Advances in Bioscience and Biotechnology
Vol.4 No.2(2013), Article ID:28417,8 pages DOI:10.4236/abb.2013.42030
Lignocellulolytic activities of a novel strain of Trichoderma harzianum
![]()
Enzyme Technology Laboratory, Biotechnology Division, Department of Botany, University of Calicut, Malappuram, India
Email: *sailasben@yahoo.co.in
Received 11 December 2012; revised 12 January 2013; accepted 20 January 2013
Keywords: Trichoderma harzianum; Morphology; Submerged Fermentation; Yellowish-Green Pigment
ABSTRACT
This study describes a novel dark-green spore producing strain of Trichoderma harzianum exhibiting higher activities of cellulase, hemicellulase and ligninase on specific plate assays. To assess the cellulase production in detail, basal salt medium (BSM) was supplemented with synthetic [carboxymethyl cellulose (CMC), glucose, sucrose, dextrose, lactose or maltose] and natural (flours of banana, banana peel, jack seed, potato or tapioca) carbon as well as nitrogen (yeast extract, beef extract, peptone, NaNO3 or NH4NO3) sources. Temperature and pH optima were 28˚C and 4, respectively for the growth of the fungus in CMCBSM with 146 U/ml cellulase activity. Flours of potato and banana supported comparable yields of cellulase to that of CMC (147 U/ml and 168 U/ml, respectively), while sodium nitrate was the preferred nitrogen source (150 U/ml). The water soluble yellowish-green pigment (a probable siderophore) extracted from the spores showed an absorption maximum at 414 nm. To comprise, this fungus shows the complete lignocellulolytic potential which offers great industrial significance, especially for the ethanol production from the lignocellulosic waste coupled with the production of a new pigment.
1. INTRODUCTION
Lignocellulosic materials are composed of cellulose, hemicellulose and lignin. Lignocarbohydrate degrading microbes produce various and multiple forms of cellulases, hemicellulases, pectinases and ligninases (lignases), which are increasingly used in the bioprocessing of plant materials, bio-fuel, feed, chemical feedstocks, silage and feed additives [1,2]. The ligninolytic system is an extracellular enzymatic complex that includes lignin peroxidase, manganese peroxidase and a copper containing phenoloxidase, known as laccase [3]. Cellulose and hemicelluloses are the principal sources of fermentable sugars in lignocellulosic feedstock; however, nature has designed woody tissue for effective resistance to microbial attack. Hemicelluloses, the second most common polysaccharides in nature, represent about 20% - 35% of lignocellulosic biomass. Like lignin, hemicellulose also constitutes a physical barrier which surrounds the cellulose fibers which can protect the cellulose from enzymatic attack. Hemicelluloses include xylan, glucuronoxylan, arabinoxylan, glucomannan and xyloglucan. Besides glucose, sugar monomers present in hemicelluloses include xylose, mannose, galactose, rhamnose and arabinose. Hemicellulose mostly contains D-pentose sugars and occasionally small amounts of L-sugars as well. Xylanases hydrolyse hemicellulose and they are of great interest to the paper and pulp industry, because of their bleach-boosting properties—which reduces environmentally unfriendly chlorine consumption [4,5]. Cellulolytic enzymes are the third most important industrial enzyme due to its versatile applications in various industries such as paper and pulp, textile and detergent industries. The resurgence in the utilization of biomass for the production of bio-ethanol and other value-added organic compounds has attracted major attention of researchers globally towards cellulases [6].
Majority of the reports on commercial production of cellulases utilize submerged fermentation (SmF), because of its ease of controlling the conditions. However, in nature, the growth and cellulose utilization of aerobic microorganisms harbouring cellulases probably resemble solid-state fermentation (SSF) than a liquid culture [7]. Ten-fold reduction in production cost has been indicated by SSF than SmF [8].
Trichoderma harzianum is a filamentous soil fungus known as an effective biocontrol agent for several plant pathogenic fungi [9]. T. harzianum secrets a well-balanced cellulolytic complex, which efficiently hydrolyzes cellulosic substrates into monomeric glucose. Due to its elevated cellulolytic activity, T. harzianum has considerable potentials in biomass hydrolysis applications [10]. However, cellulases from T. harzianum are less-studied, which have not yet been characterized biophysically and biochemically [11]. Both bacteria and fungi are known to produce cellulase using complex cellulosic substrates, however, fungal enzymes are generally complete comprising all the lignocellulosic activities [12]. Furthermore, the fungal enzymes are usually preferred because they are extra-cellular, adaptive and usually secreted in large quantities (up to 2% by weight), during growth in submerged batch fermenters. This is in sharp contrast to many bacterial enzymes which exist as tight multi-enzyme complexes, often membrane-bound, from which it is difficult to recover individual active enzyme species [13]. The appropriate physicochemical conditions play an important role in the enzyme production by microorganisms.
Considering these facts, the focus of the present work is to explore the lignocellulolytic potentials of a new strain of T. harzianum, coupled with the pigment produced by it.
2. MATERIALS AND METHODS
2.1. Microorganism
Pure fungal culture (T. harzianum) purchased from the Kerala Agriculture University, Mannuthi (Receipt no: 19 dated 17/12/2011) was used for the present study. Stock cultures were maintained on potato dextrose agar (PDA) slants at 4˚C. Analytical grade chemicals from Merck India Ltd. and Himedia were used.
2.2. Morphological Characterization
Well-grown T. harzianum culture from PDA slants was stained for studying its morphological characteristics. Lacto phenol cotton blue stain was used for the purpose. The slides were observed under the binocular microscope (Magnus MLX). The photographs were taken using Image Analyser (Nikon Eclipse E400, Towa optical, Japan) fitted with Nikon digital camera (DXM 1200F, Japan).
2.3. Screening of T. harzianum for Cellulase Activity
Basal Salt Medium (BSM) supplemented with 0.5% carboxy methyl cellulose (CMC) (NaNO3-2; KH2PO4-1, KCl-0.5, MgSO4·7H2O-0.5, protease peptone-2, Agar- 20 g/L) was used for screening [7]. Four days old T. harzianum on BSM-CMC agar medium in the Petri-plates was flooded with Gram’s Iodine solution and incubated for 15 min at 28˚C. Then, excess stain was drained off to observe the clear zone showing cellulase activity.
2.4. Screening of T. harzianum for Hemicellulase Activity
BSM supplemented with beech wood xylan (0.5%) was used for screening hemicellulase activity. 5 days old T. harzianum from PDA slants was sub-cultured on BSMxylan agar medium and incubated for 4 days at 28˚C. Then the plate was flooded with 10 ml Congo-red (0.1%) solution, incubated for 15 min, then excess stain drained off and 10 ml 1 M NaCl was added for destaining.
2.5. Screening of T. harzianum for Lignolytic Activity
5 days old T. harzianum from PDA slants was sub-cultured on BSM-lignin agar medium (0.5% lignin) and incubated at 28˚C. T. harzianum culture was grown for 5 - 6 days on BSM-lignin medium for checking lignase activity.
2.6. Cellulase Production by SmF of T. harzianum
5 days old T. harzianum on PDA medium was used for preparing the spore suspension. Culture in sterile ddH2O was stirred well on a magnetic stirrer for making a uniform spore suspension. The number of spores in 1 ml of suspension was counted by Neubauer haemocytometer. To investigate the maximum cellulase production in liquid medium by T. harzianum, various parameters like effects of incubation period, pH, temperature, CMC concentration, synthetic carbon sources, natural carbon sources and nitrogen sources were optimized.
BSM supplemented with CMC (0.5%) was used for initial studies. 10 µL of spore suspension (~2.6 × 108 spores/ml) was used for 1 ml medium throughout the studies. All flasks were incubated in an environmental shaker (Scigenics Biotech, India) at 150 rpm, 28˚C for 8 days. The culture broth was centrifuged at 9000 × g for 15 min at 4˚C and the supernatant was used for enzyme assay. Different parameters such as pH (1, 2, 3, 4, 5, 6, 7 and 8), temperature (25˚C, 28˚C, 31˚C, 33˚C, 35˚C and 37˚C), CMC concentrations (0.75%, 1.0%, 1.25% and 1.5%), various synthetic carbon sources (0.5% glucose, lactose, maltose, dextrose and sucrose), natural carbon sources (0.5% banana flour, banana peel flour, tapioca flour, potato flour and jack seed flour) and nitrogen sources (0.2% peptone, beef extract, yeast extract, sodium nitrate and ammonium nitrate) were studied for optimizing the maximum production of cellulase.
2.7. Cellulase Assay
Cellulase was assayed by employing the 3,5-dinitro salicylic acid (DNS) method of Miller [14]. One unit of enzyme activity is defined as the amount of protein (cellulase) required to liberate 1 µmol of reducing sugar (Dglucose) from CMC per min under the assay conditions. After fermentation culture broth was centrifuged at 9000 × g for 10 min at 4˚C and supernatant was used for enzyme assays. Reaction mixture contains 0.5 ml of 1% CMC in 0.1 M sodium citrate buffer (pH, 4.8) and 0.5 ml of enzyme solution was added, incubated at 50˚C for 30 min. 3 ml DNS was added for stopping the reaction and incubated in a boiling water bath for 5 min and cooled. The absorbance was measured at 540 nm (Elico double beam BL 200 Bio-spectrophotometer).
Cellulase activity was calculated using the formula, ΔE × Vf /Δt×∑ × Vs × d. Where, ΔE = absorbance at 540 nm, Vf = final volume of reaction mixture including DNS, Vs = crude supernatant (ml) containing cellulase used, Δt = incubation time for of hydrolysis, ∑ = extinction coefficient of glucose (0.0026), d = diameter of cuvette.
2.8. Pigment Extraction
Pigment (from spores) of T. harzianum was extracted from 5 days old fungus on PDA agar plates using different solvents like acetone, chloroform, hexane, methanol, ethanol or distilled water. The mixture was stirred well on a magnetic stirrer and followed by the centrifugation for 15 min at 9000 × g for 4˚C. The supernatant was separated and the absorption spectrum of extracted pigment of T. harzianum in each solvent was monitored using spectrophotometer.
2.9. Statistics
All studies were conducted at least three times to check the reproducibility of the results, and average values were given with standard deviation. Microsoft Excel was used to draw the figures.
3. RESULTS AND DISCUSSION
T. hazianum strain procured from the Kerala Agricultural University, Mannuthi was used for this study. Many mycelial fungi are known to have high potentials for cellulase production. We wanted to explore whether T. harzianum embodies the potential for lignocellulolytic activities for which we adopted the plate assay first. The results were promising. It showed a tri-phasic lignocellulytic activity, i.e., lignolytic (on lignin), hemicellulolytic (xylan) and cellulolytic (CMC) activities on respective plate assays. Further, we wanted to explore the cellulase production profiles in various media combinations with different carbon sources including CMC and natural substrates such as flours of potato, tapioca, jack seed and banana. Various culture parameters like pH, temperature, effects of CMC concentration and of nitrogen source were also studied for the cellulase production. All the results obtained are detailed below.
3.1. Morphological Characterization of T. harzianum
T. harzianum grew profusely by producing branching mycelia on PDA plate, within 24 h of inoculation with spores. The fungus at its mycelial stage was whitish, then upon sporulation, the color changed to greenish. Well developed fruiting bodies with spores appeared after 4 days of incubation at 28˚C. Lucid morphology of mycelium of the fungus with conidiophores, phialides and sporogonium were observed using Image Analyzer after staining with Lactophenol Cotton Blue (Figures 1(A) and (B)). The images of spores were also observed using phase contrast microscope (Figure 1(C)). The mycelia profusely branched with clustered fruiting bodies (phialides), however the phialides were not prominent as in T. viride [7]. Interestingly, though not frequent, the spherical sporogonia were seen attached on the mycelia along with frequent phialides. The greenish spores were spherical, smooth and biconcave—resembling human red blood corpuscles. From these morphological characteristics, the strain used in this study was confirmed of T. harzianum.
3.2. Screening for Lignocellulolytic Activity
Plate assays for T. harzianum comprises the degradation of lignocelluloses with the production of lignocellulolytic enzymes. Plate assay with Gram’s iodine showed a clear zone around the fungal growth showing cellulase activity (Figure 2(a)). T. harzianum started growing on xylan medium within 24 h of inoculation with spores at the central portion of the plate. Xylan degradation around the colonies appeared as yellow and opaque zone against a reddish purple colouration around the colonies for intact xylan towards the periphery on the plate (Figure 2(b)). T. harzianum started to grow well on lignin medium after 24 h of incubation. Maximum growth occurred after 5 - 6 days, which clearly shows its lignolytic activity (Figure 2(c)). Cellulolytic enzymes produced by fungi can be easily screened by staining techniques or by measuring the amount of reducing sugar (glucose) liberated with the dinitrosalicyclic acid reagent method [15]. Thus, we employed DNS method for the quantitative estimation of the cellulase (β-glucosidase) in liquid media.
3.3. Cellulase Production by SmF
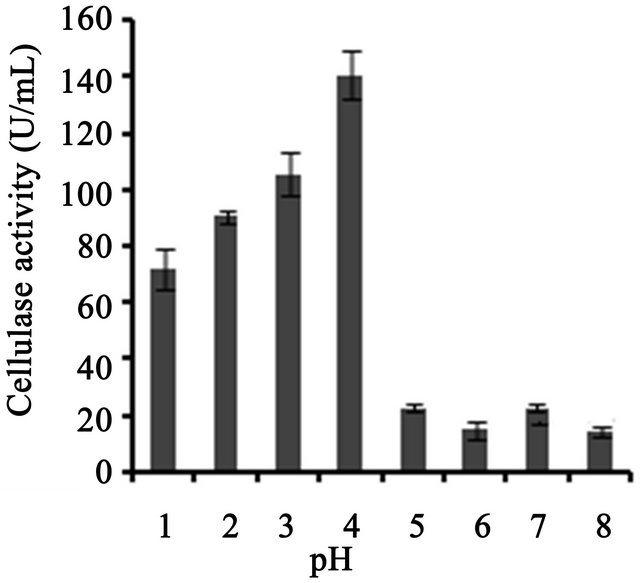
Cellulase production profile at differing parameters in BSM supplemented with various carbon and nitrogen source showed in Figure 3. T. harzianum was able to produce cellulase at an optimum pH of 4, which was about 140 U/ml, slightly higher than that obtained at pH
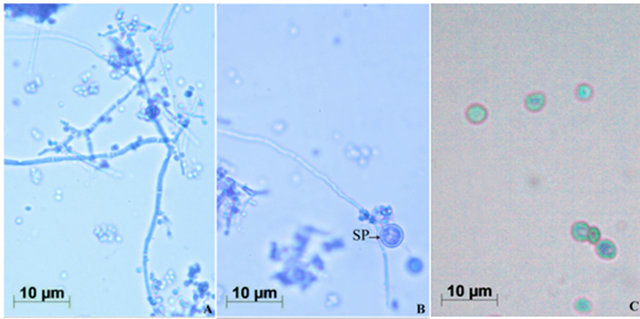
Figure 1. Morphology of T. harzianum. (A) Mycelia bearing conidiophores and conidiospores, after staining with lactophenol cotton blue (Images by Image Analyzer, Scale bar = 10 µM (Nikon Eclipse E400, Towa Optical, Japan, fitted with Nikon digital camera, DXM 1200F, Japan); (B) Mycelia showing globular sporogonium (SP); and (C) Conidiospores under Phase-Contrast Microscope (Scale bar = 10 µM, Leica M80, Germany).
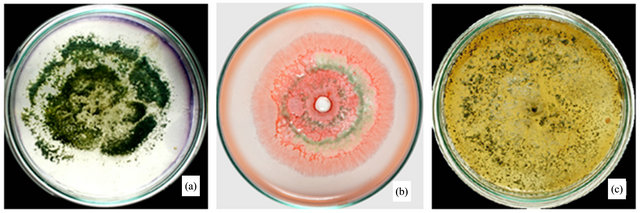
Figure 2. Lignocellulolytic activities of T. harzianum. (a) Iodine plate assay showing cellulase activity on 4th day of incubation at 28˚C; (b) Congo-red plate assay showing xylanase activity on 4th day of incubation at 28˚C; and (c) Growth of T. harzianum on lignin agar medium on 6th day of incubation at 28˚C.
3 (Figure 3(a)). Temperature on microbial growth and enzyme production appeared to have great influence. The maximum cellulase production (146 U/ml) was observed at 28˚C and pH 4. As the temperature increased, cellulase activity decreased progressively to 117.8 U/ml at 31˚C; 88.8 U/ml at 33˚C to 13.8 U/ml at 35˚C (Figure 3(b)). This may be due to the fact that higher temperature reduces the metabolic activity of the fungus or denatures the enzymes. It was noted that cellulase production was increased upon increasing the concentration of CMC up to an optimum level (1.25%) (Figure 3(c)). When CMC in the production medium was replaced with different carbon sources—natural and synthetic (0.5%)—maximum cellulase activity was obtained with banana flour (168 U/ml) followed by potato and tapioca flours (Figure 3(d)). However, lower activity was found with jack seed flour and banana peel (63 and 42 U/ml, respectively). Maximum cellulase activity observed with dextrose was about 76.7 U/ml, at 28˚C and pH 4, followed by maltose or lactose in the medium (Figure 3(e)).With different nitrogen sources (0.2%), maximum activity was observed for the medium containing sodium nitrate (150 U/ml) (Figure 3(f)). The least cellulase activity (6.9 U/ml) was observed with beef extract.
Optimization of the time course is of prime importance for cellulases biosynthesis by fungi [16].
CMC is a selective carbon source for the enrichment culture of cellulase producing microorganisms. The cellulase activity of the Trichoderma sp was about 167 U/ml with the substrate CMC [17]. Incubation period on cel-
 (a)
(a)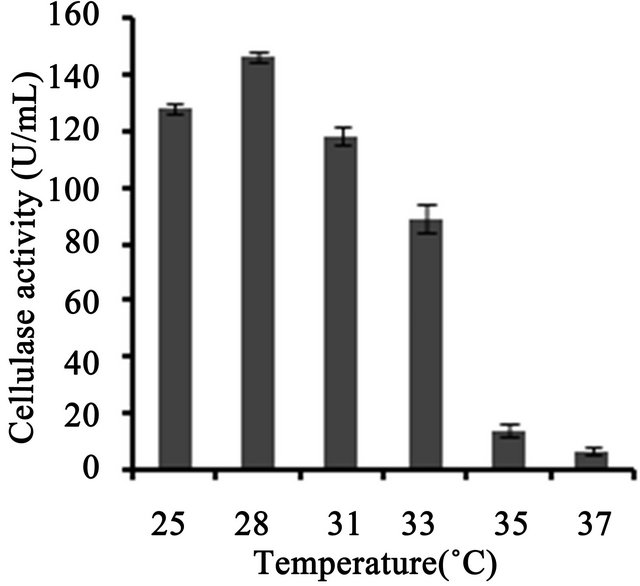 (b)
(b)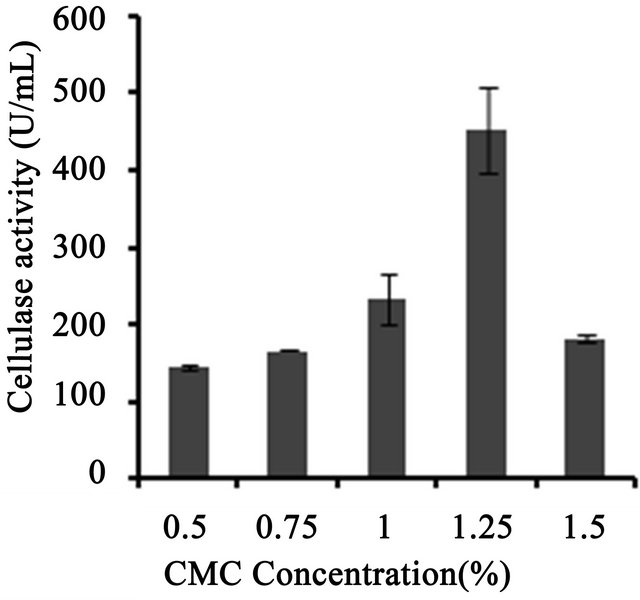 (c)
(c)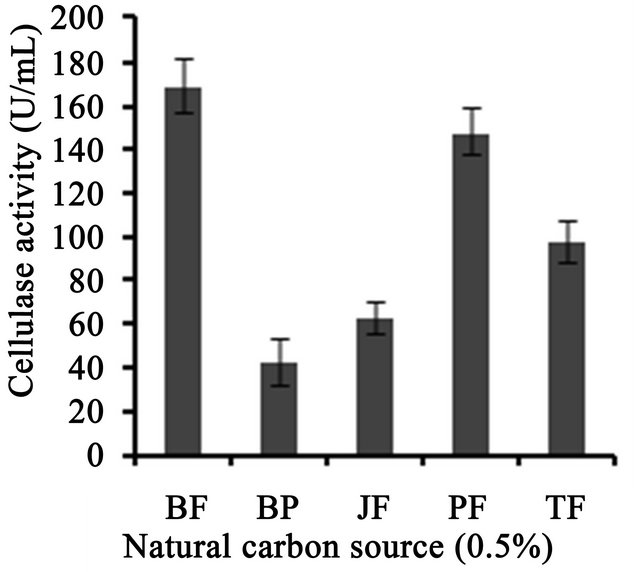 (d)
(d)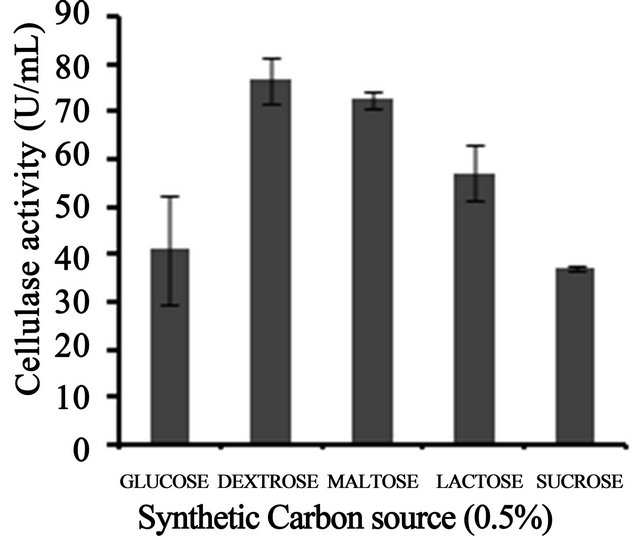 (e)
(e)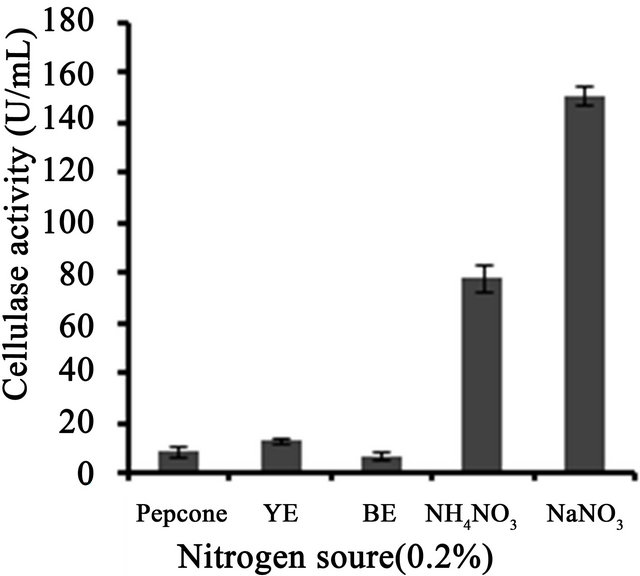 (f)
(f)
Figure 3. Optimization of parameters for cellulase production. (a) Effect of pH on cellulase production from T. harzianum on BSM supplemented with 0.5% CMC on 6th day of incubation at 150 rpm and 28˚C; (b) Effect of temperature on cellulase production from T. harzianum on BSM supplemented with 0.5% CMC on 6th day of incubation at 150 rpm and 28˚C; (c) Effect of CMC on cellulase production from T. harzianum on BSM supplemented with CMC (0.5%, 0.75%, 1.00%, 1.25%, 1.5%) on 6th day of incubation at 150 rpm and 28˚C; (d) Cellulase production of T. harzianum on BSM supplemented with different natural carbon sources (BF-banana flour, BP-banana peel flour, JF-jack seed flour, PF-potato flour, TF-tapioca flour (0.5%) on 6th day of incubation, 150 rpm, pH 4 and 28˚C; (e) Cellulase production profile of T. harzianum on BSM supplemented with different synthetic carbon sources (0.5%) on 6th day of incubation, pH 4, 150 rpm and 28˚C; and (f) Cellulase production of T. harzianum on BSM supplemented with different nitrogen sources (peptone, YE-Yeast extract, BF-Beef extract, NH4NO3, NaNO3 (0.5%) on 6th day of incubation, 150 rpm, pH 4 and 28˚C.
lulase production was found to be a major factor, and maximum cellulolytic activity was obtained on sixth day of incubation (82.8 U/ml). T. viride showed decrease in cellulase activity after 72 h growth, which might be due to the depletion of the nutrients and accumulation of other byproducts like proteases in the fermentation medium [7]. However, Mekala et al. [18] showed maximum cellulase yield (25.6 U per gram dry substrate, gds) at an incubation period of 67 h from T. ressei strain RUT C30, which further showed that crude inducer was found to be very effective for enhancing cellulose production and reducing the incubation period. We showed that beyond the pH value of 4, the production of cellulases decreased which might be due to the fact that cellulases are acidic proteins and are greatly affected by the neutral pH values [19]. Cellulase activity of T. harzianum was observed to be increased at acidic pH 4 up to 140 U/ml and then decreased at higher pH values. This behavior was in accordance with the activity of T. harzianum strain Ce 17A which showed maximum exoglucanase production at a pH range of 3 - 5 and then decreased above pH 5 [11]. Mekala et al. [18] showed that cellulase production was maximum at 33˚C incubation and decreased with high temperature. High temperature may also lead to inhibition of microbial growth.
We also investigated the effects of different substrates such as banana flour, tapioca flour, banana peel and jack seed flour on cellulase production. Maximum cellulase activity was obtained with banana flour (168 U/ml), followed by potato and tapioca flours. Singhania et al. [6] studied the efficacy of natural substrates like wheat bran, corn comb residue, orange peel, rice straw, sugarcane bagasse, etc. on cellulase production, and maximum cellulase production (154.58 U/gds)—was obtained with pre-treated sugar cane bagasse which was similar to our result, irrespective of the unit difference (U/ml in our study, which in calculation would be much more than U/gds). Similarly, the effects of fermentation conditions, such as moisture content, pH, temperature and aeration on cellulase production by T. harzianum using a mixture of wheat straw (80%) and bran (20%) were investigated, which showed the maximium activity of about 18 IU filter paper activity (FPU) and 198 IU endoglucanase activity/gds [20]. The influences of different carbohydrates: glucose, xylose, CMC, microcrystalline cellulose (avicel) and cellobiose were evaluated as carbon sources for the production of cellulase from the white rot fungus, Phlebia gigantea that colonizes conifer wood; which showed maximum activity of 112 IU/ml and 2.8 IU/ml in the presence of CMC and avicel [21]. Castro et al. [10] reported the ability of T. harzianum strain IOC-4038 to produce cellulases on a novel material (xylan free and cellulose rich) generated from sugarcane bagasse, named partially delignified cellulignin. The extract produced by T. harzianum under submerged conditions reached 0.745, 0.097 and 0.559 U/ml β-glucosidase, FPU and endoglucanase activities, respectively. Cellulase production was carried out by SSF using corncob residue—a lingo-cellulosic waste from the xylose industry—as the substrate for T. reesei strain ZU-02 which showed highest cellulase activity of about 158 IFPU/g [22]. T. reesei was co-cultured with A. phoenicis using dairy manure as a substrate to produce cellulase which showed 0.64 IU/ml β-glucosidase activity and 1.54 FPU/ml [23]. Studies on production of cellulases and hemicellulases by P. echinulatum, grown on pretreated sugar cane bagasse and wheat bran by SSF established the potentials of P. echinulatum for endoglucanase [32.89 ± 1.90 U/gdm (grams dry medium)], beta-glucosidase (58.95 ± 2.58 U/gdm) and xylanase (10 U/gdm) production by SSF [24]. In comparison, our study we showed that strain of T. harzianum we described in this study showed higher cellulase activity (168 U/ml) in BSM supplemented with 0.5% banana flour at 28˚C and pH 4, which sounds industrially significance of this novel strain.
3.4. Pigment Extraction
The present work also concentrated on the extraction of pigment from T. harzianum strain using different solvents such as acetone, chloroform, ethanol, hexane, methanol or distilled water. The pigment was soluble in methanol with pale yellowish-green coloration (Figure 4(a)), which gave an absorption spectrum showing maximum peak at 414 nm (Figure 4(b)).
In a study by Kiss et al. [25], the pigment produced by T. harzianum in the fermentation broth was separated and the main fractions were tentatively identified by reverse-phase thin-layer chromatography—fourier transforminfrared spectroscopy (RP-TLC-FT-IR), RP-HPLC-diode array detection and RP-HPLC-MS. They showed that the pigments of T. harzianum show an increasing absorbance towards the UV, unlike the maximum we described in this study. Sharma et al. [26] studied the pigment from three fungi, viz., T. virens, Alternaria alternata and Curvularia lunata for textile dyeing. It was an attempt to isolate and optimize the fermentation conditions of these fungi. T. viride is shown to produce variously coloured pigments, which range from bluish-green [7], green [27], yellow [28] to brown [29]. Very recently, Xiaoyil et al. [28] showed optimization of fermentation conditions in liquid state for a T. viride strain to produce yellow pigment, the viridin. From this, we conclude that the pigment produced by the strain is new, which needs further studies.
4. CONCLUSION
Fungal cellulase is one of the hot enzymes, owing to its high demand in biodiesel industry. Statistical tools like RSM (Response surface methodology) and ANNs (Artificial neural networks) can be employed for the better production and characterization of enzyme in future studies. The results reported in this work indicate that T. harzianum can successfully be cultivated under submerged fermentation for the production of lignocellu-
 (a)
(a) (b)
(b)
Figure 4. Characterization of pigment. (a) Pigment extracted from spores of T. harzianum in methanol (9000 × g, 15 min); and (b) Absorption spectrum of pigment of T. harzianum extracted as above (by ELICO BL 200 Double Beam Bio-spectrophotometer).
lolytic enzymes. Its potential under SSF yet to be explored. The optimum culture conditions obtained through this study could be employed for maximizing the cellulase production. Thus, this study gives a detailed account of different parameters which affecting the cellulase production and optimum cultural conditions for maximum enzyme yield. The present study gives clear evidences for the complete lignolytic and hemicellulase activities of this new strain of T. harzianum. The presence of a pigment produced on sporulation also enhances the significance of this new strain.
5. ACKNOWLEDGEMENTS
The authors are thankful to the Kerala State Council for Science, Technology and Environment for a research grant, No. (T) 422/SRS/ 2009/CSTE.
REFERENCES
- Behrendt, C.J., Blanchette, R.A., Akhtar, M., Enebak, S., Iverson, S. and Williams, D. (2000) Biomechanical pulping with Phlebiopsis gigantea reduced energy consumption and increased paper strength. Tappi Journal, 83, 65.
- Blanchette, R.A., Behrendt, C.D., Williams, D., Iverson, S., Akhtar, M. and Enebak, S.A. (1998) A new approach to effective biopulping: Treating logs with Phlebiopsis gigantean. 7th International Conference on Biotechnology in the Pulp and Paper Industry, A51-A54.
- Ruiz-Duenas, F.J. and Martínez, A.T. (2009) Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microbial Biotechnology, 2, 164-177. doi:10.1111/j.1751-7915.2008.00078.x
- Balakrishnan, H., Choudhury, M.D., Srinivasan, M.C. and Rele, M.V. (1992) Cellulase-free xylanase production from an alkalophilic Bacillus species. World Journal of Microbioliology Biotechnology, 8, 627-631. doi:10.1007/BF01238802
- Viikari, L., Kantelinen, A., Sundquist, J. and Linko, M. (1994) Xylanases in bleaching: from an idea to the industry. FEMS Microbiology Reviews, 13, 335-350. doi:10.1111/j.1574-6976.1994.tb00053.x
- Singhania, R.R., Sukumaran, R.K. and Pillai, A. (2006) Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian journal of Biotechnology, 5, 332-336.
- Neethu, K., Rubeena, M., Sajith, S., Sreedevi, S., Priji, P., Unni, K.N., Sarath Josh, M.K., Jisha, V.N., Pradeep, S. and Benjamin, S. (2012) Advances in Bioscience and Biotechnology, 3, 1160-1166. doi:10.4236/abb.2012.38142
- Tengerdy, R.P. (1996) Cellulase production by solid substrate fermentation. Indian Journal of Scientific Research, 55, 313-316.
- Viterbo, A., Haran, S., Friesem, D., Ramot, O. and Chet, I. (2001) Antifungal activity of a novel endochitinase gene (chit36) from Trichoderma harzianum Rifai TM. FEMS Microbiology Letters, 200, 169-174. doi:10.1111/j.1574-6968.2001.tb10710.x
- Castro, A.M., Pedro, K.C., Cruz, J.C., Ferreira, M.C., Leite, S.G. and Pereira, N. (2010) Trichoderma harzianum IOC-4038: A promising strain for the production of a cellulolytic complex with significant β-glucosidase activity from sugarcane bagasse cellulignin. Applied Biochemistry and Biotechnology, 162, 2111-2122. doi:10.1007/s12010-010-8986-0
- Colussi, F., Garcia, W., Rosseto, F.R., Mello, B.L.S., Net, M.O. and Polikarpov, I. (2011) Effect of pH and temperature on the global compactness, structure, and activity of cellobiohydrolase Cel7A from Trichoderma harzianum. Journal of European Biophysics, 40, 89-98.
- Stockton, B.C., Mitchell, D.J. and Grohmann K. (1991) Optimum β-D glucosidase supplementation of cellulose for efficient conversion of cellulose to glucose. Biotechnology Letters, 13, 57-62. doi:10.1007/BF01033518
- Berry, D.R. and Paterson, A. (1990) Enzymes in food industry. In: Suckling, C.J., Ed., Enzyme Chemistry, Impact and Applications, 306-351.
- Miller, G.L. (1959) Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426-428. doi:10.1021/ac60147a030
- Sazci, A., Erenler, K. and Radford, A. (1986) Detection of cellulolytic fungi by using Congo red as an indicator: A comparative study with the dinitrosalicyclic acid reagent method. Journal of Applied Microbiology, 61, 559- 562. doi:10.1111/j.1365-2672.1986.tb01729.x
- Kuhad, R.C., Singh, A. and Eriksson, K.E.L. (1997) Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Advances in Biochemical Engineering/Biotechnology, 57, 45-125. doi:10.1007/BFb0102072
- Gashe, B.A. (1992) Cellulase production and activity by Trichoderma sp. A-001. Journal of Applied Microbiology, 73, 79-82. doi:10.1111/j.1365-2672.1992.tb04973.x
- Mekala, N.K., Singhania, R.R., Sukumaran, R.K. and Pandey (2008) Cellulose production under solid-state fermentation by Trichoderma ressei RUT C30: Statistical optimization of process parameters. Applied Biochemistry and Biotechnology, 151, 122-131. doi:10.1007/s12010-008-8156-9
- Juhasz, T.Z., Szengyel, N., Szijarto and Reczey, K. (2004) Effect of pH on cellulases production of Trichoderma reesei RUT C30. Applied Biochemistry and Biotechnology, 201, 113-116.
- Deschamps, F., Giuliano, C., Asther, M., Huet, M.C and Roussost, S. (1985) Cellulase Production by Trichoderma harzianum in static and mixed solid-state fermentation reactors under non aseptic condition. Biotechnology and Bioengineering, 27, 1385-1388. doi:10.1002/bit.260270917
- Niranjane, A.P., Madhou, P. and Stevenson, T.W. (2006) The effect of carbohydrate carbon sources on the production of cellulase by Phlebia gigantean. Enzyme and Microbial Technology, 40, 1464-1468. doi:10.1016/j.enzmictec.2006.10.041
- Xia, L. and Cen, P. (1999) Cellulase production by solid state fermentation on lignocellulosic waste from the xylose industry. Process Biochemistry, 34, 909-912. doi:10.1016/S0032-9592(99)00015-1
- Wen, Z., Liao, W. and Chen, S. (2005) Production of cellulase/β-glucosidase by the mixed fungi culture Trichoderma reesei and Aspergillus phoenicis on dairy manure. Process Biochemistry, 40, 3087-3094. doi:10.1016/j.procbio.2005.03.044
- Camassola, M. and Dillon, A.J.P. (2007) Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. Journal of Applied Microbiology, 103, 2196-2204. doi:10.1111/j.1365-2672.2007.03458.x
- Kiss, G.C., Forgacs, E., Cserhati, T. and Vizcaino, J.A. (2000) Colour pigments of T. harzianum preliminary investigations with thin-layer chromatography—Fourier transform infrared spectroscopy and high-performance liquid chromatography with diode array and mass spectrometric detection. Journal of Chromatography A, 896, 61-68.
- Sharma, D., Gupta, C., Aggarwal, S. and Nagpal, N. (2012) Pigment extraction from fungus for textile dyeing. Indian Journal of Fibre and Textile Research, 37, 68-73.
- Betina, V. (1995) Photoinduced conidiation in Trichoderma viride. Folia Microbiologica, 40, 219-224. doi:10.1007/BF02814196
- Xiaoyi1, Z., Yingde, C.U., Ning, L.U., Xin, Y.U. and Meimei, Z. (2012) Optimization of fermentation condition in liquid culture of T. viride to produce yellow pigment. Center for Improved Engineering and Science Education, 61, 3205-3212.
- Chitale, A., Jadhav, D.V., Waghmare, S.R., Sahoo, A.K. and Ranveer, R.C. (2012) Production and characterization of brown coloured pigment from Trichoderma viride. Electronic Journal of Environmental Agricultural and Food Chemistry, 11, 529-537.
NOTES
*Corresponding author.

