Advances in Chemical Engineering and Science
Vol.07 No.02(2017), Article ID:75385,9 pages
10.4236/aces.2017.72015
Solubility Product of Ni-Struvite, NH4NiPO4∙6H2O, at 25˚C
Hans E. Lundager Madsen
Chemistry Department, University of Copenhagen, Copenhagen, Denmark

Copyright © 2017 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 22, 2017; Accepted: April 10, 2017; Published: April 13, 2017
ABSTRACT
Solubility product of a sparingly soluble salt is an important parameter in both pure and applied physical chemistry such as determination of values of thermodynamic functions or environmental implications of components of the substance. This paper presents the determination of the solubility product of ammonium nickel phosphate hexahydrate (Ni-struvite) at 25˚C by analysis of equilibria attained from both supersaturated and undersaturated solutions, i.e. precipitation and dissolution, respectively. Writing the dissolution process as NH4NiPO4・6H2O → NH3 + Ni2+ + 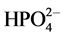 + 6 H2O, the value pKsp = 11.03 ± 0.03 is found for both precipitation and dissolution. The solubility is a little lower than that of the isomorphous Mg salt. This is to be expected from the lattice dimensions of the two phases, the crystals of Ni-struvite being slightly more compact.
+ 6 H2O, the value pKsp = 11.03 ± 0.03 is found for both precipitation and dissolution. The solubility is a little lower than that of the isomorphous Mg salt. This is to be expected from the lattice dimensions of the two phases, the crystals of Ni-struvite being slightly more compact.
Keywords:
Ammonium Nickel Phosphate, Ni-Struvite, Solubility Product

1. Introduction
Phosphates of divalent metals often show complex structures and great variability in chemical composition. Numerous examples were given in work by Bassett and Bedwell [1] . Their first paper in the series concerns monohydrogen phosphates as well as double salts with potassium or ammonium and from 0 to 7 mol of water of crystallization per mol of salt. An important representative is the biogenic mineral struvite, MgNH4PO4∙6H2O, typically found in guano [2] [3] .
A number of years ago we studied the crystallization of phosphates of some transition metals including Co, Ni and Cu and in particular double salts with ammonium [4] [5] . In this connection we needed, among others, the solubility product of ammonium nickel phosphate hexahydrate, NH4NiPO4∙6H2O:
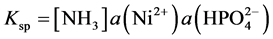 (1)
(1)
This compound is often named Ni-struvite, because it is isomorphous with struvite. Our way of writing the solubility product has been chosen for two reasons: 1) The activity coefficient of an uncharged species like NH3 is close to unity, and 2) 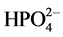 is the most abundant phosphate species in neutral and weakly basic solution, its concentration being orders of magnitude higher than that of
is the most abundant phosphate species in neutral and weakly basic solution, its concentration being orders of magnitude higher than that of  except at high pH. We then avoid involving the third dissociation constant of phosphoric acid, which is not known with so high precision as the two others.
except at high pH. We then avoid involving the third dissociation constant of phosphoric acid, which is not known with so high precision as the two others.
Solubility products of simple salts comprising only one type of cation and one type of anion are often found in standard tables [6] and databases [7] , whereas data for mixed salts are much more difficult to retrieve, if they exist at all. For unknown reasons we never published any details on the value for Ni-struvite quoted in our paper on crystal habit [4] , and nobody else seems to have done so. Recent literature, however, points to a certain interest in this and related substances in different fields as examplified by spectroscopy [8] , microbiology [9] and pollution control [10] [11] , where information on solubilities seems important for understanding the mechanism of incorporation of the mineral in microbes and evaluation as fertilizer struvite obtained from Ni-containing wastewater. We therefore found it worthwhile to reconsider the data and carry out a revision using new values of equilibrium constants.
2. Experimental
2.1. Apparatus
Colorimetric measurements were carried out on a Zeiss PM QII spectrophoto- meter. Precipitates were examined by optical microscopy either in situ, through the bottoms of the flasks, with a Zeiss Axiovert 25 inverted microscope, or ex situ with a Zeiss Jenapol polarizing microscope.
2.2. Reagents and Solutions
All reagents and solutions were prepared from Merck analytical grade chemicals except nickel chloride solution, which was prepared from Riedel-deHaën analytical grade nickel chloride hexahydrate. The water used for precipitation and dissolution experiments was demineralized water further purified by passing through an activated carbon filter and a Silhorko mixed-bed ion-exchange column. The conductivity of the water was close to that reported for pure water. Solutions for nickel determinations are described below under Analysis.
Ni-struvite for dissolution experiments was synthesized according to Bassett and Bedwell [1] . (NH4)2HPO4, 60 g, was dissolved in 1.8 L demineralized water in a 2-L crystallizer thermostatted at 25˚C. A solution of 12 g NiCl2・6H2O in 200 mL water was added under constant stirring at a rate of about 0.1 mL/min using a peristaltic pump. The crystallizer was left overnight at constant temperature and stirring. After filtration the following day on a glass filter the precipitate was washed with ethanol and acetone and dried in the air. The yield was 99% of that calculated from the amount of nickel chloride used. Microscopy revealed crystals of typical struvite morphology, i.e. somewhat elongated, hemihedral crystals belonging to the orthorhombic pyramidal class mm [4] [12] .
2.3. Precipitation and Dissolution Experiments
Precipitation experiments were carried out by mixing 25 mL of a nickel chloride solution with 25 mL of ammonium phosphate solution containing NH4H2PO4 and (NH4)2HPO4 in various proportions in a 100-mL Erlenmeyer flask. The flasks were covered with Parafilm and kept in the thermostat at 25˚C at least overnight. The precipitates were examined with the inverted microscope; if a precipitate did not consist entirely of well-developed crystals of the usual struvite morphology, that experiment was discarded. Otherwise samples of the clear supernatant solutions were withdrawn for nickel analysis.
Dissolution experiments were carried out by pouring 250 mL of either water, an ammonium dihydrogen phosphate solution or a mixed solution of ammonium phosphate and ammonium chloride over 0.5 g Ni-struvite in a polyethylene bottle with screw cap. The bottles were rotated end-over-end in a thermostat at 25˚C at least overnight. Afterwards, the bottles were left to stand in the thermostat until the solid had settled, and then samples were withdrawn for nickel analysis.
2.4. Analysis
Two different methods were used for determination of equilibrium concentrations of nickel. In the precipitation experiments nickel was determined by colorimetry on the dimethylglyoxime complex according to Mitchell and Mellon [13] . The reagents are, in the order added, a saturated aqueous solution of bromine, concentrated ammonia, 95% ethanol and a 0.1% alcoholic solution of dimethylglyoxime. Absorbance was measured at a wavelength of 440 nm. Calibration was made with nickel chloride solution standardized by complexometric titration as described below.
For nickel determination in dissolution experiments complexometric titration was applied, using the following reagents: EDTA disodium salt 0.05 M, methyl red indicator solution, NaOH 0.1 M, ammonia buffer pH 10 (70 g NH4Cl, 550 mL concentrated NH3, water up to 1 L), solid eriochrome black T indicator 1% in solid NaCl, and MgSO4 0.05 M. To a sample of the Ni-containing solution was added a known amount of EDTA in excess and a little methyl red. Any acidity was neutralized with NaOH, and an amount of ammonia buffer comprising about 10% of the total volume was added. A small amount of eriochrome black T indicator was added with a spatula, and the excess of EDTA was titrated with MgSO4 until color change from green to greyish or bluish; overtitration will result in red color. Standardization of EDTA and MgSO4 was carried out with precipitated CaCO3 (Merck analytical reagent) dissolved in HCl.
3. Calculations and Results
Total concentrations of ammonium and phosphate in the equilibrated solutions were calculated from initial concentrations and the amounts of Ni-struvite precipitated or dissolved, obtained from the results of nickel analyses. For each experiment the values of [NH3], a(Ni2+) and a(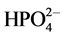 ) in Equation (1) were calculated from the known total concentrations of chloride, nickel, ammonium and phosphate and literature values for the equilibrium constants, i.e. dissociation constants of phosphoric acid and ammonium as well as stability constants of complexes of nickel ion with ammonia, phosphate and chloride [7] . The resulting system of nonlinear equations was solved by a Newton-Raphson iteration using a previously described computer program [14] , which also yields pH and ionic strength I. Activity coefficients of ions were calculated from I with the Debye-Hückel equation
) in Equation (1) were calculated from the known total concentrations of chloride, nickel, ammonium and phosphate and literature values for the equilibrium constants, i.e. dissociation constants of phosphoric acid and ammonium as well as stability constants of complexes of nickel ion with ammonia, phosphate and chloride [7] . The resulting system of nonlinear equations was solved by a Newton-Raphson iteration using a previously described computer program [14] , which also yields pH and ionic strength I. Activity coefficients of ions were calculated from I with the Debye-Hückel equation
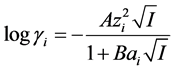 (2)
(2)
A and B are temperature-dependent parameters equal to 0.5115 and 0.3291, respectively, at 25˚C, and zi is the charge and 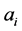 the radius of the ion in units of the electron charge and Å, respectively. Values of the latter are given in Kielland’s paper [15] ; those not found there are estimated from values for similar ions.
the radius of the ion in units of the electron charge and Å, respectively. Values of the latter are given in Kielland’s paper [15] ; those not found there are estimated from values for similar ions.
A total of 12 precipitation and 16 dissolution experiments were carried out. Of the latter, 3 results deviated so much from the mean that it was decided to make a statistical test for extreme deviations. The test yielded significant to highly significant deviation, so these results were not included in calculation of mean and standard deviation. One of the cases concerns the experiment with dissolution in pure water, yielding a solubility product significantly lower than the average. Probably the rate of dissolution in the absence of acidity is so low that the solution is not yet saturated at the time of sampling. Another cause could be uncertainty of the analysis, as the concentrations were very low. In the other two cases slight, but still significant deviation in the direction of higher solubility was found; this will be discussed below. In addition to the ordinary precipitation experiments we analyzed the mother liquor from Ni-struvite synthesis, so that the total adds up to 13 of each kind of experiment. In nine of the dissolution experiments only ammonium dihydrogen phosphate was added at concentrations ranging from 0.2 to 10 mM. Table 1 and Table 2 show the amounts of salts added in the ordinary precipitation experiments and in the dissolution experiments as well as equilibrium concentrations of nickel found by analyses of the saturated solutions.
In the mother liquor from the synthesis chloride concentration was 0.0504 M, and the equilibrium concentration of Ni was 2.3 µM. Ionic strength was high in this solution, I = 0.6, but otherwise the values ranged from 0.0015 to slightly below 0.1. In this range we can trust the validity of the Debye-Hückel Equation (2) for calculation of activity coefficients. Values of pKsp = −log Ksp are plotted
Table 1. Initial concentrations of salts added and equilibrium concentrations of Ni, all in mM, in precipitation experiments.
Table 2. Initial concentrations of salts added and equilibrium concentrations of Ni, all in mM, in dissolution experiments.
against calculated pH in Figure 1. Mean value and standard deviation of the whole sample are indicated on the graph.
A remarkable fact is that the result from the mother liquor, indicated with a filled symbol, does not deviate significantly from the rest in spite of the high ionic strength. As a whole, no significant dependence on ionic strength was found; regression analysis yielded a correlation coefficient << 1. The mean value with its standard deviation was found as
Figure 1. Solubility product of Ni-struvite at 25˚C versus calculated pH. Dashed line: mean value. Dotted lines: standard deviation. Filled symbol: value from mother liquor of synthesis.
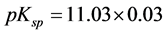
Finally we notice that the mean values for each of the two series of experi- ments are very similar, being 11.036 for precipitation and 11.034 for dissolution. Thus the general criterion for equilibrium, that the same value of the equilibrium constant should be found on approach from either side, is fulfilled in this case. This is by no means trivial, as the literature has many examples of a significantly higher value of Ksp from precipitation than from dissolution.
4. Discussion
The solubility of Ni-struvite is a little lower than that of struvite, for which we have found pKsp = 9.94 [16] . More recent research by other investigators [17] [18] [19] [20] [21] give values in the range 9.5 - 10.3 with an overall average of 9.99, i.e. not significantly different from our result and still below the value for Ni-struvite. The standard free energy change for the transformation
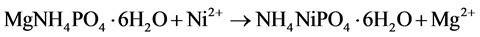
equals ΔG = −6.22 kJ/mol as found from our results. Table 3 shows calculated solubilities of the two solids in pure water as well as concentrations in a solution saturated with respect to both. The dependence of solubilities on pH is shown in Figure 2; it is supposed that pH is regulated by the addition of hydrochloric acid.
From further calculations using the literature value for the solubility product of the trinickel phosphate (TNP) Ni3(PO4)2∙8H2O [7] , it turned out that a saturated solution of Ni-struvite in pure water is highly supersaturated with respect to this compound. Some of the systems of the dissolution experiments of the present study, including those yielding deviating solubility products, were supersaturated with respect to TNP as well, though not so much. Crystallization
Figure 2. Calculated solubilities of struvite and Ni-struvite at 25˚C as functions of pH adjusted with HCl.
Table 3. Concentrations (mM) of saturated solutions in water of struvite, Ni-struvite and both.
of a new phase like TNP, which could explain deviating results for the solubility product, is more easily overlooked in dissolution than in precipitation with the procedures used in the present study.
Both metal ions, Mg2+ and Ni2+, are likely to be found as hexaquo species in both the solid phase and in solution except, for the latter, at high solution concentration of ammonia. Ni-struvite is isomorphous with struvite with all three axes a, b and c being shorter by 0.5% on the average [22] [23] . The electrostatic (Madelung) energy is thus slightly more negative for Ni-struvite, whence we should expect a lower solubility for this substance in agreement with observations. On the other hand, in view of the similarity of the structures, the possibility of mixed crystals with both Mg and Ni should not be neglected in evaluating the practical use of struvite precipitation in polluted systems [10] [11] .
5. Conclusion
The previously published value of pKsp [4] agrees with the present one within experimental uncertainty as demonstrated by the results presented above. The solubility product may thus be useful in estimates of dissolved nickel in soil and other systems containing ammonia and phosphate. Similar studies have been attempted with the analogous cobalt salt, but the strong color of Co(II) has turned out to be a problem in the analyses, so no reliable results have yet been obtained.
Acknowledgements
The work has been supported by grants from the Danish National Scientific Research Council and the Carlsberg Foundation for purchase of microscopes.
Cite this paper
Madsen, H.E.L. (2017) Solubility Product of Ni-Struvite, NH4NiPO4∙6H2O, at 25˚C. Advances in Chemical Engineering and Science, 7, 206- 214. https://doi.org/10.4236/aces.2017.72015
References
- 1. Bassett, H. and Bedwell, W.L. (1933) Studies of Phosphates. Part I. Ammonium Magnesium Phosphate and Related Compounds. Journal of the Chemical Society, 854-870.
https://doi.org/10.1039/jr9330000854 - 2. Dana, E.S. (1932) A Textbook of Mineralogy. 4th Edition, Wiley, New York, 718.
- 3. Nriagu, J.O. and Moore, P.B. (1984) Phosphate Minerals. Springer, Berlin, 113.
- 4. Abbona, F., Angela-Franchini, M., Croni Bono, C. and Lundager Madsen, H.E. (1994) Effect of Ammonia Excess on the Crystal Habit of NiNH4PO4 ·6H2O (Ni-Struvite). Journal of Crystal Growth, 143, 256-260.
- 5. Lundager Madsen, H.E. (2005) Crystal Growth Kinetics of Copper Phosphate from Acid Solution at 37°C. Journal of Crystal Growth, 275, e191-e196.
- 6. Smith, R.M. and Martell, A.E. (1976) Critical Stability Constants. Plenum, New York.
https://doi.org/10.1007/978-1-4757-5506-0 - 7. Martell, A.E., Smith, R.M. and Motekaitis, R.J. (1998, 2004) NIST Critically Selected Stability Constants of Metal Complexes Database. NIST Standard Reference Database, Vol. 46, Ver. 5.0 and 8.0, Standard Reference Data Program, National Institute of Standards and Technology, U.S. Department of Commerce, Gaithersburg.
- 8. Cahil, A., Najdoski, M. and Stefov, V. (2007) Infrared and Raman Spectra of Magnesium Ammonium Phosphate Hexahydrate (Struvite) and Its Isomorphous Analogues. IV. FTIR Spectra of Protiated and Partially Deuterated Nickel Ammonium Phosphate Hexahydrate and Nickel Potassium Phosphate Hexahydrate. Journal of Molecular Structure, 834, 408-413.
- 9. Haferburg, G., Kloess, G., Schmitz, W. and Kothe, E. (2008) ‘‘Ni-Struvite”—A New Biomineral Formed by a Nickel Resistant Streptomyces Acidiscabies. Chemosphere, 72, 517-523.
- 10. Uysal, A., Yilmazel, Y.D. and Demirer, G.N. (2010) The Determination of Fertilizer Quality of the formed Struvite from Effluent of a Sewage Sludge Anaerobic Digester. Journal of Hazardous Materials, 181, 248-254.
- 11. Ryu, H.-D., Lim, C.-S., Kang, M.-K. and Lee, S.-I. (2012) Evaluation of Struvite obtained from Semiconductor Wastewater as a Fertilizer in Cultivating Chinese Cabbage. Journal of Hazardous Materials, 221-222, 248-255.
- 12. Abbona, F. and Boistelle, R. (1978) Growth Morphology and Crystal Habit of Struvite Crystals (MgNH4PO4 6H2O). Journal of Crystal Growth, 46, 339-354.
- 13. Mitchell, A.M. and Mellon, M.G. (1945) Colorimetric Determination of Nickel with Dimethylglyoxime. Industrial and Engineering Chemistry Analytical Edition, 17, 380-382.
https://doi.org/10.1021/i560142a012 - 14. Berland, Y., Olmer, M., Grandvuillemin, M., Lundager Madsen, H.E. and Boistelle, R. (1988) In Vitro and Clinical Study of Oxalate Influence on Calcium Oxalate Crystal Formation. Journal of Crystal Growth, 87, 494-506.
- 15. Kielland, J. (1937) Individual Activity Coefficients of Ions in Aqueous Solutions. Journal of the American Chemical Society, 59, 1675-1678.
https://doi.org/10.1021/ja01288a032 - 16. Abbona, F., Lundager Madsen, H.E. and Boistelle, R. (1982) Crystallization of Two Magnesium Phosphates, Struvite and Newberyite: Effect of pH and Concentration. Journal of Crystal Growth, 57, 6-14.
- 17. Aage, H.K., Andersen, B.L., Blom, A. and Jensen, I. (1997) The Solubility of Struvite. Journal of Radioanalytical and Nuclear Chemistry, 223, 213-215.
https://doi.org/10.1007/BF02223387 - 18. Babic-Ivancic, V., Kontrec, V.J., Kralj, D. and Brecevic, L. (2002) Precipitation Diagrams of Struvite and Dissolution Kinetics of different Struvite Morphologies. Croatica Chemica Acta, 75, 89-106.
- 19. Michalowski, T. and Pietrzyk, A. (2006) A Thermodynamic Study of Struvite plus Water System. Talanta, 68, 594-601.
- 20. Bhuiyan, M.I.H., Mavinic, D.S. and Beckie, R.D. (2007) A Solubility and Thermodynamic Study of Struvite. Environmental Technology, 28, 1015-1026.
https://doi.org/10.1080/09593332808618857 - 21. Hanhoun, M., Montastruc, L., Azzaro-Pantel, C., Biscans, B., Frèche, M. and Pibouleau, L. (2011) Temperature Impact Assessment on Struvite Solubility Product: A Thermodynamic Modeling Approach. Chemical Engineering Journal, 167, 50-58.
- 22. Abbona, F., Calieri, M. and Ivaldi, G. (1984) Synthetic Struvite, MgNH4PO4 6H2O: Correct Polarity and Surface Features of some Complementary Forms. Acta Crystallographia B, 40, 223-227.
https://doi.org/10.1107/S0108768184002020 - 23. Blachnik, R., Wiest, T., Dülmer, A. and Reuter, H. (1997) The Crystal Structure of Ammonium Hexaaquanickel (II) Phosphate. Zeitschrift für Kristallographie, 212, 20-23.
https://doi.org/10.1524/zkri.1997.212.1.20




