Green and Sustainable Chemistry
Vol.1 No.3(2011), Article ID:7075,6 pages DOI:10.4236/gsc.2011.13015
Cerium (IV) Ammonium Nitrate (CAN) Mediated Reactions IV. A Highly Efficient Synthesis of N,N’-Diarylsubstituted Formamidines in Water at Ambient Temperature
1Chemistry Department, Faculty of Science, El-Minia University, El-Minia, Egypt
2Applied Science Department, College of Technological Studies, Public Authority of applied Education and Training, Safat Kuwait
3Current Address: Department of Chemistry, Faculty of Science for Girls, King Abdul-Aziz University, Jeddah, Kingdom of Saudi Arabia
E-mail: *kusadek@yahoo.com
Received March 29, 2011; revised May 9, 2011; accepted May 23, 2011
Keywords: CAN, Triethylorthoformate, Aromatic Amines, Water, Ambient Temperature
Abstract
A green, simple and highly efficient synthesis of N,N-diarylsubstituted formamidines has been developed employs reaction of triethylorthoformate (TEO) with aromatic amines catalyzed by cerium (IV) ammonium nitrate (CAN) in water at ambient temperature. This method offers the advantages of proceeding under environmentally friendly technique with high yields and simplicity either in conducting the reaction or handling the products.
1. Introduction
Formamidines are of considerable interest in fields related to organic and medicinal chemistry. They act as starting materials in several synthetic approaches mainly in the synthesis of heterocycles [1-5]. Moreover, amidines were considered as precursors of acid functions [6,7] Furthermore, they possess antimalarial activity [8] which is effective against chloroquino-resistant strain [9]. Classes of compounds containing amidine structures have found enormous applications as building blocks in polymer synthesis [10,11], bleaching agents for paper [12], ultraviolet light absorbers [13], protecting groups for primary amines [14,15] and other several applications [16-19].
Many of known methods for the synthesis of formamidines require long reaction times and/or a multi-step procedures. Thus, Taylor et al. [15] have reported a general synthetic method for the synthesis of formamidines by the reaction of triethylorthoformate or orthoacetate with a number of aliphatic and aromatic amines in acetic acid under reflux at 140˚C - 150˚C for 1.5 - 94 hours. Although the reduction of carbodiimides with sodium borohydride in isopropanol perform a convenient synthetic method of the corresponding formamidines [20] but this protocol suffers from the use of toxic organic solvents either in conducting the reaction or working-up the product. The exchange of N,N-dimethylformamidines or acetamidines by a variety of amines has been reported to be the most popular method for the synthesis of symmetrical and unsymmetrical formamidines [21]. This approach suffers from drawbacks of high temperatures and the use of protic organic solvents (mostly methanol or ethanol) for a period of 1 - 48 hours [22]. The beneficial effect of base in non-protic solvents for this transformations has also been reported [23]. A revisiting of the effect of acid in the amine exchange of N,N-- dimethylformamidines has been recently reported [18] which allowing the preparation of highly electron-rich compounds that are difficult to obtain under standard conditions. In their study, they have claimed that such exchange is highly dependent on the nucleophilicity of the corresponding amine (e.g. weak nucleophiles such as aniline did not react at all and pyrrolidine gave a poor yield). They also emphasized that, minor products derived from loss of the imine component rather than dimethylamine, followed by addition exchange reactions can often be observed. Unexpected formation of N,N–-disubstituted formamidines by the reaction of an amine with sodium hydride and triflouroacetic anhydride in dimethylformamid has been recently reported [24]. Accordingly, the development of a simple, high yield and green protocol for the synthesis of such scaffold will be of interest for researches and scientists in such area.
Cerium (IV) ammonium nitrate (CAN) is a convenient and widely used catalyst for affecting a broad spectrum of synthetic transformations for its many advantages such as solubility in water, inexpensiveness, eco-friendly nature, simplicity in handling and convenient work-up which make CAN an interesting and potential catalyst in organic synthesis [25]. Although, the utility of CAN as a one-electron oxidant was extensively utilized in literature [26,27] its use as a Lewis acid catalyst in C-N bond formation is somehow limited [28]. In this connection, it is worth mentioning that CAN is a useful alternative to the expensive lanthanide triflates. Also, Ce salts are the ones that have the lowest affinity for oxygen, making them potential complementary to other extensively studied Lewis acids [29].
In continuation to our efforts utilizing a simple and green technologies for organic synthesis [30] we reported herein, for the first time, a convenient, green, highly efficient synthesis of N,N–-diarylsubstituted formamidines in water at ambient temperature performing CAN in catalytic amount.
2. Results and Discussion
With the aim of optimizing experimental conditions, we initially explored the reaction of aniline (1a) (1 mol) and triethylorthoformate (2) (1 mol) in water (20 mL) in the presence of CAN (10 mol%) at ambient temperature (27˚C). The reaction was promoted by stirring for 15 minutes after which a solid was formed affording N,N–- diphenylformimidamide (3a), in moderate yield (60%). Upon duplication of aniline molar ratio the yield was highly improved up to 93%. We also explored the effect of catalyst molar ratio on overall yield. Our investigations clearly revealed that addition of (10 mol%) of CAN to the reaction mixture containing 2:1 molar ratios of amine 1 and triethylorthoformate (2) in water at ambient temperature was optimal for the formation of the target molecule 3. Finally, irrespective of the aryl substituent, reactions utilizing a variety of aromatic amines took place in reasonable yields (Table 1).
In order to extend the scope of the reaction, heterocyclic amine 1h was examined and the reaction proceeds easily with a good yield. However, attempted conden
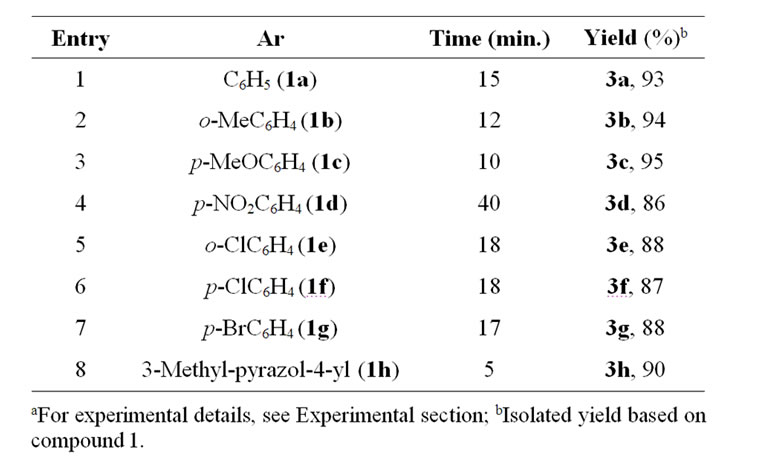
Table 1. Synthesis of N,N--diarylsubstituted formamidines 3a-h from aromatic and heterocyclic amines and triethyl orthoformatea.
sation of aliphatic amines and triethylorthoformate was unsuccessful. Starting materials were recovered even after prolonged stirring. This may be rationalized for the fact that the equilibrium position for the reaction of an aliphatic amines with triethylorthoformate is probably in favor of the starting materials. Although, syn-anti isomerization is possible but the anti-isomer was favored based on the fact that syn-isomer is being sterically crowded21 (Scheme 1).
A proposed mechanism for the formation of 3 is shown in Scheme 2. It is clear from the sequence of steps that CAN as a Lewis acid activate ethoxy groups and enhance the C-O bond cleavage to generate carbenium ions that are stabilized by resonance facilitating subsequent nucleophilic displacements by aromatic amines.
3. Conclusions
In conclusion, we have developed, for the first time, a convenient synthesis of N,N–-diarylsubstituted formamidines in water at ambient temperature catalyzed with (CAN). On the basis of the experimental results in our study, this annulations reaction proved to be green, simple and highly efficient procedure for the target molecules which make it a useful and important addition for such area of research.
4. Experimental Section
4.1. General Procedures
Commercially available chemicals were used as received. IR spectra were recorded with a Schimadzu 470 spectrophotometer in KBr disks. The 1H NMR (400 MHz) and 13C NMR (100 MHz) were run on a Bruker DPX spectrometer with DMSO-d6 as solvent and TMS as an internal standard; Chemical shifts (δ) were reported in ppm. Mass
spectra were measured on VG Autospec Q MS 30 and MS 9 (AEI) spectrometers with EI 70 ev. Elemental analyses were performed by by using of LECO CHNS-932 Elemental Analyzer. Melting points were measured on a Gallenkamp apparatus in open capillary tubes. The purity of the products was checked by TLC (Merck Kieselgel 60 F254 )
4.2. Experimental Section
4.2.1. General Procedure for the Synthesis of N,N–-Disubstituted Formamidines 3a-h
To a stirred mixture of each of aromatic or heteroaromatic amines 1a-f (20 mmol), triethylorthoformate (10 mmol) in water (20 mL) was added cerium (IV) ammonium nitrate (10 mol%). The reaction mixture was stirred at room temperature (27˚C) for 10 - 40 minutes. For liquid amines a solid was formed, while for solid amines, they dissolved in the reaction medium and then resolidified forming the final product. The resulting solid was collected by filtration and recrystallized from ethanol to afford analytically pure samples 3a-h. Compounds 3a-f have been described previously [31-37] and were found identical with our samples synthesized by our protocol.
4.2.2. N,N–-Diphenylformimidamide (3a)
Pale yellow crystals 0.183 g, 93% yield; mp 142˚C - 143˚C. IR (KBr pellet)): 3422 (NH), 3050 (arom.CH), 1665 (C=N) cm–1; MS: m/z (%) = 197 (M+ + 1, 8), 196 (M+, 69), 195 (25), 119 (3), 104 (15), 93 (100), 77 (42), 66 (7), 65 (7); 1H NMR (DMSO-d6, 400MHz): 9.70 (s, 1H), 8.27 (s, 1H), 7.39 - 6.90 (m, 10H). 13C NMR (DMSO-d6, 100 MHz): 163.2, 152.6, 144.4, 130.1, 129.5, 127.2, 122.6, 122.3, 115.7. Anal. Calcd. for C13H12N2 (196.1): C, 79.56%; H, 6.16%; N, 14.27%. Found: C, 79.44%; H, 6.11%; N, 14.31%.
4.2.3. N,N–-Bis(2-Tolylformimidamide (3b)
Buff crystals 0.210 g, 94% yield; mp 161˚C - 162˚C. IR
(KBr pellet): 3439 (NH), 3018 (arom. CH), 2939 (aliph. CH), 1662 (C=N) cm–1.; MS: m/z (%) = 225 (M+ + 1, 10), 224 (M+, 62), 223 (M+ – 1, 12), 209 (13), 133 (3), 118 (30), 107 (100), 91 (24), 65 (10); 1H NMR (DMSO-d6, 400 MHz): 8.76 (s, 1H), 7.80 (s, 1H), 7.05 (m, 5H), 6.85 (m, 3H), 2.41 (s, 3H), 2.17 (s, 3H). Anal. Calcd. C15H16N2 (224.30): C, 80.32%; H, 7.19%; N, 12.49%. Found: C, 80.44%; H, 7.13%; N, 12.31%.
4.2.4. N,N–-Bis(4-Methoxyphenyl)formimidamide (3c)
Colourless crystals 0.243 g, 95% yield; mp 254˚C - 255˚C. IR (KBr pellet): 3426 (NH), 3060 (arom. CH), 2925 (aliph. CH), 1663 (C=N) cm–1. ; MS: m/z (%) = 257 (M+ + 1, 10), 256 (M+, 63), 134 (16), 123 (100), 108 (82)77 (10); 1H NMR (DMSO-d6, 400 MHz): 8.17 (br, 2H), 7.32 (d, J = 7 Hz, 2H), 6.94 (d, J = 7 Hz, 2H), 6.80 (d, J = 8 Hz, 2H), 6.74 (d, J =7 Hz, 2H), 3.68 (s, 3H), 3.60 (s, 3H). 13C NMR (DMSO-d6, 100 MHz): 163.2, 159.3, 153.2, 144.7, 136.6, 122.1, 117.6, 115.5, 115.1, 55.7, 55.3. Anal.Calcd for C15H16N2O2 (256.3) C, 70.29%; H, 6.29%; N, 10.93%. Found: C, 70.31%; H, 6.13%; N, 10.88%.
4.2.5. N,N–-Bis(4-Nitrophenyl)formimidamide (3d)
Greenish crystals 0.233 g, 86% yield; mp 144˚C - 145˚C. IR (KBr pellet): 3422 (NH), 3040 (arom. CH), 1668 (C=N) cm–1; MS (M+ ) = 271.1; 1H NMR (DMSO-d6, 400 MHz): 9.72 (s, 1H), 8.09 (s, 1H), 7.36 - 7.02 (m, 8H). Anal.Calcd for C13H9N3O4 (271.35): C, 54.55%; H, 3.52%; N, 19.57%. Found: C, 54.52%; H, 3.49%; N, 19.58%.
4.2.5. N,N–-Bis(2-Chlorophenyl)formimidamide (3e)
Buff crystals 0.233 g, 88% yield; mp 148˚C - 149˚C. IR (KBr pellet): 3439 (NH), 3050 (arom. CH), 1664 (C=N) cm–1. MS: m/z (%) = 266 (M+, 13), 265 (M+, 5), 264 (M+, 18), 229 (35), 140 (5), 138 (14), 127 (100), 111 (18), 75 (10), 65 (3); 1H NMR (DMSO-d6, 400 MHz): 9.16 (s, 1H), 8.45 (s, 1H), 7.37 (d, J = 8.4 Hz, 2H), 7.02 (m, 3H), 6.96 (t, J = 8.4 Hz,3H). Anal.Calcd. for C13H10Cl2N (264.14): C, 58.89%; H, 3.80%; N, 10.57%; Cl, 26.74%. Found: C, 58.82%; H, 3.78%; N, 10.66%; Cl, 26.55%.
4.2.6. N,N–-Bis(4-Chlorophenyl)formimidamide (3f)
Redish crystals 0.230 g, 87% yield; mp 185˚C - 186˚C. IR (KBr pellet): 3420 (NH), 3014 (arom. CH), 1660 (C=N) cm–1. MS: m/z (%) = 266 (M+, 49), 265 (M+, 17), 264 (M+, 79), 140 (7), 138 (25), 127 (100), 111 (35), 99 (8), 75 (16); 1H NMR (DMSO-d6, 400 MHz): 9.79 (s, 1H), 8.09 (s, 1H), 7.26 - 7.08 (m, 8H). Anal. Calcd. for C13H10Cl2N2 (264.14): C, 58.89%; H, 3.80%; N, 10.57%; Cl, 26.74%. Found: C, 58.82%; H, 3.78%; N, 10.66%; Cl, 26.55%.
4.2.7. N,N–-Bis(4-Bromophenyl)formimidamide (3g)
Buff crystals 0.311 g, 88% yield; mp 188˚C - 189˚C. IR (KBr pellet): 3437 (NH), 3051 (arom. CH), 1660 (C=N) cm–1. MS: m/z (%) = 356 (M+, 18), 354 (M+, 38), 352 (M+, 19), 182 (12), 171 (100), 155 (18), 92 (11), 76 (12), 64 (7); 1H NMR (DMSO-d6, 400 MHz): 9.82 (s, 1H), 8.10 (s, 1H), 7.36 - 6.98 (m, 8H).) Anal. Calcd. for C13- H10Br2N2 (354.04): C, 44.10%; H, 2.85%; N, 7.91%;Br, 45.14%. Found: C, 44.21%; H, 2.78%; N, 7.88%; Br, 45.23%.
4.2.8. N,N–-Bis(5-Methyl-1H-pyrazol-3-yl)- formimidamide (3h)
Pink crystals 0.178 g, 88% yield; mp 282˚C - 283˚C. IR (KBr pellet): 3422 (NH), 3015 (arom. CH), 2935 (aliph. CH), 1660 (C=N) cm–1. 1H NMR (DMSO-d6, 400 MHz): 12.66 (s, 1H), 12.23 (s, 1H), 9.15 (s, 1H), 8.08 (s, 1H), 2.35 (s, 3H), 2.30 (s, 3H). Anal. Calcd. for C9H10N6 (204.23): C, 52.93%; H, 5.92%; N, 41.15%. Found: C, 52.89%; H, 5.91%; N, 41.23%.
5. Acknowledgements
This research was financed by the financial support of the Public Authority for Applied Education and Training (Transform Grant TS-07-11) of Kuwait.
6. References
[1] G. V. Boyd, “Chemistry of Amidines and Imidates,” J. Wiley, New York, 1991, p. 367.
[2] K. Ziegleskylakakis, S. Nill, J. F. Pan and U. Andrea “SOxygenation of Thiourea Results in the Formation of Genotoxic Products,” Environmental and Molecular Mutagenesis, Vol. 31, No. 4, 1998, pp. 362-373. doi:10.1002/(SICI)1098-2280(1998)31:4<362::AID-EM9>3.0.CO;2-K
[3] V. K. S. Leung, T. Y. K. Chan and V. T. F.Yeung, “Amitraz Poisining in Humans,” Clinical Toxicology, Vol. 37, No. 4, 1999, pp. 513-514. doi:10.1081/CLT-100102523
[4] T. Goto, H. Sakashita, K. Murakami, M. Sugiura, T. Kondo and C. Fukaya, “Novel Histamine H3 Receptor Antagonists: Synthesis and Evaluation of Formamidine and S-Methylisothiourea Derivatives,” Chemical & Pharmaceutical Bulletin, Vol. 45, No. 2, 1997, pp. 305-311.
[5] G. Wulff and R. Schönfeld, “Polymerizable-AmidinesAdhesion Mediators and Binding Sites for Molecular Imprinting,” Advanced Materials, Vol. 10, No. 12, 1998, pp. 957-959. doi:10.1002/(SICI)1521-4095(199808)10:12<957::AID-ADMA957>3.0.CO;2-4
[6] G. Haberhauer and F. Rominger, “Synthesis of a New Class of Imidazole-Based Cyclic Peptides,” Tetrahedron Letters, Vol. 43, No. 36, 2002, pp. 6335-6338. doi.org/10.1016/S0040-4039(02)01365-5
[7] G. Haberhauer and F. Rominger, “Synthesis and Structures of Imidazole Analogues of Issoclynum Cyclopeptide,” European Journal of Organic Chemistry, Vol. 2003, No. 16, 2003, pp. 3209-3218. doi:10.1002/ejoc.200300207
[8] S. Delarue, S. Girault, F. D. Ali, L. Maes, P. Grellier and C. Sergheraert, “One-Pot Synthesis and Antimalarial Activity of Formamidine Derivatives of 4-Aminoquinolie,” Chemical & Pharmaceutical Bulletin, Vol. 49, No. 8, 2001, pp. 933-937. doi:10.1248/cpb.49.933
[9] W. M. Watkins, D. G. Sixsmith, H. G. Spencer, D. A. Boriga, D. M. Karjuki, T. Kipingor and D. K. Koech, “Effectvness of Amodiaquine as Treatment for Chloroquine Resistant Plasmodium Infections in KENYA,” Lancet, Vol. 324, No. 8373, 1984, pp. 357-359. doi:10.1016/S0140-6736(84)90410-0
[10] H. Komber, H. H. Limbach, F. Bohme and C. Kunner, “NMR Studies of the Tautomerism of Cyclo-tris(4-R- 216-Pyridylformamidines) in Solution and in the Solid State,” Journal of the American Chemical Society, Vol. 124, No. 40, 2002, pp. 11955-11963. doi:10.1021/ja0202762
[11] A. V. Tenkovvtsev, A. V. Yakimanski, M. M. Dudkina, V. V. Lukoshkin, H. Komber, L. Haussler and F. Bohme, “Ionic Complexes of Bis (Hydroxyarylidine) Alkanones with Strong Polymeric Bases as a New Class of ThirdOrder Nonlinear Optical Chromophors,” Macromolecules, Vol. 34, No. 20, 2001, pp. 7100-7107. doi:10.1021/ma0020234
[12] V. R. Parthasarathy, “Elemental Chlorine Free (ECF) and Totally Chlorine Free (TCF) Bleaching of Colored Broke,” Tappi Journal, Vol. 80, No. 7, 1997, pp. 159-169.
[13] J. A. Virgilio, W. Emanuel, H. Fairfield, I. D. Cohen and N. Brooklyn, USA Patent No. 005. 243.055A, 1993.
[14] H. Rudyk, M. H. Knaggs, S. Vasiljevic, J. Hope, C. Birkett and I. H. Gilbert, “ Synthesis and Evaluation of Analogues of Cengo Red as Potential Compounds against Transimissible Spongiform Encephalopathies,” European Journal of Medicinal Chemistry, Vol. 38, No. 6, 2003, pp. 567-579. doi:10.1016/S0223-5234(03)00081-3
[15] F. Poch, “Acces Aux Alkyl (Ou Aryl)-4Diamino-3,6 Pyrazolo[3,4-b]: Pyridines Substituees en 5 par un Groupe Sr ou Cl,” Tetrahedron, Vol. 42, No. 16, 1986, pp. 4461- 4469.
[16] P. S. Furth, M. S. Reitman, R. Gentles and A. F. Cook, “Solid-Phase Synthesis of Novel Amino-Ether Derivatives,” Tetrahedron Letters, Vol. 38, No. 38, 1997, pp. 6643-6646. doi:10.1016/S0040-4039(97)01579-7
[17] P. S. Furth, M. S. Reitman and A. F. Cook, “Annovel Formamidine Linker for Use in Solid-Phase Synthesis,” Tetrahedron Letters, Vol. 38, No. 31, 1997, pp. 5403- 5406. doi:10.1016/S0040-4039(97)01200-8
[18] A. I. Meyers, M.A. Gonzalez, V. Struzka, A. Akahane, J. Guiles and J. S. Warmus, “Chiral Formamidines. Assymetric Synthesis of 1,2-Disubstituted Tetrahydroisoquinolines,” Tetrahedron Letters, Vol. 32, No. 40, 1991, pp. 5501-5504. doi:10.1016/0040-4039(91)80068-H
[19] M. A. Matulenko and A. I. Meyers, “Total Synthesis of (-)-Tetrahydropalmatine via Chiral Formamidine Carbanions: Unexpected Behaviour with Certain Ortho-Substituted Electrophiles,” The Journal of Organic Chemistry, Vol. 61, 1996, pp. 573-580.
[20] E. C. Taylor and W. A. Ehrhart, “A Convenient Synthesis of N,N’-Disubstituted Formamidines and Acetamidines,” The Journal of Organic Chemistry, Vol. 27, 1962, pp. 1108-1112.
[21] K. Kaji, H. Matsubara, H. Nagashima, Y. Kikugawa and S. I. Yamada, “Synthesis of Formamidines from Carbodiimides with Sodium Borohydride in Isopropanol,” Chemical & Pharmaceutical Bulletin, Vol. 26, No. 7, 1978, pp. 2246-2249.
[22] V. G. Granik, “Advances in the Chemistry of Amidines,” Russian Chemical Reviews, Vol. 52, No. 4, 1983, pp. 337-360. doi:10.1070/RC1983v052n04ABEH002824
[23] A.S. Ripak, D. D. Diaz, K. P. Sharpless and M. G. Finn, “Expanded Chemistry of Formamidine Ureas,” Organic Letters, Vol. 6, No. 1, 2004, pp. 43-46. doi:10.1021/ol035995f
[24] D. D. Diaz, W. G. Lewis and M. G. Finn, “Acid-Mediated Amine Exchange of N,N-Dimethylformamidines: Preparation of Electron-Rich Formamidines,” ChemInform, Vol. 37, No. 2, 2005, pp. 2214-2218.
[25] A. Mekhalifa, R. Mutter, W. Heal and B. Chen, “Unexpected Formation of N,N-Disubstituted Formamidines from Aromatic Amines, Formamides and Trifluroacetic Anhydride,” Tetrahedron, Vol. 62, No. 24, 2006, pp. 5617-5625. doi:10.1016/j.tet.2006.03.099
[26] S. C. Roy, C. Guin, K. K. Rana and G. Maiti, “Ceric Ammonium Nitrate Mediated Selective Bromoalkoxylation of Activated Cinnamyl Compounds Using Lithium Bromide,” ChemInform, Vol. 32, No. 2, 2001, pp. 226- 227.
[27] S. C. Roy and B. A. Banergie, “A Mild and Efficient Method for the Chemoselective Synthesis of Acylas from Aldehydes and Their Deprotections Catalysed by Ceric Ammonium Nitrate,” Synlett, Vol. 2002, No. 10, 2002, pp. 1677-1678.
[28] J. S. Yadav, B. V. S. Reddy, K. B. Reddy, K. S. Raj and A. R. Prasad, “Ultrasound-Accelerated Synthesis of 3,4- Dihydropyrimidine-2(1H)-Ones with Ceric Ammonium Nitrate,” Journal of the Chemical Society, Perkin Transaction 1, pp. 1939-1941. doi:10.1039/b102565c
[29] V. Nair, S. B. Paricker, L. G. Nair, T. G. George and A. Augustine, “Carbon-Heteroatom Bond Forming Reactions by Cerium (IV) Ammonium Nitrate: An Overview,” Synlett, Vol. 2, 2003, pp. 156-165. doi:10.1055/s-2003-36775
[30] T. Imamoto, M. Nishiura, Y. Yamanoi and K. Yamaguchi, “Single X-Ray Analyses of a Series of Hexamethylphosphoramide-Coordinate Complexes of Rare Earth Triflates Existence of Tetrad Effects in the Coordinate Bonds,” Chemistry Letters, Vol. 25, No. 10, 1996, pp. 875-877. doi:10.1246/cl.1996.875
[31] K. U. Sadek, F. Al-Qalaf, M. M. Abdelkhalik and M. H. Elnagdi, “ Cerium (IV) Ammonium Nitrate as an Efficient Lewis Acid for the One-pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Their Corresponding 2- (1H)-Thiones,” Journal of Heterocyclic Chemistry, Vol. 47, No. 2, 2010, pp. 284-286.
[32] R. A. Mekheimer, A. A. Hameed and K. U. Sadek, “Solar Thermochemical Reactions, Four Component Synthesis of Polyhydroquinoline Derivatives Induced by Solar Thermal Energy,” Green Chemistry, Vol. 10, No. 5, 2008, pp. 592-593. doi:10.1039/b715126h
[33] R. A. Mekheimer and K. U. Sadek, “Microwave Assisted Reactions: Three Component Process for the Synthesis of 2-Amino-2-Chromenes under Microwave Heating,” Journal of Heterocyclic Chemistry, Vol. 46, No. 2, 2009, pp. 149-151. doi:10.1002/jhet.13
[34] R. A. Mekheimer, A. M. Abdelhameed, S. A. Mohamed and K. U. Sadek, “ Green Three Component, Highly Efficient Synthesis of 2-Amino5,6,7,8-Tetrahydro-4H-Chromen-3-Carbonitriles in Water at Ambient Temperature,” Green Chemistry Letters and Reviews, Vol. 3, No. 3, 2010, pp. 161-163.
[35] D. Nori-Shargh, A. Shiroudi, A. R. Oliaey and F. Deyhimi, “Ab Initio Study and NBO Analysis of the Allylic Rear-Rangements (Hetero Claisen and Cope Rearrangements) and Decarboxylation Reactions (Retro Carbonyl ene Reaction) of Allylformate and Allyldithioformate,” Journal of Molecular Structure: THEOCHEM, Vol. 824, No. 1-3, 2007, pp. 1-7. doi:10.1016/j.theochem.2007.08.008
[36] R. M. Roberts, “Ortho Esters, Imidic Esters and Amidines. The Identity of N-Phenyl-N’-p-Tolylformamidine,” Journal of the American Chemistry Society, Vol. 72, No. 8, 1950, pp. 3603-3608. doi:10.1021/ja01164a078
[37] R. M. Roberts and R. H. de Wolfe, “Ortho Esters, Imidic Esters and Amidines. IV. The Mechanism of the Reaction of Aniline with Ethyl Orthoformate,” Journal of the American Chemistry Society, Vol. 76, No. 9, 1954, pp. 2411-2414. doi:10.1021/ja01638a033



