Advances in Chemical Engineering and Science
Vol. 2 No. 3 (2012) , Article ID: 20832 , 7 pages DOI:10.4236/aces.2012.23044
Recovery of Pentachlorophenol from Aqueous Solution via Silicone Rubber Membrane
Faculty of Applied Bioscience, Kanagawa Institute of Technology, Atsugi, Japan
Email: *sawai@bio.kanagawa-it.ac.jp
Received April 17, 2012; revised May 18, 2012; accepted May 29, 2012
Keywords: Persistent Organic Pollutants (POPs); Soil Pollution; Membrane Separation; Polymer Membrane; Partition Coefficient
ABSTRACT
Although pentachlorophenol (PCP) has been widely employed as a biocide for over 60 years, its production and use are currently severely curtailed in many countries due to its extreme toxicity. In recent years, the contamination of both soil and surface waters by PCP has become a concern. In this study the permeation characteristics of PCP penetrating silicone rubber membranes (SRM) were studied, in order to determine the feasibility of separation of PCP from water via the permeation and chemical desorption (PCD) method. It was found that efficient separation and recovery of PCP could be obtained using an acidic feed solution and an alkaline recovery solution. The permeation rate of PCP into the SRM was optimized when the feed solution was maintained at a pH of 4 or lower. The SRM thickness did not significantly affect the permeation rate, indicating that the rate determining step for the process is the initial movement of the PCP into the SRM. The activation energy for the penetration process was determined to be quite high, and thus thermal controls will play an important role in the recovery of PCP by this method. The membrane distribution coefficient (mc) for PCP moving into SRM was large and showed a strong correlation to permeation rates reported previously, confirming that PCD is a suitable technique for the separation and recovery of PCP from aqueous solution.
1. Introduction
Pentachlorophenol (PCP), or its sodium salt, has been extensively used since the 1930’s as a herbicide, algaecide, germicide, fungicide, molluscicide, defoliant and wood preservative, due to its action as a potent biocide [1-3]. In addition to its innate toxicity, technical or commercial grade PCP also contains approximately ten percent impurities, consisting of several potentially hazardous chlorinated aromatic compounds, primarily the more highly chlorinated dibenzo-p-dioxin and dibenzofuran congeners [4]. In the 1970s, the toxicity of PCP towards the liver and kidney was confirmed and its reproductive and developmental toxicities were also reported [5]. Following this, between 1978 and 1984, many countries either restricted or banned the production and use of PCP, due to its potential adverse effects on human health [6]. In the 1990s, the endocrine disrupting effects of PCP were also recognized [7,8]. Although it is now largely banned, PCP is still commonly found as a contaminant in air, water and soil worldwide, due to its widespread use in the past [6,9]. Remediation of contaminated sites is complicated by the fact that chlorinated phenols, such as PCP, are chemically stable. Although there have been attempts to remove PCP from soil by bioremediation, such treatment requires a very long duration and typically does not produce acceptably clean sites [10-12]. Thus the development of improved treatment technologies for the remediation of PCP-contaminated soil and water is of interest.
We have previously investigated the permeation and chemical desorption (PCD) methodology for the separation and recovery of pollutants, using nonporous materials such as silicone rubber membranes (SRM) [13,14]. In the PCD method, two solutions with different chemical properties are separated by a nonporous membrane. The compound to be recovered (in the so-called feed side solution) has significant affinity for the membrane material and penetrates through the membrane. Upon exiting to the recovery side solution, this same compound is chemically modified such that it no longer has an affinity for the membrane and is thus trapped. To date, this technique has been demonstrated to be effective in the recovery of various contaminants including iodine, phenols and anilines [13-18]. Both 4-substituted phenols and anilines have been recovered from aqueous solutions using either NaOH or HCl, respectively, for neutralization [15]. A comparison of the relative efficiencies of the PCD and pervaporation (PV) methods has been reported, using a tube-type apparatus. The removal rates of phenols by the PCD method were much greater than those by the PV method, demonstrating the efficient separation and recovery of compounds with low-volatility via PCD [15]. The rate at which phenols and anilines permeate into the SRM, the most important step in the PCD method, has been found to be well correlated to their concentration in the membrane [16]. Livingston et al. have also described a membrane aromatic recovery system (MARS) for recovering anilines and phenols using an SRM [17,18]. The MARS process operates on a very similar principle to that of the PCD method. Recently, the successful scale-up and operation of the MARS process following pilot-plant trials have been reported [19].
In this study the permeation characteristics of PCP through SRMs using the PCD method were investigated, as a first step in developing the technology to separate and remove PCP contamination in water and soil.
2. Materials and Methods
2.1. PCD Method
Figure 1 illustrates the basic principles of the PCD method. A solution of PCP dissolved in an acidic solvent is placed in the feed cell, while the recovery cell is filled with an alkaline solution. Dissolved PCP molecules in the feed solution, being protonated at the hydroxyl group and thus uncharged, will tend to penetrate into the hydrophobic SRM. Once these PCP molecules permeate the membrane and emerge in the alkaline recovery solution, the hydroxyl group of the molecule is deprotonated to produce the charged phenolate anion (ROH → RO−). This charged phenolate species is poorly adsorbed by the SRM, thus does not tend to migrate back to the feed cell. As a consequence, PCP dissolved in an acidic solution in the feed cell, with an alkaline solution in the recovery cell, is eventually concentrated to the recovery cell.
2.2. Experimental Procedure
Figure 1 illustrates the flat membrane apparatus assembled by us for this study. The SRM (A.S. One Co. Ltd., Osaka, Japan) was fixed between two cells made of glass
(capacity of 250 ml) and held in place by a flange. The thickness of the SRM varied from 0.05 to 0.3 mm, and had an effective area of 1.7 × 10−3 m2. The membrane material used was confirmed to be polydimethylsiloxane with fumed silica filler by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR). A 0.04 mM solution of PCP (Wako Pure Chemicals Co. Ltd., Osaka, Japan) was prepared in distilled water. The pH of this PCP solution was adjusted to the desired value using either dilute HCl or NaOH (0.1 M) and 250 ml of this PCP solution was then transferred to the feed side cell. In a previous study [15], the permeation rate of phenols to the SRM was optimized when the recovery solution concentration of NaOH was at least 50 times greater than that of the feed solution of the phenols. For this reason, 250 ml of 20 mM NaOH was added to the recovery side cell, ensuring that the concentration of the deprotonating species is significantly greater than that of the target compound PCP. The solutions on both sides of the membranes are constantly stirred via magnetic stir bars.
High performance liquid chromatography (HPLC) with UV detection was used to measure the PCP concentrations in the cells. Samples of 1 ml were withdrawn from both cells at regular intervals and their PCP concentrations were determined under the conditions listed in Table 1. All work was conducted with the experiment-
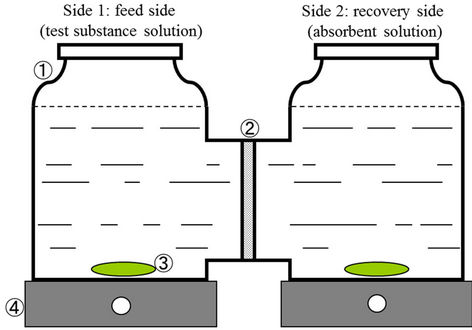
Figure 1. The PCD apparatus. The apparatus was contained in a thermostatic chamber at ambient pressure. Legend: (1) Glass cell; (2) Silicone membrane; (3) Stir bar; (4) Stir plate.
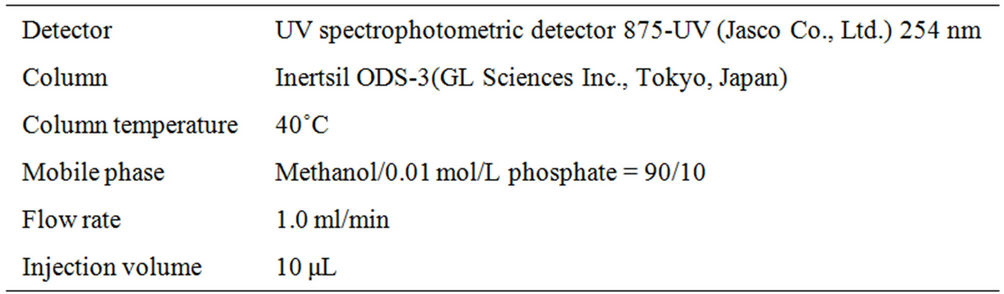
Table 1. HPLC measurement conditions.
tal apparatus contained in a thermostatic chamber.
2.3. Measurement of Membrane Distribution Coefficient mc
A 3 cm square section of 0.05 mm thick SRM was immersed for 24 h in a weighed bottle containing 10 ml of PCP solution of concentration ranging from 0.1 to 0.4 mM. A duration of 24 h was used since previous work had demonstrated that transport equilibrium through the membrane was reached after this time period. The equilibrium concentration of PCP (C1) was then determined by HPLC. The SRM was subsequently removed from the solution, drained well, and immersed for 24 h in 20 mM NaOH after which the concentration of PCP released from the SRM into the NaOH solution (C2) was determined by HPLC. The concentration of PCP in the SRM (C3) was estimated by:
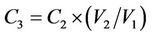 (1)
(1)
where V1 and V2 are the volumes of the SRM and NaOH solution, respectively. The membrane distribution coefficient, mc, was obtained by dividing C3 into C1. These distribution experiments were performed at 25˚C.
3. Results and Discussion
3.1. pH of the Feed Solution
The permeation of PCP through the SRM, with a NaOH solution in the recovery cell, was investigated using different pH values of the PCP solution in the feed cell. Figure 2 shows the percent PCP recovery at different feed cell pH values after 6 h when using 20 mM NaOH as the recovery solution. The SRM thickness was 0.05 mm, and a temperature of 25˚C was used. The recovery remained constant up to pH 4, with almost 90% of the PCP migrating to the recovery side after 6 h. The recovery decreased with further increases in pH, and little permeation was observed at pH 10 or higher.
As noted, when a phenol penetrates the SRM from the feed cell and enters the alkaline solution in the recovery cell, the phenol (R-OH) deprotonates to form the phenolate anion (R-O−). The associated electrolytic dissociation constant, Ka, can be expressed as follows:
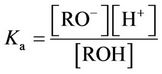 (2)
(2)
Using Equation (2), the molar ratio of non-ionized phenol to the combined sum of non-ionized and ionized phenol (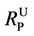 ) can be calculated at any value of pH as follows:
) can be calculated at any value of pH as follows:
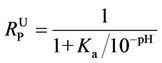 (3)
(3)
According to the Hazardous Substances Data Bank [20], the value of pKa for PCP is 4.47. The values of  calculated based on Equation (3) are shown as a solid line in Figure 2. This line demonstrates that PCP is essentially all in the non-ionized state below pH 4 and, conversely, is completely ionized above pH 8. The variation in percent recovery is closely correlated with that of the calculated values of
calculated based on Equation (3) are shown as a solid line in Figure 2. This line demonstrates that PCP is essentially all in the non-ionized state below pH 4 and, conversely, is completely ionized above pH 8. The variation in percent recovery is closely correlated with that of the calculated values of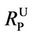 .
.
These results indicate that non-ionized PCP in the feed solution will dissolve and penetrate the SRM, whereas the ionized form (the phenolate) will not, since the negatively charged phenolate is poorly adsorbed by the mem-
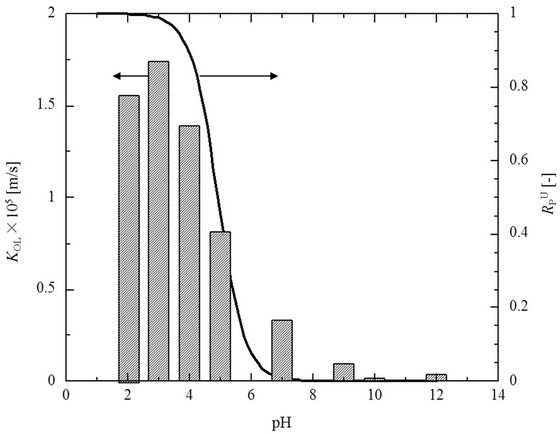
Figure 2. The variation of overall mass transfer coefficient (KOL) and electrolytic dissociation of PCP (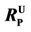 obtained from Equation (4)) with pH (SRM thickness 0.05 mm, feed side cell pH 2, 25˚C).
obtained from Equation (4)) with pH (SRM thickness 0.05 mm, feed side cell pH 2, 25˚C).
brane material. As a consequence, placing an acidic solution of PCP in the feed cell with an alkaline solution in the recovery cell creates an efficient system for the recovery of PCP. As noted, such a system spontaneously works to concentrate PCP on the recovery side, since phenolate in the recovery cell cannot return to the feed side through the SRM. The phenolate thus concentrated in the recovery cell can be subsequently collected by neutralization operation.
3.2. pH of the Recovery Solution and Membrane Permeation of PCP
Figure 3 shows typical changes in PCP concentration in the feed solution as a function of time. The PCP concentration, as expressed by ln(C/C0), decreases linearly with time as the phenol penetrates through the membrane from the feed side to the recovery side. Assuming that the amount of deprotonating species on the recovery side (the NaOH) is in large excess compared to the target compound (PCP), and that the concentration of non-ionized PCP on the recovery side is zero, the PCP concentration (C) on the feed side can be represented by [21, 22]:
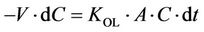 (4)
(4)
where A, V and KOL are the effective membrane area, liquid volume in the feed cell and overall mass transfer coefficient, respectively. Integrating Equation (4) under the initial conditions of t = 0 and C = C0, gives:
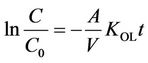 (5)
(5)
This equation suggests that ln(C/C0) will be linearly proportional to t and Figure 3 in fact shows such a rela-
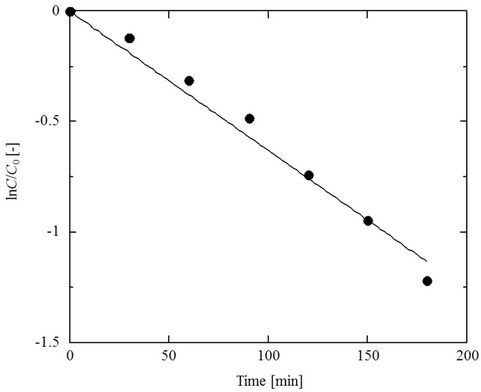
Figure 3. The variation of feed side PCP concentration over time in the PCD apparatus (SRM thickness 0.05 mm, feed side cell pH 2, 25˚C).
tionship. A similar tendency was observed under other conditions, allowing determination of KOL from the slopes of the fitted lines.
3.3. Effect of SRM Thickness
Figure 4 shows variations in the PCP transfer coefficient KOL as a function of the SRM thickness. The effect of increasing membrane thickness is moderate; even when the thickness of the SRM is multiplied six fold, the decrease in KOL is only about 15%. In practical applications of this method, where water used to wash PCP-contaminated soil will produce the feed solution, the durability of the membrane will become a very important factor. The apparent minimal impact of membrane thickness on the permeation rate will allow the design of the process so as to place a priority on the durability of the membrane, which will be very advantageous in practice.
3.4. Effect of Temperature
The temperature dependence of the membrane permeability (P) of rubbery polymers such as SRM above their glass transition temperature is described by the van’t Hoff-Arrhenius equation [23]:
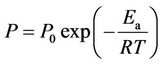 (5)
(5)
where P0, Ea, R and T are the constant specific to the polymer, the activation energy for the permeation, the gas constant and temperature, respectively. Specific values of P for PCP permeation were obtained by employing the resistances-in-series model, which has been used to describe the transport of molecules through a membrane with liquid films on either side. In this model, the term 1/KOL represents the overall mass transfer resistance
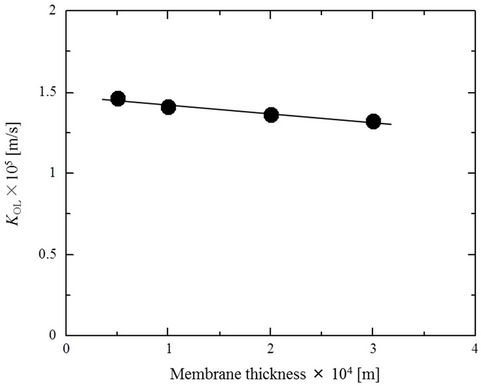
Figure 4. SRM thickness effects on the overall PCP mass transfer coefficient (KOL) (feed side cell pH 2, 25˚C).
and can be written as:
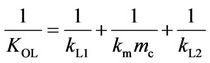 (6)
(6)
where kL1 and kL2 are the liquid boundary film mass transfer coefficients of the feed and recovery sides, respectively, and km and mc are the mass transfer coefficient in the membrane and the membrane/aqueous solution distribution coefficient, respectively. When chemical absorption occurs as described for this method, the mass transfer resistance of the recovery side (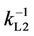 ) is negligible [21-23] and km relates to diffusivity, D, and film membrane thickness, d, as shown in Equation (7).
) is negligible [21-23] and km relates to diffusivity, D, and film membrane thickness, d, as shown in Equation (7).
 (7)
(7)
Equation (6) can thus be re-written as:
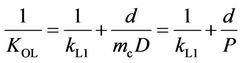 (8)
(8)
where P = mcD. Equation (8) describes a linear relation between d and 1/KOL. When values of 1/KOL are plotted against d, the value of P can therefore be obtained from the slope of the line.
Figure 5 shows the manner in which calculated P values for PCP vary with temperature (the van’t HoffArrhenius plot). The value of P increases with temperature, due to temperature-enhanced mobility of the polymer chains which allows the PCP molecules to more readily diffuse into the membrane. A suitably linear relationship was obtained for the data (R2 = 0.93) with a calculated value for activation energy (Ea) of 92 kJ/mol. Compared to the respective Ea values of 17 and 15.6 kJ/mol reported previously for phenol and 4-chloroaniline [17,18], the Ea value determined for PCP appears very high. The penetration of PCP into SRM is thus quite
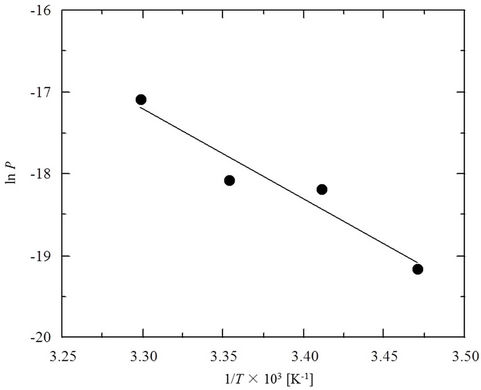
Figure 5. Arrhenius plot for the permeation of PCP into SRM (SRM thickness 0.05 mm, feed side cell pH 2).
temperature sensitive, indicating that temperature control will be a very important operational factor in this process.
3.5. Distribution Coefficient of PCP in the SRM mc
Previously, it was determined that the permeation rate (KOL) for 4-substituted phenols and anilines correlated with the logarithm of the distribution coefficient (logmc) [16]. In this research, the mc value for PCP moving through SRM was determined experimentally. The value of logmc for PCP was found to be 2.6 at pH 2 and it was further determined that this value did not change when the initial PCP concentration in the feed solution was varied over a range of 0.01 to 0.04 mM. The value of logmc thus obtained for PCP was compared with those derived for phenols and anilines in the previous study [16] in order to determine if there was a correspondence.
A good linear relationship between KOL and logmc is evident in Figure 6. The derived value of logmc for PCP fits well into the extrapolation of the straight line produced from previous data (R2 = 0.94). This indicates that the membrane distribution coefficient is a very important factor in regard to permeation of the SRM in this technique. The mc value of PCP is obviously relatively high, indicating that PCP moves readily into the SRM. As noted earlier, however, deprotonation of PCP in the recovery cell prevents reverse migration back through the SRM.
Pervaporation has also been able to separate volatile compounds, such as alcohols, on a large scale by the ap-
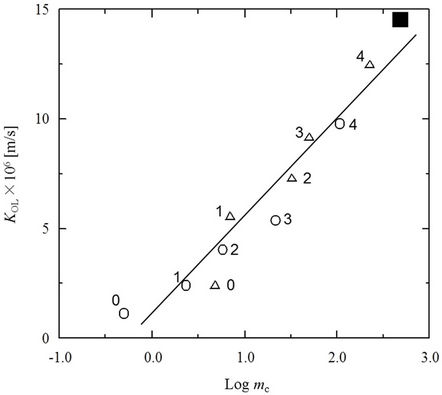
Figure 6. Variation of overall mass transfer coefficient (KOL) with membrane distribution coefficient (mc) for phenols (○), anilines (△) and PCP (■). (The values of KOL for phenols and anilines were obtained in a previous study [16]. The numbers 0 - 4 refer to the number of carbons in the alkyl chain of the 4-substituted phenol and aniline derivatives.
plication of vacuum to the recovery side of the process [24-27]. However, this method has not been widely applied for the industrial scale remediation of low volatile organic pollutants in water. Furthermore, results to date [15] have demonstrated that the recovery of phenols by the PCD method proceeds at a significantly greater rate as compared to removal by the PV method, suggesting that PCD is a superior technique for the separation and recovery of PCP.
4. Conclusions
After The permeation characteristics of PCP migrating into silicone rubber membranes were investigated in an attempt to better understand the utility of applying the PCD method to PCP remediation. The results of this work lead to the following conclusions:
1) A system comprised an acidic PCP solution contained in a feed cell, combined with an alkaline solution in the recovery cell, offers an efficient process for the recovery of PCP;
2) The apparent minimal effect of membrane thickness on the permeation rate will allow process design incorporating a suitable degree of membrane durability which offers the advantage of a robust system;
3) The penetration rate of PCP into the SRM is highly temperature sensitive, indicating that temperature control will be a very important operational factor in this process;
4) The mc value for PCP moving into the SRM is relatively high, an indicator that PCD offers a suitable and efficient means for the separation and recovery of PCP.
After PCP has been removed from aqueous solution by PCD, it may be collected by neutralization of the recovery solution. This PCD process demonstrably allows the efficient recovery of PCP and is of significant interest and deserving of further study. Our group is pursuing further work in this field and has initiated a pilot study investigating the use of a silicone hollow-fiber membrane manufactured from the same silicone rubber used in this study.
5. Acknowledgements
This study was supported by the City Area Program 2004 of the Ministry of Education, Culture, Sport, Science and Technology.
REFERENCES
- U. Heudorf, S. Letzel, M. Peters and J. Anger, “PCP in the Blood Plasma: Current Exposure of the Population in Germany, Based on Data Obtained in 1998,” International Journal of Hygiene and Environmental Health, Vol. 203, No. 2, 2000, pp. 135-139. doi:10.1078/S1438-4639(04)70018-8
- J. Ge, J. Pan, Z. Fei, G. Wu and J. P. Giesy, “Concentrations of Pentachlorophenol (PCP) in Fish and Shrimp in Jiangsu Province, China,” Chemosphere, Vol. 69, No. 1, 2007, pp. 164-169. doi:10.1016/j.chemosphere.2007.04.025
- H. J. Geyer, I. Scheunert and F. Korte, “Distribution and Bioconcentration Potential of the Environmental Chemical Pentachlorophenol (PCP) in Different Tissues Humans,” Chemosphere, Vol. 16, No. 4, 1987, pp. 887-899. doi:10.1016/0045-6535(87)90022-1
- B. A. Schwetz, P. A. Keeler and P. J. Gehring, “The Effect of Purified and Commercial Grade Pentachlorophenol on Rat Embryonal and Fetal Development,” Toxicology and Applied Pharmacology, Vol. 28, No. 1, 1974, pp. 151-161. doi:10.1016/0041-008X(74)90140-9
- World Health Organization, “Pentachlorophenol Environmental Health Criteria 77,” International Programme on Chemical Safety, Geneva, 1987.
- W. Zheng, X. Wang, H. Yu, X. Tao, Y. Zhou and W. Qu, “Global Trends and Diversity in Pentachlorophenol Levels in the Environment and in Humans: A Meta-Analysis,” Environmental Science & Technology, Vol. 45, No. 11, 2011, pp. 4668-4675. doi:10.1021/es1043563
- K. J. van den Berg, “Interaction of Chlorinated Phenols with Thyroxine Binding Sites of Human Transthyretin, Albumin and Thyroid Binding Globulin,” Chemico-Biological Interactions, Vol. 76, No. 1, 1990, pp. 63-75. doi:10.1016/0009-2797(90)90034-K
- O. Frances, L. Ilka, K. Werner and R. Edwin, “Endocrine Disrupting Effects of Herbicides and Pentachlorophenol; in Vitro and in Vivo Evidence,” Environmental Science & Technology, Vol. 43, No. 6, 2009, pp. 2144-2150. doi:10.1021/es8028928
- J. Qu and M. Fan, “The Current State of Water Quality and Technology Development for Water Pollution Control in China,” Critical Reviews in Environmental Science and Technology, Vol. 40, No. 6, 2010, pp. 519-560. doi:10.1080/10643380802451953
- H. B. Lee and T. E. Peart, “Organic Contaminants in Canadian Municipal Swage Sludge. Part I. Toxic or Endocrine-Disrupting Phenolic Compounds,” Water Quality Research Journal of Canada, Vol. 37, No. 4, 2002, pp. 681-696.
- S. T. Chen and P. M. Berthouex, “Use of an Anaerobic Sludge Digestion Process to Treat Pentachlorophenol (-PCP-) Contaminated Soil,” Journal of Environmental Engineering, Vol. 129, No. 12, 2003, pp. 1112-1119. doi:10.1061/(ASCE)0733-9372(2003)129:12(1112)
- M. Walter, K. S. H. Boyd-Wilson, D. McNaughton and G. Northcott, “Laboratory Trials on the Bioremediation of Aged Pentachlorophenol Residues,” International Biodeterioration & Biodegradation, Vol. 55, No. 2, 2005, pp. 121-130. doi:10.1016/j.ibiod.2004.09.002
- M. Kikuchi, K. Sato and T. Minami, Japan Patent No. 293472, 2001.
- M. Kikuchi, K. Sato and T. Minami, Japan Patent No. 331228, 2002.
- J. Sawai, N. Ito, T. Minami and M. Kikuchi, “Separation of Low Volatile Organic Compounds, Phenol and Aniline Derivatives, from Aqueous Solution Using Silicone Rubber Membrane,” Journal of Membrane Science, Vol. 252, No. 1-2, 2005, pp. 1-7. doi:10.1016/j.memsci.2004.06.018
- J. Sawai, K. Higuchi, T. Minami and M. Kikuchi, “Removal and Permeation Characteristics of 4-Substituted Phenol and Aniline Derivatives in Aqueous Solution Using a Silicone Rubber Membrane,” Chemical Engineering Journal, Vol. 152, No. 1, 2009, pp. 133-138. doi:10.1016/j.cej.2009.04.003
- S. Han, F. Castelo and A. G. Livingston, “Membrane Aromatic Recovery System (MARS)—A New Membrane Process for the Recovery of Phenols from Wastewaters,” Journal of Membrane Science, Vol. 188, No. 1-3, 2001, pp. 219-233. doi:10.1016/S0376-7388(01)00377-5
- F. C. Ferreira, S. Han and A. G. Livingston, “Recovery of Aniline from Aqueous Solution Using the Membrane Aromatic Recovery System,” Industrial & Engineering Chemistry Research, Vol. 41, No. 11, 2002, pp. 2766- 2774. doi:10.1021/ie010746l
- F. C. Ferreira, S. Han, A. Boam, S. Zhang and A. G. Livingston, “Membrane Aromatic Recovery System (MARS): Lab Bench to Industrial Pilot Scale,” Desalination, Vol. 148, No. 1-3, 2002, pp. 267-273. doi:10.1016/S0011-9164(02)00709-9
- US National Library of Medicine, “Hazardous Substances Data Bank (HSDB) 2000-32,” 1989. http://qsar.cerij.or.jp/SHEET/S2000_32.pdf
- N. Watanabe and T. Miyauchi, “The Permeation of Iodine through a Diaphragm Type Liquid Membrane—The Diffusion Coefficient of Iodine in Poly (Dimethylsiloxane),” Kagaku Kogaku Ronbunshu, Vol. 2, No. 3, 1976, pp. 262- 265. doi:10.1252/kakoronbunshu.2.262
- M. Imai, S. Furisaki and T. Miyauchi, “Separation of Volatile Materials by Gas Membrane,” Industrial & Engineering Chemistry Process Design and Research, Vol. 21, No. 3, 1982, pp. 421-426. doi:10.1021/i200018a013
- S. C. George, M. Knörgen and S. Thomas, “Effect of Nature and Extent of Crosslinking on Swelling and Mechanical Behavior of Styrene-Butadiene Rubber Membranes,” Journal of Membrane Science, Vol. 163, No. 1, 1999, pp. 1-17. doi:10.1016/S0376-7388(99)00098-8
- K. W. Boddekker, G. Bengtson and E. Bode, “Pervaporation of Low Volatility Aromatics from Water,” Journal of Membrane Science, Vol. 53, No. 1-2, 1990, pp. 143-158. doi:10.1016/0376-7388(90)80010-J
- M. Hoshi, M. Kogre, T. Saitoh and T. Nakagawa, “Separation of Aqueous Phenol through Polyurethane Membranes by Pervaporation,” Journal of Applied Polymer Science, Vol. 65, No. 3, 1997, pp. 469-479. doi:10.1002/(SICI)1097-4628(19970718)65:3<469::AID-APP6>3.0.CO;2-F
- P. Wu, R. W. Feild, R. England and B. J. Brisdon, “A Fundamental Study of Organofunctionalised PDMS Membranes for the Pervaporative Recovery of Phenolic Compounds from Aqueous Streams,” Journal of Membrane Science, Vol. 190, No. 2, 2001, pp. 147-157. doi:10.1016/S0376-7388(01)00408-2
- M. Czaplicka, “Photo-Degradation of Chlorophenols in the Aqueous Phase Solution,” Journal of Hazardous Materials, Vol. 134, No. 1-3, 2006, pp. 45-59. doi:10.1016/j.jhazmat.2005.10.039
NOTES
*Corresponding author.

