target cells is constant. This assumption is adequate in healthy individuals but the production of monocytes can vary, especially during infection [10] . In fact, the data in [10] show that monocyte levels are actually elevated during dengue infection, which is rather counter-intuitive. In general, the production is controlled by the Macrophage Colony Stimulating Factor (M-CSF). We account for this additional aspect in our model and show that this modification allows for better agreement with the data.
The remainder of the paper is organized as follows: in Section 2 we formulate the homogeneous viral infection model. Section 3 is the analysis of the model’s equilibria. Section 4 contains the parameter sensitivity analysis and comparisons with previous models. In Section 5 we make some concluding remarks.
2. The Model
Within this section, we formulate a model of population growth of the dengue virus within the human body based on the model in [9] . The model starts with the beginning of the detectable viremia period. It is assumed that one serotype of dengue virus circulates within the infected host and that the virus infects the monocyte cell population of the host.
In [9] , the authors studied the following model:
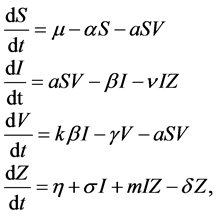 (1)
(1)
where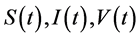 , and
, and 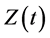 represent the density of susceptible monocytes, infected monocytes, free virus particles and immune cells in
represent the density of susceptible monocytes, infected monocytes, free virus particles and immune cells in 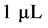 blood at time
blood at time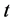 , respectively. The production of susceptible monocytes is assumed to be a constant
, respectively. The production of susceptible monocytes is assumed to be a constant 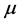 and they also have a constant death rate
and they also have a constant death rate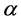 . This model always shows that monocyte population significantly decreases during infection, which is not always the case [10] . In order to obtain a model that more closely resembles the data in [10] , we have chosen to model the production of monocytes dynamically. First, we account for the fact that the primary catalyst for monocyte production is a cytokine called the Macrophage Colony Stimulating Factor (M-CSF). Other components of the blood are also controlled in a similar way. For example, the production of erythrocytes is controlled by the hormone erythropoietin. In several previous works, including [11] [12] [13] , it was assumed that the rate of production was proportional to the hormone concentration. We will make the same assumption here and will require that in the absence of infection, the rate of production is indeed the constant
. This model always shows that monocyte population significantly decreases during infection, which is not always the case [10] . In order to obtain a model that more closely resembles the data in [10] , we have chosen to model the production of monocytes dynamically. First, we account for the fact that the primary catalyst for monocyte production is a cytokine called the Macrophage Colony Stimulating Factor (M-CSF). Other components of the blood are also controlled in a similar way. For example, the production of erythrocytes is controlled by the hormone erythropoietin. In several previous works, including [11] [12] [13] , it was assumed that the rate of production was proportional to the hormone concentration. We will make the same assumption here and will require that in the absence of infection, the rate of production is indeed the constant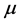 . This changes the first equation in (1) to
. This changes the first equation in (1) to
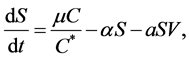 (2)
(2)
where 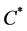 is the normal concentration of M-CSF. We are now required to model the dynamics of the M-CSF production. First, we will model how the body regulates its control under normal conditions, i.e., no infection. In this case, the production’s purpose is to maintain a normal monocyte count [14] , which we will call
is the normal concentration of M-CSF. We are now required to model the dynamics of the M-CSF production. First, we will model how the body regulates its control under normal conditions, i.e., no infection. In this case, the production’s purpose is to maintain a normal monocyte count [14] , which we will call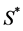 . We want a function that increases when
. We want a function that increases when 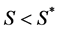 and decreases when
and decreases when 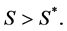 To achieve this, we
To achieve this, we
have chosen the function 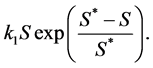
It is also known that M-CSF production increases as a result of susceptible cells being infected [15] [16] . Therefore, we will assume that the rate of increased production is proportional to the rate of infection,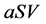 . Thus we will have the term
. Thus we will have the term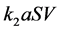 , where
, where 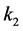 is the constant of proportionality. Finally, M-CSF has a natural decay rate which we will call
is the constant of proportionality. Finally, M-CSF has a natural decay rate which we will call 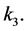 This results in the equation
This results in the equation

The infection of susceptible monocytes depends on the successful invasion rate 
is assumed constant as


It is assumed that the immune cells are produced at a constant rate 

current level of infection 



With these assumptions, we formulate the model for with-in host dengue viral infection with immune response and variable monocyte production rate, as the following.

We were able to find the values for normal susceptible counts and the normal M-CSF concentration, 







found in [10] , which we will discuss in detail in Section 4. Since we can express 


All model parameters are assumed to be positive.
3. Model Equilibria and Analysis
We will focus on the disease-free equilibrium




The Jacobian of the model is expressed below

Substituting the disease-free equilibrium into the Jacobian matrix results in

And the tedious calculation of 

which, conveniently, is a product of three linear polynomials and a quadratic. Finding the roots of these four polynomials gives us the expressions for the eigenvalues given below.

where
This allows us to formulate the following theorem:
Theorem 1. If 

Proof. Recall that all parameter values are positive. Upon inspection, we can clearly see that all 



which leads to the result. W
By substituting

cells are still present. We call this the “death” equilibrium since individuals do not function without monocytes. The resulting Jacobian in this case is:

Here the resulting characteristic polynomial is:

In this case also we can get expressions for eigenvalues, which are given below:

Since 

4. Parameter Values and Simulations
In this section we provide numerical simulations of different theoretical treatment techniques. We were able to find all parameters in the model in literature [9] [17] except the parameters 





where


The rest of the parameter values are given in Table 1. We should also mention that this parameter set and all of the modified ones that follow result in
4.1. Comparisons with Previous Models
As previously mentioned, this model displays dynamics of the monocyte population that are more in agreement with the data in [10] than previously studied models. Specifically, according to the data in [10] , monocytes levels are actually elevated above the normal count of 


We see that in both models with constant monocyte production the monocyte levels are never higher than the equilibrium. The new model with dynamic monocyte production does demonstrate this behavior and agrees quite well with the data in [10] .
4.2. Treatment Scenarios
There are several theoretical approaches to treating the disease. For example, the illness
Table 1. Parameter values.
Figure 1. Comparison of monocyte counts.
Figure 2. Comparison of monocyte counts.
might be less severe if the death rate of the infected cells, 


We see that while this type of treatment appears successful in reducing the viral and infected cell loads, it also prolongs the infection. Still, it seems like a promising approach. We can now compare this scenario with the previously mentioned increase in

It can be seen here that the model has nearly no sensitivity to the parameter 


Again the model reacts very little to adjusting this parameter suggesting that increasing the free viral death rate is not a useful strategy. Another logical approach is to reduce the number of new viruses produced by an infected monocyte,

One can see the most drastic reaction in this case. By reducing 
Figure 3. Infected cell and virus populations measured with varying 
Figure 4. Infected cell and virus populations measured using varying 
Figure 5. Infected cell and virus populations measured with varying 
Figure 6. Infected cell and virus populations measured using varying 
5. Conclusion
In this paper we have presented a new model for within-host dengue infection. The new approach does not assume that the monocyte production is constant throughout infection and includes a fifth equation that models the production of the primary stimulant for monocyte production, Macrophage Colony Stimulating Factor (M-CSF). By modeling the production of monocyte counts dynamically, our model has produced qualitative behavior not seen in previous models. Namely, that monocyte counts are elevated above the equilibrium during at least some period of infection. This behavior is in agreement with available data [10] . We were also able to find the net reproductive rate 


Acknowledgements
We thank the Editor and the referee for their comments. This work was partially funded by the Marquette Fellowship at Loyola University New Orleans.
Cite this paper
Thibodeaux, J.J. and Hennessey, M. (2016) A Within-Host Model of Dengue Infection with a Non- Constant Monocyte Production Rate. Applied Mathematics, 7, 2382-2393. http://dx.doi.org/10.4236/am.2016.718187
References
- 1. B?ck, A.T. and Lundkvist, ?. (2013) Dengue Viruses—An Overview. Infection Ecology and Epidemiology, 3, 19839.
https://doi.org/10.3402/iee.v3i0.19839� - 2. World Health Organization (2005) Tropical Disease Research, Making Health Research Work for Poor People, Progress 2003-2004, Geneva.
- 3. Noisakran, S., Chokephaibulkit, K., Songprakhon, P., et al. (2009) A Reevaluation of the Mechanisms Leading to Dengue Hemorrhagic Fever. Annals of the New York Academy of Sciences, 1171, E24-E35.
https://doi.org/10.1111/j.1749-6632.2009.05050.x - 4. Wang, W.K., Sung, T.L., Tsai, Y.C., Kao, C.L., Chang, S.M. and King, C.C. (2002) Detection of Dengue Virus Replication in Peripheral Blood Mononuclear Cells from Dengue Virus Type 2-Infected Patients by a Reverse Transcription-Real-Time PCR Assy. Journal of Clinical Microbiology, 40, 4472-4478.
https://doi.org/10.1128/JCM.40.12.4472-4478.2002 - 5. Ansari, H. and Hesaaraki, M. (2012) A With-In Host Dengue Infection Model with Immune Response and Beddington-DeAngelis Incidence Rate. Applied Mathematics (Irvine), 3, 177-184.
https://doi.org/10.4236/am.2012.32028 - 6. Nikin-Beers, R. and Ciupe, S.M. (2015) The Role of Antibody in Enhancing Dengue Virus Infection. Mathematical Biosciences, 263, 83-92.
https://doi.org/10.1016/j.mbs.2015.02.004 - 7. Ben-Shachar, R. and Koelle, K. (2015) Minimal Within-Host Dengue Models Highlight the Specific Roles of the Immune Response in Primary and Secondary Dengue Infections. Journal of the Royal Society Interface, 12.
- 8. Clapham, H.E., Tricou, V., Chau, N.V.V., Simmons, C.P. and Ferguson, N.M. (2014) Within-Host Viral Dynamics of Dengue Serotype 1 Infection. Journal of the Royal Society Interface, 11, 21040094.
https://doi.org/10.1098/rsif.2014.0094 - 9. Nuraini, N., Tasman, H., Soewono, E. and Sidarto, K.A. (2009) A With-In Host Dengue Infection Model with Immune Response. Mathematical and Computer Modelling, 49, 1148-1155.
https://doi.org/10.1016/j.mcm.2008.06.016 - 10. Klekamp, B.G. (2011) Assessing the Relationship of Monocytes with Primary and Secondary Dengue Infection among Hospitalized Dengue Patients in Malaysia, 2010: A Cross-Sectional Study. Graduate Theses and Dissertations, University of South Florida.
- 11. Ackleh, A.S., Deng, K., Ito, K. and Thibodeaux, J.J. (2006) A Structured Erythropoiesis Model with Nonlinear Cell Maturation Velocity and Hormone Decay Rate. Mathematical Biosciences, 204, 21-48.
https://doi.org/10.1016/j.mbs.2006.08.004 - 12. Banks, H.T., Cole, C.E., Schlosser, P.M. and Tran, H.T. (2004) Modeling and Optimal Regulation of Erythropoiesis Subject to Benzene Intoxication. Mathematical Biosciences and Engineering, 1, 15-48.
https://doi.org/10.3934/mbe.2004.1.15 - 13. Bélair, J., Mackey, M.C. and Mahaffy, J.M. (1995) Age-Structured and Two-Delay Models for Erythropoiesis. Mathematical Biosciences, 128, 317-346.
https://doi.org/10.1016/0025-5564(94)00078-E - 14. Yang, K., Salooja, N., Donahue, R.E., Hegde, U. and Linch, D.C. (1992) Human Macrophage Colony-Stimulating Factor Levels Are Elevated in Pregnancy and in Immune Thrombocytopenia. Blood, 80, 2897-2902.
- 15. Akashi, M. and Koeffler, H.P. (1992) Colony Stimulating Factors: Regulation of Production. Modern Trends in Human Leukemia IX, 83-92.
https://doi.org/10.1007/978-3-642-76829-3_16 - 16. Pang, T., Cardosa, M.J. and Guzman, G.M.G. (2007) Of Cascades and Perfect Storms: The Immunopathogenesis of Dengue Haemorrhagic Fever-Dengue Shock Syndrome (DHF/ DSS). Immunology & Cell Biology, 85, 43-45.
https://doi.org/10.1038/sj.icb.7100008 - 17. Roth, P., Dominguez, M.G. and Stanley, E.R. (1998) The Effects of Colony-Stimulating Factor-1 on the Distribution of Mononuclear Phagocytes in the Developing Osteopetrotic Mouse. Blood, 91, 3773-3783.










