Advances in Bioscience and Biotechnology
Vol. 4 No. 1 (2013) , Article ID: 26958 , 7 pages DOI:10.4236/abb.2013.41008
Antioxidant activity of Bios-p peptide analogue in HEK293T cells and three-dimensional structure prediction
![]()
1Center of Neurosciences and Peptides Biology, Universidad de La Frontera, Temuco, Chile
2Departamento de Ciencias Biologicas, Facultad de Ciencias Biologicas, Universidad Andres Bello, Talcahuano, Chile
3Laboratorio de Neurobiología Molecular y Celular II, Departamento de Neurobiología Molecular y Celular, Instituto de Neurobiología, Campus Juriquilla-Querétaro, Universidad Nacional Autónoma de México, México D.F., México
4Centro de Modelación y Computación Científica, Universidad de La Frontera, Temuco, Chile
Email: magdalenacuevas@ufro.cl
Received 25 October 2012; revised 27 November 2012; accepted 17 January 2013
Keywords: Antioxidant; Bios-p; Bauhinia bauhinoides
ABSTRACT
Studies had indicate that excessive production of reactive oxygen species (ROS) affect cellular signaling pathways, which is associated with pathological and physiological conditions such as cancer, diabetes and neurodegenerative diseases In this context, our laboratory has obtained the Bios-p, a ROS modulator, peptide analogue by sequencing from the seed of Bauhinia bauhinoides, which represents the active 12- amino acid, obtained from the inhibitor BbKI protease and we predicted the three-dimensional structure of Bios-p analogue peptide using homology modeling, being patented by the working group of Dr. Maria Luiza Vilela Oliva of UNIFESP, Brazil (a member of our cluster). The protective effect on the viability and antioxidant capacity of Bios-p was studied in HEK 293T cells under oxidative stress induced by hydrogen peroxide (H2O2) using SYTOXGREEN/DHE and luminescence assay. The three-dimensional structure of Bios-p peptide analogue was predicted by homology-based modeling using Modeller9v8. The pretreatment with different concentrations of Bios-p (1 μM - 10 μM) showed an increase of 53.83% ± 3.86% the cellular viability in under oxidative stress compared to control. Furthermore, the results to indicate that HEK293T cells by incubating for 24 h with Bios-p shown a significant decreased of basal extracellular ROS on total cell population in 89.67% ± 0.76%, compared to control in the absence of the analogue. Similarly it is observed that Bios-p has a significant antioxidant effect on extracellular ROS production when cells are subjected to oxidative stress induced by 200 μM H2O2 in 64.37% ± 4.63%, compared to control in absence of H2O2 and Bios-p. These results suggest that Bios-p has potential as antioxidant agent in cells HEK293T under H2O2-induced oxidative stress and that can protect the cells viability as concentration-dependent, and we propose a new biotechnological tool for modulate the ROS production.
1. INTRODUCTION
The excessive production of reactive oxygen species (ROS) affect cellular signaling pathways, which is associated with pathological and physiological conditions such as cancer, diabetes and neurodegenerative diseases [1] . Cellular defense mechanisms against oxidative damage include enzymatic conversion of ROS (e.g., 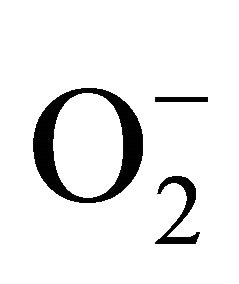 and OH−) into less reactive species, chelation of transition metal catalysts and detoxification of ROS by antioxidants [2] . Thus, application of antioxidants might be an effective therapeutic strategy to cure neurodegenerative disorders initiated by ROS. Because of this, there is growing interest in the scientific community and industry to obtain naturally occurring substances aimed at the prevention and treatment of these pathologies.
and OH−) into less reactive species, chelation of transition metal catalysts and detoxification of ROS by antioxidants [2] . Thus, application of antioxidants might be an effective therapeutic strategy to cure neurodegenerative disorders initiated by ROS. Because of this, there is growing interest in the scientific community and industry to obtain naturally occurring substances aimed at the prevention and treatment of these pathologies.
It has also reported a number of possible bioactivities that might have, including antimicrobial capacity, hypertensive, antioxidant and anticancer [3]. Accordingly, biotechnological products are among various naturally occurring substances that are receiving growing attention from the viewpoint of antioxidation. The antioxidant peptides can be isolated from different sources such as marine sources [4] and plants [5]. Some of them have been accepted to be one of the important candidates for the development of effective and non-toxic medicines with antioxidant actions. The presence of His (H) within the peptide sequence is characteristic of antioxidant peptides, together with the presence at the N-terminal residues Leu (L) or Pro (P) [6]. According to the findings by Chan and Decker (1994) [7], the structure-activity relationship of antioxidants in their own sequence His, is attributed to the ability of hydrogen donation and/or metal chelation capacity of the imidazole group.
The plants, the subject of a growing number of natural product researches, are now considered as efficient producers of biologically active and/or chemically novel compounds. One source plants used for extraction of bioactive polypeptides is the genus Bauhinia, which has more than 600 species of wide distribution in tropical and subtropical forests [8]. Many proteins have been isolated from its seeds, and in particular bauhinioides species [9] . Thus, functional and biologically active peptides and derivatives obtained from seeds, as well as being a contribution to their nutritional value, have the ability to exert physiological functions that native protein sequenced from a large active, can be obtained by scalable production processes industrial, or by synthesis, by fermentation or by methods of recombinant protein. In this context, our laboratory has utilized the Bios-p peptide analogue, which represents the active 12-amino acid active site, obtained by sequencing from the inhibitor BbKI protease present in the seed of Bauhinia bauhinoides and patented by the working group of Dr. Maria Luiza Vilela Oliva of UNIFESP, Brazil. In this study we investigated the protector effect and antioxidant capacity of Bios-p peptide in the cellular model HEK293T when these were subjected to oxidative stress induced by hydrogen peroxide (H2O2). On the other hand, it is known that the function of the bioactive peptide is dependent on its amino acid sequence and three dimensional structures that these possess [10] and binding with cell membrane components. Because of this, we have obtained a threedimensional structure of Bios-p using modeling basedhomology.
2. MATHERIALS AND METHODS
2.1. Cellular Lines and Culture Conditions
HEK293 cells were cultured in D-MEM medium supplemented with 10% FBS (Hyclone) and penicilin-streptomicin 1% (Invitrogen). Cell growth was done at 5% CO2 and 37˚C changing the culture medium every three days.
2.2. Incubation of HEK293T Cells with Bios-p Peptide Analogue
The cells were grown during 24 h in six-well plates and allowed to reach to 50% confluence. Then, were incubated with Bios-p analogue peptide at different concentrations of 0,1; 1 and 10 µg/ml during 24 h at 37˚C. Additionally cells were treated with H2O2 200 µM for 15 min at 37˚C to induce the oxidative stress.
2.3. Cells-Viability Assessment
The cells were incubated with the probe SYTOX Green 0.5 µM (Molecular Probes, Eugene, OR) at 37˚C for 15 min in darkness. The cells were analyzed in a confocal microscope at 510 nm emission of SYTOX Green. The percentage of viable cells was calculated by manually counting the number in the x-y reference frame visualized in ten different fields.
2.4. Production of ROS Extracellular
The production of ROS extracellular were measured by luminescence assay by incubating the cells with luminol 200 µM (5-amino-2, 3 hydro-1, 4-ftalazinediona, Sigma Chemical Co., St. Louis, MO) for 15 min at 37˚C in darkness and immediately quantifying the luminescence in a luminometer Luminoskan mark (Thermos Scientifics, China), expressing the results as relative luminescence units (RLU). In each analysis, a negative control without the addition of luminol and positive control cells treated with H2O2 200 µM, were added.
2.5. Anion Superoxide Intracellular Production
The cells were incubated with the probes DHE/SYTOX Green (2 µM and 0.5 µM respectively) (Molecular probes, Eugene, OR) at 37˚C for 15 min in darkness. The results obtained with this probe have been validated as a measure of the ability of cells to generate ROS, specifically definitive identification of the superoxide anion. The cells were analyzed in a confocal microscope at 510 and 670 nm emissions of SYTOX Green and DHE, respectively [11]. The percentage of viable cells producing superoxide anion was calculated by manually counting the number in the x-y reference frame, and dividing by the total number of cells visualized in ten different fields.
2.6. Homology Modeling
The three-dimensional structure of Bios-p peptide analogue was predicted by homology-based modeling using Modeller9v8 [12]. BLAST-P was used to identify the potential template structures for molecular modeling. The templates are in Protein Data Bank (PDB) WEB page, with PDB IDs 2GO2/2GZB. The protein models were validated using prochek [13] and Anolea [14].
2.7. Statistical Analysis
The data for the different functional parameters evaluated were expressed as mean + SEM. The data were analyzed with GraphPad Prism version 5.02 for Windows (GraphPad Software, San Diego, CA, USA). The differences between the groups were analyzed using the one way analysis of variance (ANOVA) followed by Tukey multiple comparison tests. P values < 0.05 were considered as significant.
3. RESULTS AND DISCUSSION
3.1. Bios-p Protects the Cells-Viability
Was evaluated the protective effect of Bios-p peptide analog against H2O2-induced cytotoxicity in the HEK 293T cellular model (Figure 1). When added to cell culture H2O2 200 µM without pre-treatment with Bios-p, was observed a decreased of cell viability product of H2O2-cytoxicity. However, the pre-treatment with different concentrations of Bios-p (1 - 10 μM) showed a increase of 53.83% ± 3.86% the cellular viability in under oxidative stress compared to control. As shown in the Figure 1(a), the protective effect on the viability of Bios-p is concentration-dependent (P < 0.05). Additionally, we determined the effective concentration of Bios-p to maintain viable cells by 50% (EC50) when these are subjected to H2O2-induced oxidative stress. The EC50 was determined in 7.51 µM ± 0.09 µM.
Furthermore, we directly examined the viability effect when the cells were pretreatment with the EC50 of Bios-p (7.51 µM) during 6-12-18 and 24 h. As shown in the
Figure 1(b) the incubation with Bios-p significantly increased the viability cells (P < 0.05) when the cells are under oxidative stress with H2O2. Moreover, the results show that by incubating the cells at 37˚C during 6, 12, 18 and 24 h with Bios-p (7.51 µM), was not observed a significant effect on the protection of viability respect to control in the absence of Bios-p when these cells are not in conditions of oxidative stress (P > 0.05).
3.2. Bios-p Reduces Extracellular ROS Production
Was evaluated the antioxidant capacity of Bios-p on the basal extracellular ROS production in HEK293T cells and extracellular ROS production when the cells are subjected to oxidative stress induced by H2O2. Extracellular ROS production was measured by luminescence assays incubating the cells with luminol (5- amino-2,3-dihydro-1,4-ftalazinediona) and immediately quantifying luminescence. These measurements are highly sensitive, allowing the presence of different types of reactive oxygen species simultaneously (HO·, 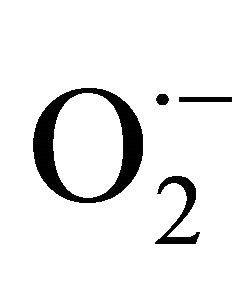 , H2O2, 1O2) [15].
, H2O2, 1O2) [15].
The Figure 2(a) shows the results of the measurement of basal production of ROS by incubating with Bios-p (0.1, 1.0 and 10 μM) for 24 h at 37˚C. As noted, the addition of Bios-p decreases significantly the number of cells producing basal ROS concentration-dependent manner.
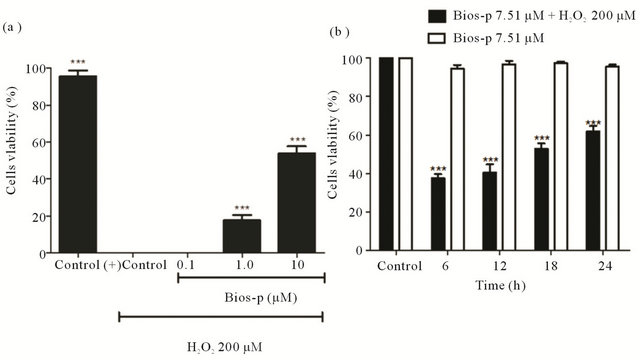
Figure 1. Cell viability effect of Bios-p on H2O2-induced cytotoxicity in HEK293T cell system (SYTOX Green assay assay). (a) Concentration effect of Bios-p incubation on cell viability. HEK293T cells were pretreated for 24 h with different concentrations of Bios-p (0; 0.1; 1; 10 µM). The cells were then treated with 200 µM H2O2 for 15 min; (b) Time effect of Bios-p incubation on cell viability. HEK293T cells were pretreated for 6, 12, 18 and 24 h with 7.51 µM of Bios-p. The cells were then treated with 200 µM H2O2 for 15 min. Results shown are means ± SD (n = 6). Significant difference (P < 0.05).
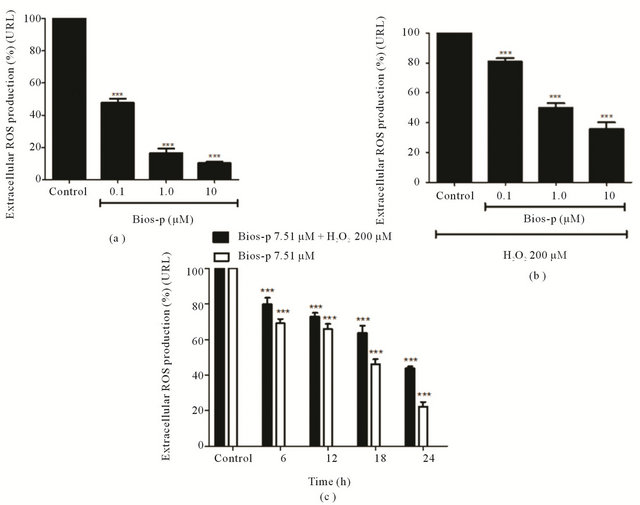
Figure 2. Antioxidant effect of Bios-p in HEK293T cell system (luminescence assay). (a) Antioxidant effect of Bios-p on H2O2-induced cytotoxicity in HEK293T cell. HEK293T cells were pretreated for 24 h with different concentrations of Bios-p (0; 0.1; 1 and 10 µM). The cells were then treated with 200 µM H2O2 for 15 min. (b) Antioxidant effect of Bios-p on HEK293T cell. HEK293T cells were pretreated for 24 h with different concentrations of Bios-p (0; 0.1; 1 and 10 µM); (c) Time effect of Bios-p incubation on antioxidant capacity. HEK293T cells were pretreated for 6, 12, 18 and 24 h with 7.51 µM of Bios-p. The cells were then treated with 200 µM H2O2 for 15 min. Results shown are means ± SD (n = 6). Significant difference (P < 0.05).
At concentrations of 10 µM is a basal production of ROS 13.33% ± 0.76%, i.e. to achieve an 86.67% decrease the number of cells producing ROS. Similar results were obtained when the cells were subjected to oxidative stress induced by H2O2 200 µM and co-treated with Bios-p (0 (control), 0.1, 1.0 and 10 µM) for 24 h. A concentration of 10 µM, the number of cells producing ROS was 35.63% + 4.63%, i.e., attainment reduce the percentage of cells producing extracellular ROS in a 64.37% (Figure 2(b)).
The Figure 2(c) shows the antioxidant effect of Bios-p (7.51 µM) on HEK293T cells subjected to oxidative stress induced by H2O2 200 µM. As time goes by incubation with Bios-p, there is a decrease in the percentage of extracellular ROS producing cells (P < 0.05) compared to control (without addition of Bios-p), reaching 55% of decreased production of ROS at 24 h of incubation with the analogue. Between 12 and 18 h of incubation with Bios-p, although there is a decrease in the percentage of extracellular ROS producing cells, this difference is not statistically significant. Similarly, it is observed that in cell cultures that were not subjected to oxidative stress, there is a decrease in extracellular ROS producing cells compared to basal control (without addition of Bios-p) (P < 0.05) without however, when comparing the responses obtained at 6 and 12 h o significant differences between them.
The same is true when comparing responses between 18 and 24 h of incubation with Bio-p. Still, these results suggest that the antioxidant capacity of Bios-p on cells subjected to oxidative stress induced by H2O2 200 μM depends on the time of incubation with the analogue. On the other hand, decreased extracellular ROS when cells are subjected to oxidative stress relates the protective effect of viability of Bios-p with its antioxidant capacity; however it is not possible to determine whether the peptide is interacting directly with ROS.
3.3. Bios-p Reduces Intracelullar ROS Production Levels
As shown in the Figure 3, by incubating the cells with only H2O2 200 µM was observed 100% of intracellular superoxide anion-producing cells. However, was observed in this case also a complete loss of viability (data not shown in this graph). The same effect was observed by incubating the cells with 0.1 µM of Bio-p and coincubating with 200 µM H2O2. Opposite effect was observed when comparing these results with the control without addition of neither peptide analogue nor H2O2. In this case, we observed a basal intracellular ROS production and viability percentage of 100%. At concentrations of 1 and 10 µg/ml, we observed a decrease of intracellular superoxide anion-producing cells, indicating that the analog peptide have an antioxidant effect, decreasing significantly the number of intracellular superoxide anion-producing cells (P < 0.05), compared to the control without the addition of Bios-p.
As seen in the above results, Bios-p has the ability to protect cells from cytotoxicity of HEK293T 200 µM H2O2 concentration and time dependent manner. Likewise, it was observed that Bios-p has the ability to regulate the levels of extra-and intracellular ROS. These results confirm that there is a direct relationship between the degree of oxidative stress that is subject to the cell
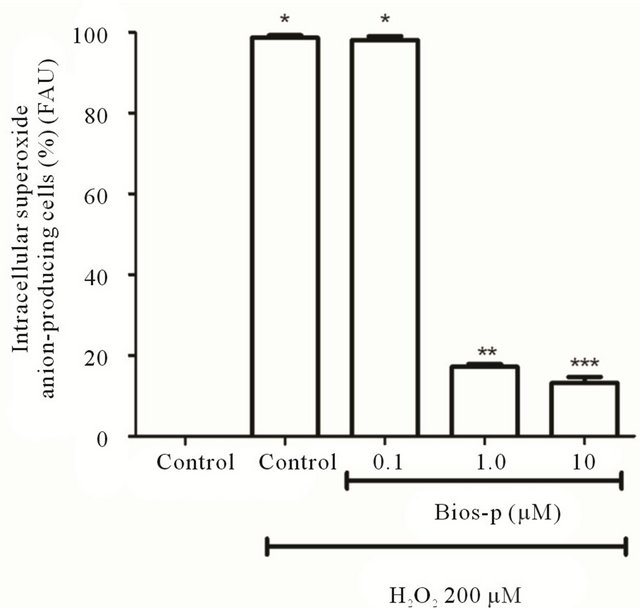
Figura 3. Antioxidant effect of Bios-p on superoxide anion levels (DHE assay). HEK293T cells were pretreated for 24 h with different concentrations of Bios-p (0; 0.1; 1 and 10 μM). The cells were then treated with 200 μM H2O2 for 15 min. Results shown are means ± SD (n = 6). Significant difference (P < 0.05).
and the damage caused to it by being in this condition. The results suggest that the increase in cell viability is because Bios-p decreased levels of basal production of ROS also generated under oxidative stress conditions, allowing the cell to maintain a redox equilibrium ensuring their viability, growth and various cellular functions [16].
3.4. Bios-p: Three Dimensional Structure Prediction
The role of bioactive peptide depends on its amino acid sequence and three-dimensional structures possessing these are essential to a deep understanding of the mode of action [10]. In this context, it is obtained a Bios-p three-dimensional structure prediction using homologybased modeling (Figure 4).
The primary sequence of Bios-p was obtained by sequencing from a peptide BbKi of 265 aa ADDIN EN.CITE ADDIN EN.CITE.DATA [9] . The primary sequence of Bios-p consist 12 amino acids in which there proline near the N-terminal and C-terminal. Furthermore, this analogue peptide has within its sequence a type of tripeptide arginine-glycine-aspartic acid (RGD), non-linear, since it has a loop between the amino acid RG-D (Figure 4(a)). The templates was obtained from Protein Data Bank and were Crystal structure of BbKI, a Kunitz-type kallikrein inhibitor (PDB ID: 2GO2) and Bauhinia bauhinioides cruzipain inhibitor (BbCI) (PDB ID: 2GZB). The percent identity of the target sequence with template is highly identical, so that the resulting model can be considered as a good approximation (84%) [17]. Was performed a structural alignment between the resulting model and temperate, and from temperate to calculate the RMSD as a parameter to evaluate the generated model (Figure 4(b)). The RMSD calculated for target-temperate overlap was 1.64 Å. The RMSD calculated in the comparison between temperate was 1.65 Å. RMSD values obtained indicate that the resulting model is very similar to temperate. According to the analysis of RMSD and the sequence identity is considered that the models generated for the peptide analog are good approximations of the 3D structure [18].
Figure 4(a) shows the three-dimensional structure of Bios-p represented by its surface colored according to electrostatic potential. As shown, the peptide analogue has two zones in its sequence a positively charged and negatively charged area. This feature would make possible the interaction of the peptide analogue with the extracellular membrane and ultrastructural components of cell membrane, acting as a regulator compound and modulator of intracellular signaling, this being one of the possible mechanisms of antioxidant action. Furthermore, in BBCi crystallographic structure revealed that maintaining the conformation of the loop RGD is important
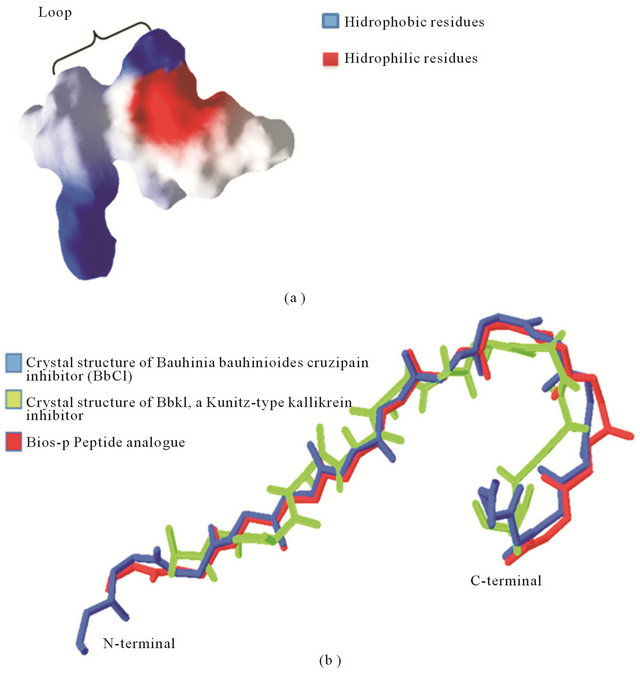
Figure 4. Three dimensional structure of Bios-p. (a) Three-dimensional structure of peptide analogue predicted by homology modelling method. The structure of the peptide is represented by its surface colored according to electrostatic potential: red, negative, white, neutral and blue, positive; (b) Structural alignment of peptide analogue predicted by homology modelling method with templates. The templates was Crystal structure of BbKI, a Kunitz-type kallikrein inhibitor (PDB ID: 2GO2) and Bauhinia bauhinioides cruzipain inhibitor (BbCI) (PDB ID: 2GZB). The C backbones of the modeled query protein were super imposed with the templates of Crystal structure of BbKI and BbCI sequence and RMSD was calculated as 1.64 Å using Swiss-pdbviewer v.4.0.1. The RMSD calculated between templates was 1.65 A.
for the protein function [17]. It has also been reported that RGD peptides have demonstrated a high affinity for membranes and membrane components this can enhance the antioxidant capacity. In addition studies indicated that RGD peptides have regulatory capacity of the antioxidant activity of the rabbit bronchial epithelial cells, by modulating the activity of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase (CAT) and the level of glutathione (GSH). This suggests that the antioxidant activity by the peptide analogue is due to the presence of RG-D tripeptide of the sequence, which also creates a pocket which allows its interaction with different membrane components thereby modulating the signal transduction system. Furthermore, it has been considered that RG-D regions as “strategic areas” since they protect areas of proteolytic breakdown [3].
4. CONCLUSIONS
We investigated the protective effect and the antioxidant activity of the Bios-p analogue peptide in vitro using HEK293T cells. These results confirm that there is a direct relationship between the degree of oxidative stress that is subject to the cell and the damage caused to it by being in this condition. The results suggest that the increase in cell viability is because Bios-p decreased levels of basal production of ROS also generated under oxidative stress conditions, allowing the cell to maintain a redox equilibrium ensuring their viability, growth and various cellular functions.
Furthermore, the three-dimensional structure obtained by computational methods according to the analysis of energy and RMSD is considered that the models generated for the peptide analog are good approximations of the 3D structure. The analysis of the structure of the peptide analog makes the structure prediction and modeling studies, is useful to provide clues to their function. The results presented in this paper can be interpreted in the context of ligand binding sites.
5. ACKNOWLEDGEMENTS
Patricia Navarrete Gómez has postdoctoral fellow from CTIC-UNAM and BECAS Chile.
REFERENCES
- Ajila, C.M. and Prasada R.U.J. (2008) Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract. Food and Chemical Toxicology, 46, 303-309. doi:10.1016/j.fct.2007.08.024
- Ames, B.N., M.K. Shigenaga, and Hagen, T.M. (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States, 90, 7915-7922. doi:10.1073/pnas.90.17.7915
- Korhonen, H. and A. Pihlanto (2003) Food-derived bioactive peptides—Opportunities for designing future foods. Current Pharmaceutical Design, 9, 1297-1308. doi:10.2174/1381612033454892
- Neves, S.A., et al. (2001) Neutrophil migration induced in vivo and in vitro by marine algal lectins. Inflammation Research, 50, 486-490. doi:10.1007/PL00000222
- Megias, C., et al. (2004) Purification of an ACE inhibittory peptide after hydrolysis of sunflower (Helianthus annuus L.) protein isolates. Journal of Agricultural and Food Chemistry, 52, 1928-1932. doi:10.1021/jf034707r
- Chen, J.Y., et al. (1998) α-Tocopherol content and oxidative stability of egg yolk as related to dietary α-tocopherol. Journal of Food Science, 63, 919-922. doi:10.1111/j.1365-2621.1998.tb17927.x
- Chan, K.M. and Decker, E.A. (1994) Endogenous skeletal muscle antioxidants. Critical Reviews in Food Science and Nutrition, 34, 403-426. doi:10.1080/10408399409527669
- Vaz, A.M.S.F. and Tozzi, A.M.G.A. (2005) Sinopse de Bauhinia sect. pauletia (Cav.) DC. (leguminosae: Caesalpinioideae: Cercideae) no Brasil. Revista Brasileira de Botânica, 28, 477-491. doi:10.1590/S0100-84042005000300006
- Cagliari, C.I., et al. (2003) Action of Bauhinia bauhinioides synthetic peptides on serine proteinases. Biochemical and Biophysical Research Communications, 311, 241-245. doi:10.1016/j.bbrc.2003.09.203
- Se-Kwon, K., et al. (2011) Proteins and peptides as antioxidants, in bioactive food proteins and peptides. CRC Press, Boca Raton, 97-116.
- Fink, B., et al. (2004) Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. American Journal of Physiology—Cell Physiology, 28, C895-C902. doi:10.1152/ajpcell.00028.2004
- Eswar, N., et al. (2006) Comparative protein structure modeling using Modeller. Current Protocols in Bioinformatics—Current Protocols, Chapter 5, Unit 5-6.
- Laskowski, R.A., et al. (1993) PROCHECK: A program to check the stereochemical quality of protein structures. Journal of Applied Crystallography, 26, 283-291. doi:10.1107/S0021889892009944
- Melo, F., et al. (1997) ANOLEA: A www server to assess protein structures. Proceedings International Conference on Intelligent Systems for Molecular Biology, 5, 187-190.
- Villegas, J., et al. (2005) Influence of reactive oxygen species produced by activated leukocytes at the level of apoptosis in mature human spermatozoa. Fertility and Sterility, 83, 808-810. doi:10.1016/j.fertnstert.2004.09.022
- Blanchetot, C. and Boonstra, J. (2008) The ROS-NOX connection in cancer and angiogenesis. Critical Reviews in Eukaryotic Gene Expression, 18, 35-45. doi:10.1615/CritRevEukarGeneExpr.v18.i1.30
- Hansen, D., et al. (2007) Crystal structure of a novel cysteinless plant Kunitz-type protease inhibitor. Biochemical and Biophysical Research Communications, 360, 735- 740. doi:10.1016/j.bbrc.2007.06.144
- Vitkup, D., et al. (2001) Completeness in structural genomics. Nature Structural & Molecular Biology, 8, 559- 566. doi:10.1038/88640

