World Journal of Condensed Matter Physics
Vol. 2 No. 4 (2012) , Article ID: 24687 , 4 pages DOI:10.4236/wjcmp.2012.24035
Synthesis and Characterization of Cr Doped ZnO Nanocrystals
![]()
Advanced Materials Research Laboratory, Department of Physics, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.
Email: *drbndole.phy@gmail.com
Received June 7th, 2012; revised July 6th, 2012; accepted July 18th, 2012
Keywords: Wurtzite Structure; Average Grain Size; Scherrer’s Formula; FTIR Spectra
ABSTRACT
Samples of chromium doped ZnO were synthesized using co-precipitation technique at room temperature. Structural and optical properties of Cr doped ZnO samples were investigated by X-ray diffraction technique (XRD and UV-Visible spectroscopy (UV-Vis) respectively. X-ray diffraction (XRD) patterns confirm that the samples have hexagonal (wurtzite) structure with no additional peak which suggests that Cr ions go to the regular Zn sites in the ZnO crystal structure. The lattice constants were calculated using X-ray diffraction data and it is found that lattice parameters decrease with increasing Cr content. The average grain size was calculated using Scherrer’s formula for pure and Cr doped ZnO samples and it is observed that grain size is in the range 11 to 17 nm. Band gap of Zn1–xCrxO samples has been evaluated using UV-Vis spectrometer. It is found that the band gap decreases as Cr increases; it is attributed to the s-d and p interactions and the smaller average grain size. It indicates that incorporation of Cr ions into the ZnO matrix. The chemical species of the grown crystals were identified by Fourier transform infrared spectroscopy (FTIR). From FTIR spectra it is observed that IR peaks corresponding to the Zn-O bands. Such results are presented in this paper quantitatively and qualitatively.
1. Introduction
In recent years, much attention has been paid to Diluted Magnetic Semiconductors (DMSs) formed by the partial replacement of cations in a non-magnetic semiconductor by magnetic transition metal ions for spintronic applications. Spintronics is a field which investigates the applications of carrier spins transport as well as charge transport in a new generation of devices such as spin-valve transistors [1], spin light-emitting diodes [2] and logic devices [3]. To realize these devices it is necessary to develop semiconducting materials that show ferromagnetic behavior at room temperature. Among the different types of wide-band-gap semiconductors ZnO, with a direct band gap of 3.37 eV and a large exciton binding energy of 60 meV, has become one of the most important functional semiconducting materials for electro-optical devices like UV light emitters [4], piezoelectric transducers [5] and gas sensors [6]. Since then, many systems of TM-doped ZnO (TM = Co, Mn, Ni, etc.) have been studied by different methods [7-10]. However, the reported experimental results on the studies of Cr-doped ZnO have been very conflicting. Some studies reveal that the magnetic behavior of Cr-doped ZnO appears to be very sensitive to the deposition method. For example, Ueda et al. [11] did not observe any ferromagnetic behavior for Cr-doped ZnO film grown by pulse laser deposition, whereas Roberts et al. [12] prepared Crdoped ZnO via magnetron sputtering and obtained ferromagnetic ordering at 9.5 at% doping concentration. Furthermore, the results of Jin et al. [13] showed no ferromagnetic behavior for Cr-doped ZnO film at low temperatures even down to 3 K. Lee et al. [14] did not find ferromagnetism in solgel synthesized Zn1−xCrxO thin films; however, ferromagnetism appeared when the same films were co-doped with Li. We report a detail study on the structural and optical properties of Zn1−xCrxO nanocrystals synthesized by co-precipitation technique at room temperature.
2. Experimental
2.1. Sample Preparation
Samples with compositional formula of Zn1–xCrxO, with x = 0.00 and 0.10 were prepared by co-precipitation route in an alcoholic medium (methanol). In this procedure, to prepare pure ZnO, Zinc acetate dihydrate (with 99+% purity, kemphasol make, A. R. Grade) was dissolved in methanol (100 ml) and KOH (purity 99+%, sd FiNECHEM Limited make, A. R. Grade) was dissolved in methanol (100 ml). Both the solutions were mixed by constant magnetic stirring by heating at 52˚C for 2 h. The precipitate separated from the solution by filtration, washed several times with distilled water and ethanol then dried in air at 127˚C to obtain ZnO nanocrystals. For the synthesis of Cr doped ZnO nanocrystals, Zinc acetate dihydrate and Chromium acetate tetrahydrate (with 99% purity, kemphasol make, A. R. Grade) were dissolved in methanol (100 ml) and KOH was dissolved in methanol (100 ml). Both the solutions were mixed by constant magnetic stirring by heating at 52˚C for 2 h. The precipitate separated from the solution by filtration, washed several times with distilled water and ethanol then dried in air at 127˚C to obtain Cr doped ZnO nanocrystals.
2.2. Characterization
The crystalline structure, phase purity and size of the nanoparticles were determined by X-ray diffraction (XRD) using X-ray diffractometer (Model: PW-3710) employing CuKα radiation. Fourier Transform Infrared (FTIR) spectra of the samples were recorded using JASCO FI-IR 460 spectrometer in the range 400 - 4000 cm−1 by KBr pellet technique. Absorption spectra of the samples in the UV-visible spectral region were recorded using UVVIS-NIR (JASCO V 570) spectrophotometer.
3. Results and Discussion
3.1. X-ray Diffraction Study
Figure 1 shows XRD patterns with distinct diffraction peaks, corresponded to the (100), (002), (101), (102), (110), (103), (200), (112) and (201) lattice planes, reveal that as prepared nanocrystals have a wurtzite (hexagonal) structure. It is seen from the XRD patterns that the peaks of ZnO shift towards higher angles with increasing Cr concentration of ZnO samples. Such shifts of the XRD peaks reveal a lattice expansion due to the Cr substitution for Zn in the ZnO crystal lattice. It shows the small reduction in the lattice constant as Cr concentration increases. Table 1 shows the lattice constant of Cr doped ZnO was slightly decreased with increasing Cr concentration. It may be due to the larger ionic radius of Zn as compared to Cr ions. The volume of the unit cell was also decreased with increasing Cr concentration. It indicates that Cr ions go to Zn site in ZnO structure.
The broadening of XRD peaks attributed nano-sized formation of Cr doped ZnO samples. The average grain size of Cr doped ZnO nanocrystals are estimated using Debye Scherer’s formula. It is found that the average grain size is in the range of 11 - 17 nm. The average
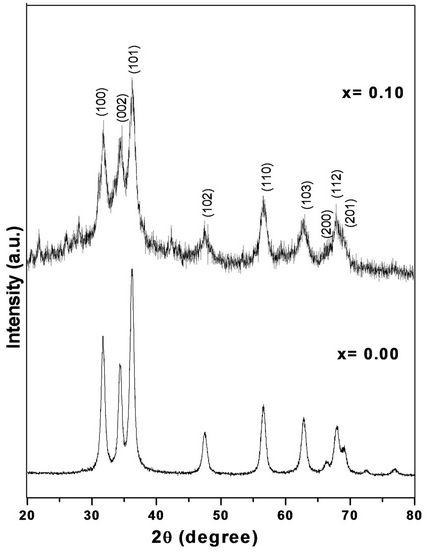
Figure 1. X-ray diffraction patterns of Cr doped ZnO at room temperature.
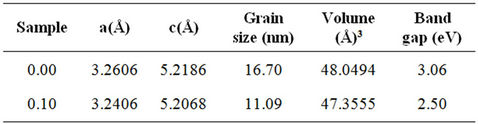
Table 1. Lattice parameters, grain size, volume cell and band gap at room temperature for Zn1–xCrxO system.
grain size, lattice parameter and absence of impurity phases with increasing Cr concentration for all samples could be attributed to the ionic radii of Zn and Cr ions. This is mainly because of the nucleation and subsequent growth rate with increasing Cr due to the difference of ionic radii of Zn and Cr ions.
3.2. Fourier Transform Infrared (FTIR) Analysis
FTIR spectra of pure and Cr doped ZnO nanoparticles are shown in the Figure 2. The broad peak in higher energy in the region at 3400 - 3600 cm–1 is due to OH stretching or it may be due to the M-OH-M. The peak in the range 1400 - 1757 cm–1 is due to OH bending of adsorbed moisture in the sample and all other peaks are attributed to the characteristic of the material. The bands appeared near at 2000 - 2200 cm–1 indicates the CO adsorption on the surface of oxide. Similarly the bands at 780 - 980 cm–1 might be due to the peroxide formation (M-O-O-M) and peak in the range of 900 - 1200 cm–1 is
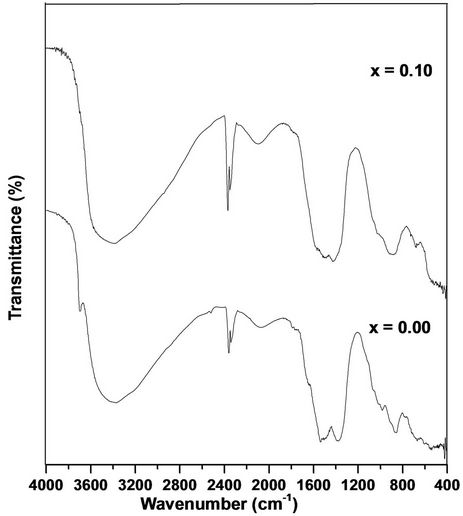
Figure 2. FTIR spectra of Zn1–xCrxO samples.
due to the M-O-M bonding. The FTIR spectrum of the main absorption band is due to Zn-O stretching of ZnO in the range of 600 - 400 cm–1. FTIR spectra of pure sample of the present investigation are similar to that of Cr doped ZnO samples and are in good agreement with the reported values [15-19].
3.3. Optical Absorption and Optical Band Gap
The room temperature UV-Vis spectra of undoped and Cr doped ZnO samples are shown in Figure 3. The maximum absorption for all samples is observed, which indicates red shift from the bulk ZnO samples. This red shift absorption edge is due to the small size of the particles. The band gap was calculated by plotting theabsorption plot (Energy, E) versus (αhν)2. It is found that the band gaps of Cr doped ZnO samples are 2.50 - 3.06 eV. Figure 4 shows the α-absorption is shifted slightly towards low energy as Cr content increases. The band gap is found to decrease with increasing Cr concentration. These values are tabulated in Table 1. The decrement in Eg with increasing Cr content is attributed to the s-d and p interactions and smaller average grain size.
4. Conclusion
Chromium doped ZnO nanocrystals were synthesized by co-precipitation method. Lattice parameters were determined using X-ray diffraction data and it is found that they show the wurtzite structure. The volume of unit cell decreases with increasing Cr concentration, it may be due to the smaller ionic radius of Cr ions as compared to Zn
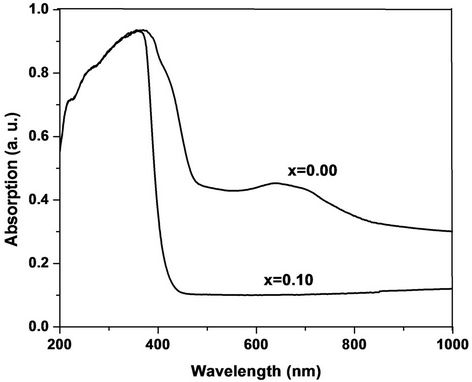
Figure 3. Absorption vs wavelength of Zn1–xCrxO samples.
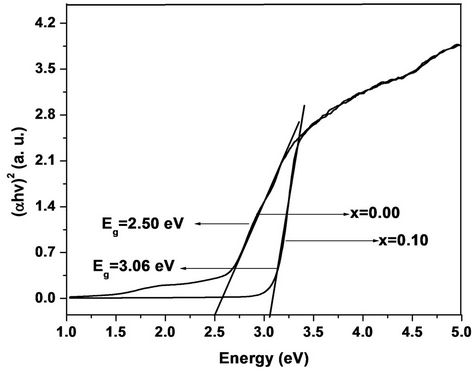
Figure 4. Energy vs (αhν)2 of Zn1–xCrxO samples.
ions. The grain size was calculated using Scherrer’s formula. It is found that the average grain size is in the range 11 - 17 nm. The chemical groups of the samples have been identified by FTIR spectra. The absorption peak is shifted towards low energy side as Cr content increases. It may be owing to the s-d and p interactions and smaller average grain size of Cr doped ZnO samples.
5. Acknowledgements
Authors would like to thank Dr. Mahavir Singh, Professor, Department of Physics, H. P. University, Shimla (H. P.) for providing facilities, Dr. S. S. Shah for his encouragement and University Grants Commission, New Delhi for financial support through project F-37-563/2009/SR.
REFERENCES
- M. Tanaka and Y. Higo, “Large Tunneling Magnetoresistance in GaMnAs/AlAs/GaMnAs Ferromagnetic Semiconductor Tunnel Junctions,” Physical Review Letters, Vol. 87, No. 2, 2001, pp. 26602-26606. doi:10.1103/PhysRevLett.87.026602
- S. J. Pearton, C. R. Abernathy, M. E. Overberg, G. T. Thaler, D. P. Norton, N. Theodoropoulou, A. F. Hebard, Y. D. Park, F. Ren, J. Kim and L. A. Boatner, “Wide Band Gap Ferromagnetic Semiconductors and Oxides,” Journal of Applied Physics, Vol. 93, No. 1, 2003, pp. 1-13. doi:10.1063/1.1517164
- Y. Z. Wang, H. Liu, Z. Q. Li, X. X. Zhang, R. K. Zheng and S. P. Ringer, “Role of Structural Defects on Ferromagnetism in Amorphous Cr-Doped TiO2 Films,” Applied Physics Letters, Vol. 89, No. 4, 2006, Article ID: 042511. doi:10.1063/1.2240139
- W. Z. Xu, Z. Z. Ye, Y. J. Zeng, L. P. Zhu, B. H. Zhao, L. Jiang, J. G. Lu, H. P. He and S. B. Zhang, “ZnO LightEmitting Diode Grown by Plasma-Assisted Metal Organic Chemical Vapor Deposition,” Applied Physics Letters, Vol. 88, No. 17, 2006, pp. 173506-173509. doi:10.1063/1.2199588
- Z. C. Tu and X. Hu, “Elasticity and Piezoelectricity of Zinc Oxide Crystals, Single Layers, and Possible Single-Walled Nanotubes,” Physical Review B, Vol. 74, No. 3, 2006, Article ID: 035434. doi:10.1103/PhysRevB.74.035434
- P. Mitra, A. P. Chatterjee and H. S. Maiti, “ZnO Thin Film Sensor,” Materials Letters, Vol. 35, No. 1-2, 1998, pp. 33-38. doi:10.1016/S0167-577X(97)00215-2
- G. Lawes, A. S. Risbud, A. P. Ramirez and R. Seshadri, “Absence of Ferromagnetism in Co and Mn Substituted Polycrystalline ZnO,” Physical Review B, Vol. 71, No. 4, 2005, Article ID: 045201. doi:10.1103/PhysRevB.71.045201
- A. S. Risbud, N. A. Spaldin, Z. Q. Chen, S. Stemmer and R. Seshadri, “Magnetism in Polycrystalline Cobalt-Substituted Zinc Oxide,” Physical Review B, Vol. 68, No. 20, 2003, pp. 205202-205209. doi:10.1103/PhysRevB.68.205202
- C. N. R. Rao and F. L. Deepak, “Absence of Ferromagnetism in Mnand Co-doped ZnO,” Journal of Materials Chemistry, Vol. 15, No. 5, 2005, pp. 573-578. doi:10.1039/b412993h
- S. Thota, T. Dutta and J. Kumar, “On the Sol-Gel Synthesis and Thermal, Structural, and Magnetic Studies of Transition Metal(Ni, Co, Mn) Containing ZnO Powders,” Journal of Physics: Condensed Matter, Vol. 18, No. 8, 2006, pp. 2473-2478. doi:10.1088/0953-8984/18/8/012
- K. Ueda, H. Tabata and T. Kawai, “Magnetic and Electric Properties of Transition-Metal-Doped ZnO Films,” Applied Physics Letters, Vol. 79, No. 7, 2001, pp. 988-990. doi:10.1063/1.1384478
- B. K. Roberts, A. B. Pakhomov, V. S. Shutthanandan and K. M. Krishnan, “Ferromagnetic Cr-Doped ZnO for Spin Electronics via Magnetron Sputtering,” Journal of Applied Physics, Vol. 97, No. 10, 2005, Article ID: 10D310- 3. doi:10.1063/1.1847914
- Z. Jin, T. Fukumura, M. Kawasaki, K. Ando, H. Saito, T. Sekiguchi, Y. Z. Yoo, M. Murakami, Y. Matsumoto, T. Hasegawa and H. Koinuma, “High through Put Fabrication of Transition-Metal-Doped Epitaxial ZnO Thin Films: A Series of Oxide-Diluted Magnetic Semiconductors and Their Properties,” Applied Physics Letters, Vol. 78, No. 24, 2001, pp. 3824-3826. doi:10.1063/1.1377856
- H. J. Lee, S. Y. Jeong, J. Y. Hwang and C. R. Cho, “Ferromagnetism in Li Co-Doped ZnO:Cr,” Europhysics Letters, Vol. 64, No. 6, 2003, pp. 797-802. doi:10.1209/epl/i2003-00628-6
- S. Kurian, S. Sebastian, J. Mathew and K. C. George, “Structural and Electrical Properties of Nano-Sized Magnesium Aluminate,” Indian Journal of Pure and Applied Physics, Vol. 42, No. 12, 2004, pp. 926-933.
- B. S. Rema Devi, R. Raveendran and A. V. Vaidyan, “Synthesis and Characterization of Mn2+-Doped ZnS Nanoparticles,” Pramana, Vol. 68, No. 4, 2007, pp. 679-687.
- S. Maensiri, P. Laokul and V. Promarak, “Synthesis and Optical Properties of Nanocrystalline ZnO Powders by a Simple Method Using Zinc Acetate Dihydrate and Poly (Vinyl Pyrrolidone),” Journal of Crystal Growth, Vol. 289, No. 1, 2006, pp. 102-106. doi:10.1016/j.jcrysgro.2005.10.145
- S. Suwanboon, “Structural and Optical Properties of Nanocrystalline ZnO Powder from Sol-Gel Method,” Science Asia, Vol. 34, No. 1, 2008, pp. 31-34. doi:10.2306/scienceasia1513-1874.2008.34.031
- B. N. Dole, V. D. Mote, V. R. Huse, Y. Purushotham, M. K. Lande, K. M. Jadhav and S. S. Shah, “Structural Studies of Mn Doped ZnO Nanoparticles,” Current Applied Physics, Vol. 11, No. 3, 2011, pp. 762-766. doi:10.1016/j.cap.2010.11.050
NOTES
*Corresponding author.

