Advances in Chemical Engineering and Science
Vol. 2 No. 1 (2012) , Article ID: 16703 , 12 pages DOI:10.4236/aces.2012.21008
High-Resolution 1H NMR Investigations of the Oxidative Consumption of Salivary Biomolecules by a Tooth-Whitening Formulation: Relevance to Safety Issues
1Institute for Materials Research and Innovation (IMRI), University of Bolton, Bolton, UK
2Warwick Dentistry, Warwick Medical School, University of Warwick, Warwick, UK
Email: *mg6@bolton.ac.uk
Received July 27, 2011; revised September 19, 2011; accepted November 3, 2011
Keywords: Biomedical 1H NMR Analysis; Human Saliva; Tooth-Whitening Product; Hydrogen Peroxide; Salivary Antioxidants
ABSTRACT
A multicomponent evaluation of the oxidative consumption of salivary biomolecules by a hydrogen peroxide (H2O2)- containing tooth-whitening formulation has been performed using high-resolution proton (1H) nuclear magnetic resonance (NMR) spectroscopy. Unstimulated human saliva samples (n = 10) were treated with aliquots of supernatants derived from 1) the H2O2-containing whitening gel, 2) the corresponding tooth-whitening accelerant solution containing an amino-alcohol activator, and 3) a combination of these product agents pre-mixed in the recommended manner. 600 MHz 1H NMR spectra acquired on these samples demonstrated that H2O2 present in the whitening gel gave rise to the oxidative decarboxylation of salivary pyruvate (to acetate and CO2), the direct oxidation of trimethylamine and methionine (to trimethylamine-N-oxide and methionine sulphoxide respectively), and the indirect oxidative consumption of lactate and carbohydrates in general. Experiments conducted on a chemical model system confirmed the consumption of pyruvate by added H2O2, and also revealed that this method could be employed for determinations of the H2O2 content of tooth-whitening products. In conclusion, high-resolution 1H NMR analysis provides much valuable molecular information regarding the fate of tooth-whitening oxidants in human saliva, and also permits an assessment of the ability of endogenous antioxidants therein to protect against any soft tissue damage arising from the possible leakage of H2O2 from tooth-whitening application trays.
1. Introduction
The incorporation of hydrogen peroxide (H2O2), carbamide peroxide (CP) and peroxo-adducts of inorganic anions such as peroxoborate, peroxodisulphate and peroxocarbonate as agents for the bleaching of discoloured teeth in commercially-available tooth-whitening products such as gels, toothpastes and oral rinses has evoked much interest regarding their modes of action, redox activity in oral environments, and adverse effects and safety considerations [1,2]. However, our understanding of the capacities of salivary biomolecules to chemically react with (and hence neutralise) such peroxide-based tooth-whitening oxidants remains limited. In principal, salivary biomolecules which can act as scavengers of H2O2 (or other peroxo-adducts) serve to protect oral soft tissues against any deleterious effects exertable by these oxidants which are sometimes prone to escape from customised application trays or alternative application devices and hence make contact with these and further sensitive oral environments.
Moreover, the analysis of salivary biomolecules by conventional methods (for example, those involving gas or high-performance liquid chromatographic techniques) generally requires the time-consuming, labour-intensive determination of pre-selected components. Indeed, these methods also require much information regarding the identities of particular salivary biomolecules prior to analysis, and hence generally offer only a limited characterisation of the redox reactivity of H2O2 and/or related peroxo-adducts.
The multicomponent analytical ability of high-resolution nuclear magnetic resonance (NMR) spectroscopy, however, offers major advantages over the above conventional techniques (reviewed in [3,4]). Indeed, the development of high-field NMR spectrometers with increased resolution, dynamic range and sensitivity has given rise to rapid advances in the analysis of complexmulticomponent samples such as human biofluids (intact or otherwise), pharmaceutical formulations, dentifrices and foodstuffs, etc. NMR spectroscopy is a technique which involves the absorption of energy from the radiofrequency region of the electromagnetic spectrum to detect changes in the alignment of nuclear magnets during exposure to a powerful external magnetic field. The absorption frequencies of such nuclei [e.g., those of biologically ubiquitous hydrogen nuclei (1H)] present in the 1H NMR spectrum of a particular chemical species are critically dependent on their magnetic (and therefore, chemical) environment. Moreover, the appearance (multiplicity) of a resonance (signal) is influenced by neighbouring 1H nuclei in a well characterised manner. Furthermore, the intensity of each signal is directly proportional to the product of the number of magnetically-equivalent protons in the structural/functional group giving rise to it and the concentration of the molecule containing that group. Hence, much valuable molecular information regarding the nature and concentrations of a wide range of components present in biofluids (e.g., human plasma [5,6], urine [7,8], knee-joint synovial fluid [9] and salivary supernatants [10]) can be simultaneously obtained from high-field, high-resolution NMR investigations. The broad overlapping resonances arising from any macromolecules (e.g. proteins, polysaccharides, etc.) present in biofluid samples are routinely suppressed by the application of spinecho pulse sequences, a procedure which generates spectra containing well-resolved, sharp signals ascribable to a multitude of low-molecular mass components (endogenous or exogenous), and the molecularly-mobile portions of macromolecules such as “acute-phase” glycoprotein carbohydrate side-chains and lipoprotein-associated triacylglycerols. Biomedical NMR spectroscopy is a virtually non-invasive technique since it has little or no requirement for the pre-treatment of samples. Moreover, it does not necessarily require a detailed knowledge of sample composition prior to analysis.
Since 1) small levels of H2O2 are released into saliva during tooth-whitening treatments [the quantity being dependent on its content in the product employed, the product class, the product’s application method (such as customised trays), and also partially on the amount of saliva generated], and 2) this oxidant has the ability to chemically react with chemical species present in this and/or other oral fluids present on tooth surfaces, in this manuscript we describe the application of high-resolution 1H NMR analysis to simultaneously evaluate the oxidising actions of a commercially-available H2O2-containing tooth-whitening formulation1 towards a multitude of biomolecules present in (intact) human salivary supernatants. The reactions of H2O2 with a single-component chemical model system containing a buffered aqueous solution of the H2O2-scavenger pyruvate (which is readily 1H NMR-detectable in human saliva) was also examined. The significance of the results acquired is discussed with special reference to the capacity of endogenous antioxidants present in human saliva to offer protection against sensitivity or further adverse effects induced by H2O2 which inadvertently escapes from bleaching application trays and comes into contact with soft tissues. The mechanisms involved in the scavenging of H2O2 and/or more reactive oxygen radical species arising therefrom by salivary antioxidants/electron-donors are also discussed in detail.
2. Materials and Methods
2.1. Sample Collection and Preparation
Unstimulated human saliva samples were obtained from a total of 10 healthy participants (6 male, 4 female). In order to avoid interferences arising from the introduction of exogenous agents into the oral environment, participants were requested to collect all saliva available, i.e., (“whole”) saliva expectorated from the mouth, into a plastic universal collection tube immediately after waking in the morning on a pre-selected day. Each participant was also requested to refrain completely from oral activities (i.e., eating, drinking, tooth-brushing, oral rinsing, smoking, etc.) during the short period between awakening and sample provision (ca. 5 min). Approval for the collection of saliva specimens from human participants in this study was obtained from the University of Bolton Research Ethics Committee.
Each collection tube contained sufficient sodium fluoride (15 μmol.) in order to ensure that metabolites were not produced or consumed via the actions of micro-organisms or their enzymes present in whole saliva during periods of sample preparation and/or storage. Saliva specimens were transported to the laboratory on ice and then centrifuged immediately (4000 r.p.m. for 30 min.) on their arrival to remove cells and debris, and the resulting supernatants were stored at –20˚C for a maximum duration of 18 hr prior to treatment with tooth-whitening product supernatants and/or solutions, further storage at –20˚C as detailed below, and 1H NMR analysis. The pH values of each salivary supernatant were determined prior to 1H NMR analysis (these ranged from 6.45 - 7.44).
The tooth-whitening product investigated here consisted of a dual-syringe system, the first of which contained 12.5% (w/w) H2O2 (the active bleaching agent) and 2.50% (w/w) carbopol, and the second 95.0% (v/v) of an amino-alcohol bleaching accelerant, the remaining 5.0% (v/v) being water (Figure 1(a)). For the purpose of clinical application, the components in the first syringe are pre-mixed with those of the second in a 4:1 volume ratio so that the final applied H2O2 content is 10.0% (w/v). The pH value of the undiluted accelerant solution was 12.56.
 (a)
(a) (b)
(b)
Figure 1. (a) Double-barelled opaque syringe with mixing tip, Dappen’s dish for mixing and brush for application [the larger syringe chamber contains the 12.50% (w/w) H2O2- containing tooth-whitening gel, and the smaller one contains the 95.0% (v/v) amino-alcohol tooth-whitening activator]; (b) A thin coat of the resulting 4:1 (v/v) tooth-whitening gel: amino-alcohol tooth-whitening activator solution admixture (similar to varnish) is painted onto the tooth enamel surface.
For the clinical application of this dual-syringe toothwhitening product, a specially-designed mouth retractor is employed to retract the lips away from the teeth, and then the wy10 bleaching gel/activator mixture is painted onto tooth surface enamel as a thin coat of varnish [Figure 1(b)] in order to ensure that the bleaching agent does not gain access to areas outside the tooth surface (adequate further steps are taken to ensure that the bleaching agent components are isolated from all exposed dentine and soft tissues). This tooth-whitening product is employed with a thermal diffusion system2 which is mounted over the retractor and serves to elevate the temperature of the bleaching gel on the enamel surfaces up to 38˚C. The application is applied once-daily (of total length 40 min, and involving a further bleaching episode after 20 min with a repeated application (replenished) volume of the 4:1 tooth-whitening gel:activator solution mixture). The final pH value of the resulting 4:1 (v/v) tooth-whitening gel:amino-alcohol activator product admixture is 10.0.
2.2. Preparation of Aqueous Supernatants/Solutions Derived from the H2O2 Tooth-Whitening Gel and Tooth-Whitening Accelerant Formulations
Approximately 100 mg pre-recorded masses of the 12.5% (w/w) H2O2 bleaching gel formulation present in syringe 1 were added to a 10.00 ml volume of HPLC-grade water, the mixture rotamixed and then centrifuged (3500 r.p.m. at ambient temperature) for a 30 min. period. The clear supernatant (of pH value 5.53) was removed and then employed to treat salivary supernatant samples and aqueous solutions containing sodium pyruvate in the manners outlined below.
The 95.0% (v/v) bleaching activator (accelerant) material present in syringe 2 was diluted 1:10 with HPLCgrade water so that its final concentration was 9.50% (v/v), and this solution was then directly utilised for additions to human salivary supernatant specimens. This 9.50% (v/v) activator solution had a pH value of 11.24, and the addition of only 38 µl of this to 0.18 ml volumes of human salivary supernatants (the mixture made up to a final volume of 0.594 ml with 2H2O and H2O as detailed below) did not elevate the final pH value of the latter and the final reaction mixture above 7.70, i.e. the buffering capacity of the much larger volume salivary supernatant effectively countered the non-neutral (alkaline) pH value of the activator.
Three separate aliquots (0.18 ml) of each salivary supernatant sample were removed and 76 µl volumes of the supernatant arising from the above 12.5% (w/v) H2O2 (syringe 1) formulation was then added to the first, 38 µl of the above 9.50% (v/v) amino-alcohol accelerant formulation solution was added to the second, and a combination of 76 µl of the above H2O2-containing supernatant and 38 µl of the accelerant solution were added to the third; to each of these mixtures, a 0.30 ml aliquot of 2H2O (field frequency lock) was then added and their final volume was then adjusted to 0.594 ml with HPLCgrade H2O. The mixtures were thoroughly rotamixed and then equilibrated at a temperature of 37˚C for a 30 min. period and then stored at –20˚C for a maximum duration of 72 hr prior to 1H NMR analysis. Further 0.18 ml aliquots of each salivary supernatant sample treated with a 0.30 ml volume of 2H2O and 0.114 ml of HPLC-grade H2O [both previously “sparged” with helium (He) gas for a 30 min period] and then equilibrated and stored in the same manner served as controls.
Aqueous solutions containing sodium pyruvate (5.00 × 10–3 mol·dm–3) (Sigma Chemical Co. Ltd., Poole, Dorset, UK) were prepared in 5.00 × 10–2 mol·dm–3 phosphate buffer (pH 7.00) which was rigorously deoxygenated by purging with helium (He) gas prior to use (30 min at ambient temperature). 1.00 ml aliquots of these solutions were treated with 0, 19, 38, 57, 76 and 95 µl volumes of the above H2O2 solution, the samples thoroughly rotamixed and equilibrated at a temperature of 37˚C for 30 min, and then stored at a temperature of –20˚C for a period of 72 hr prior to 1H NMR analysis. Matching pyruvate-containing solutions treated with equivalent volumes of HPLC-grade H2O in place of the oral rinse, equilibrated at 37˚C (30 min), and stored in the same manner served as controls. This technique served as an effective means to estimate the content of this active tooth-whitening agent present in the tooth-whitening gel (syringe 1) formulation.
2.3. Proton (1H) NMR Measurements
Typically, the total 0.594 ml volume of each sample was placed in a 5-mm diameter NMR tube, and then 6.0 µl of a 1.00 × 10–2 mol·dm–3 solution of trimethylsilyl-[2,2,3, 3-2H4] propionate (TSP) in 2H2O [serving as an internal chemical shift reference (δ = 0.00 ppm) and quantitative 1H NMR standard] was added.
Proton (1H) NMR measurements on the above pre-prepared human salivary supernatant samples were conducted on a Bruker Avance AX-600 spectrometer (Queen Mary University of London Facility, London, UK) operating at a frequency of 600.13 MHz in quadrature detection mode and a probe temperature of 300 K. Each spectrum corresponded to 32 free induction decays (FIDs), 5.6 μs pulses, a 1 s pulse repetition rate, 32,768 (subsequently zero-filled to 65,536) data points and spectral width 10,500 Hz. The intense residual H2O/HOD signal (δ = 4.80 ppm) was suppressed via gated decoupling during the delay between pulses. Exponential linebroadening functions of 0.30 Hz were routinely applied to the FIDs prior to Fourier transformation, and all spectra were manually-phased and baseline-corrected. Spectra were acquired in an automated manner using a sample changer for continuous sample delivery.
Where appropriate, the broad protein resonances present in control and tooth-whitening product-treated salivary supernatant samples were suppressed by the Hahn spin-echo sequence (D[90˚x-t-180˚y-t-collect]) which was repeated 128 times (t = 68 ms). Chemical shifts were referenced to internal TSP. Where present, the methyl group resonances of acetate (singlet, δ = 1.920 ppm), alanine (doublet, δ = 1.487 ppm) and lactate (doublet, δ = 1.330 ppm) served as secondary internal chemical shift references for the saliva samples investigated.
For single-pulse, one-dimensional (1D) 1H NMR spectra acquired on control and H2O2-containing tooth whitening gel supernatant-treated solutions of pyruvate, samples were prepared by the addition of a fixed volume (0.10 ml) of a solution of TSP in 2H2O (1.00 × 10–2 mol·dm–3) to a 0.50 ml volume of the reaction mixture. Typical pulsing conditions were 64 free induction decays (FIDs) using 32,768 data points, 72˚C pulses and a 3 s pulse repetition rate to allow full spin-lattice (T1) relaxation of the protons in the samples investigated. Chemical shifts were referenced to TSP (internal; final concentration 1.67 × 10–3 mol·dm–3), and exponential line-broadening functions of 0.30 Hz were again employed for purposes of processing.
The identities of biomolecule resonances present in the salivary 1H NMR spectra acquired were routinely assigned by a consideration of chemical shift values, coupling patterns and coupling constants. The relative intensities of selected signals therein, and those of the phosphate-buffered aqueous pyruvate solutions treated with increasing volumes (0, 19, 38, 57, 76 and 95 µl) of the above solution prepared from the H2O2-containing toothwhitening gel, were determined by electronic integration via the spectrometer’s proprietory software (XWIN-NMR), and the concentrations of components detectable were determined by comparisons of their resonance areas with that of TSP (final concentration 1.00 × 10–4 mol·dm–3 in the salivary supernatants). Maintenance of the exact integral regions for each spectrum acquired was ensured.
Since the protein concentration of human saliva (1.40 g·dm–3 - 6.40 g·dm–3 [11]) is much lower than that of human blood plasma (65 g·dm–3 - 83 g·dm–3 [12]), the minimal macromolecular broad resonance envelop was not found to interfere with the observation and integration of each of the sharp low-molecular-mass resonances, and hence all of the salivary metabolite concentrations documented here represent those derived from such spectra. Furthermore, it should also be noted that the biomolecule concentrations determined in this investtigation reflect only the non-macromolecular-bound portion of these components and hence are expected to be somewhat lower than their total salivary levels.
Two-dimensional shift-correlated 1H-1H NMR (COSY) spectra of human salivary supernatants were acquired using the standard sequence of Aue et al. [13] with 2048 data points in the t2 dimension, 256 increments of t1, a 3.00 s relaxation delay, and 64 transients.
2.4. Statistical Analysis of Salivary Biomolecule Concentration Data
The statistical significance of differences observed between salivary pyruvate, methionine, lactate and trimethylamine concentrations was determined by a paired sample t-test performed on untransformed data (based on 9 degrees-of-freedom for n = 10 participants).
3. Results
3.1. High-Resolution 1H NMR Analysis of Human Salivary Supernatants
Figure 2(a) shows a typical high-field (0.72 ppm - 2.46 ppm) region of the 600 MHz single-pulse 1H NMR spectrum of an untreated human salivary supernatant sample, and corresponding partial spectra acquired subsequent to the in vitro treatment of this sample with specified aliquots of the tooth-whitening amino-alcohol activator solution, bleaching gel formulation supernatant, and a combination of equivalent added volumes of the activator and bleaching gel supernatant solutions, are shown in Figures 2(b)-(d) respectively (the volumes of each solution or supernatant added are specified above in Section 2).
As previously documented [10], 600 MHz single-pulse 1H NMR spectra of control (untreated) human salivary supernatant samples contained many prominent, sharp resonances ascribable to a wide range of low-molecularmass components. Indeed, signals assignable to short-chain organic acid anions (e.g., acetate, isoand n-butyrates, formate, fumarate, lactate, propionate, pyruvate, succinate, n-valerate and 3-D-hydroxybutrate), carbohydrates

Figure 2. Expanded 0.72 ppm - 2.46 ppm regions of the 600.13 MHz single-pulse 1H NMR spectra of a human salivary supernatant specimen (pH value 6.84). (a) Untreated; (b) Treated with the 9.50% (v/v) amino-alcohol accelerant solution; (c) Treated with the supernatant derived from the H2O2-containing tooth-whitening gel, and (d) treated with a combination of the 9.50% (v/v) amino-alcohol accelerant solution and the supernatant derived from the H2O2-containing tooth-whitening gel (as described in Section 2). For these samples, 38 µl and/or 76 µl aliquots of the amino-alcohol accelerant solution and the supernatant obtained from the tooth-whitening gel, respectively, were added to a 0.18 ml volume of human saliva, and subsequently 0.30 ml of 2H2O was added and the reaction mixture was made up to a final volume of 0.594 ml with HPLC-grade water (samples were then equilibrated at a temperature of 37˚C for a 30 min period). The disappearance of the pyruvate-CH3 resonance in the spectra shown in (c) and (d) is highlighted by rectangles. Typical spectra are shown. Abbreviations: A, Acetate-CH3; Ala I, alanine-CH3, group protons; Bu I and Bu II 3-D-hydroxybutyrate γ-CH3 and α/α’-CH2 group protons respectively; iso-But I and II, iso-butyrate-CH3 and -CH group protons respectively; n-But I, II and III, n-butyrate γ-CH3, β- and α-CH2 protons respectively; Eth I, ethanol-CH3; Lac I, lactate-CH3 group protons; Leu I and II/III, leucine δ-CH3’s and β-CH2/γ-CH respectivly; N-Ac, spectral region for acetamido methyl groups of N-acetyl sugars; Prop I and II, propionate-CH3 and -CH2 group protons respectively; Pyr, pyruvate-CH3; Suc, succinate-CH2; n-Val II, n-valerate γ-CH2 protons. Activator Peak 2 represents a major high-field region resonance arising from the added amino-alcohol tooth-whitening activator/accelerant.
such as glucose and galactose (both α- and β-anomers) are observable. The organic acid anions detectable are products arising from microbial metabolic pathways, and hence these agents (either individually or several or more in concert), particularly their salivary concentrations, may serve as chemotaxonomic indicators of microbial activity in the oral environment. Indeed, the pathogenic microorganism P. gingivalis generates high levels of acetate, n-butyrate and propionate, together with smaller amounts of iso-butyrate, iso-valerate, phenylacetate and succinate [14,15].
Also present are one or more broad resonances located at 2.04 ppm which are assignable to the acetamido methyl group protons (-NHCOCH3) of N-acetylsugars located in the molecularly-mobile branching carbohydrate side-chains of salivary glycoproteins. This assignment is consistent with those previously made for the 1H NMR spectra of other biofluids [16]. This broad resonance overlaps several sharper acetamido-CH3 group 1H resonances attributable to low-molecular-mass N-acetylsugars such as N-acetylneuraminate and N-acetylglucosamine saccharide fragments, which conceivably arise from the actions of bacterial-derived neuraminidase and hyaluronidase respectively, the latter towards ground substance hyaluronate [17]. These resonances may also be assignable to N-acetyl amino acids such as N-acetylglycine or N-acetylglutamate.
In addition, both ethanol and methanol were detectable in a large proportion of the human saliva samples subjected to 1H NMR analysis. Although ethanol is a bacterial-derived catabolite (for example, arising from carbohydrate metabolism by Streptococcus mutans) [18], the methanol present is putatively derived from the passive or direct inhalation of cigarette smoke in which this alcohol is present, a consequence of the combustion of tobacco lignin which contains many methoxy substituents in its complex, macromolecular aromatic structure.
Two-dimensional 1H-1H COSY NMR spectra of typical human salivary supernatant samples showed clear connectivities between the multiplet resonances present. For example, linkages between the alanine-CH3 and α-CH signals (doublet, δ = 1.487 ppm and quartet, δ = 3.765 ppm respectively), propionate-CH3 and -CH2 group signals (triplet, δ = 1.04 ppm and quartet, δ = 2.17 ppm respectively), the n-butyrate-CH3, β-CH2 and α-CH2 group resonances (triplet, δ = 0.91 ppm, multiplet, δ = 1.56 ppm and triplet, δ = 2.15 ppm respectively), the ethanolCH3 and -CH2 groups (triplet, δ = 1.21 ppm and quartet, δ = 3.68 ppm), the lactate-CH3 and -CHOH group resonances (doublet, δ = 1.330 ppm and quartet, δ = 4.130 ppm respectively) and the tyrosine aromatic ring protons (doublets located at 6.88 and 7.17 ppm) (data not shown).
3.2. Multicomponent 1H NMR Evaluations of the Oxidative Consumption of Salivary Components by H2O2 Present in the Tooth-Whitening Formulation Investigated
Clearly, addition of the H2O2-containing product gives rise to the complete disappearance of the pyruvate-CH3 group signal (singlet, δ = 2.388 ppm), an observation reproducible in all saliva samples tested in this manner (n = 10) [Figures 2(a) and (c)]. Further expanded 1.88 ppm - 2.46 ppm regions of these spectra are exhibited in Figures 3(a) and (c) respectively. These data are fully consistent with the oxidative consumption of salivary pyruvate by H2O2 present in the formulation (Equation (1)). Indeed, previous investigations have demonstrated that pyruvate acts as a powerful biofluid electron-donor (i.e., a water-soluble antioxidant) and is oxidatively decarboxylated to acetate and CO2 on reaction with H2O2. The mean (±SEM) pre-treatment salivary pyruvate concentration was 2.19 (±1.07) × 10–3 mol·dm–3 for the n = 10 samples collected in this study.
 (1)
(1)
An alternative mechanism for the generation of acetate and CO2 from pyruvate involves 1) the prior generation of ŸOH radical from the interaction of “catalytic” redoxactive low-molecular-mass metal ions such as Fe(II), Cu(I) and Co(II) present in human saliva with the H2O2 tooth-whitening agent, and 2) direct reaction of this aggressively-reactive oxygen radical species with this α-keto acid anion “scavenger” (Equations (2) and (3)), although with much larger concentrations of available H2O2, this oxidation process is, of course, much more likely to proceed in accordance with Equation (1). In addition to both endogenous and exogenous (dietary) sources of these metal ions, the mean concentrations of copper and cobalt ions in human saliva are 1.25 (±0.20) × 10–6 (mean ± SEM) [19] and 3.7 × 10–7 mol·dm–3 [20] respectively, the latter representing the mean of 10 out of 37 salivary determinations in which it was detectable, and presumably predominant as Co(II) in view of thermodynamic considerations and the availability of Co(III)-reducing electrondonors in this biofluid.
M(n+1)+ + e– → Mn+ (2)
Mn+ + H2O2 → M(n+1)+ + ŸOH + OH–(3)
 (4)
(4)
Since 1H NMR-detectable concentrations of acetate were detectable as a minor contaminant present in the tooth-whitening gel (syringe 1) supernatant, spectra of the H2O2-containing bleaching product-treated salivary supernatants showed elevations in the intensities of resonances ascribable to this agent. Of course, smaller increases in the acetate-CH3 group signal intensity observed

Figure 3. Corresponding expanded 1.88 ppm - 2.46 ppm regions of the spectra shown in Figure 2. Abbreviations: as Figure 2.
following treatment are attributable to the H2O2-mediated oxidative decarboxylation of pyruvate described above.
Further H2O2-induced modifications to the 1H NMR profiles of human saliva included 1) the oxidative consumption of lactate (-CH3 group doublet, δ = 1.33 ppm, -CHOH proton quartet, δ = 4.13 ppm), which is explicable by the attack of ŸOH radical on this metabolite to primarily generate pyruvate (Equation (5)) which is then consumed by excess H2O2 (Equation (1)), or further ŸOH radical (Equation (4)) [as noted above, ŸOH radical can be generated from Fenton or pseudo-Fenton reaction processes involving trace levels of “catalytic” metal ions (Equations (2) and (3)); 2) significant elevations in the salivary concentrations of formate, an organic acid anion which is known to arise from oxidative damage to carbohydrate species such as glucose, and/or hyaluronate or N-acetyl glycoprotein N-acetylsugar species by ŸOH radical produced in the same manner as that described in 1) above; 3) the oxidation of trimethylamine (TMA) (singlet, δ = 2.91 ppm) to its corresponding N-oxide, a process represented by equation 6. Mean ± SEM percentage decreases observed in the TSP-normalised intensities of the lactate-CH3 and trimethylamine-N(CH3)3 resonances were 21% ± 5% (p < 0.05) and 45% ± 9% (p < 0.01) respectively, whereas that for the increase in the formate signal was 42% ± 11% (p < 0.02). The mean ± SEM before-treatment concentrations of lactate, trimethylamine and formate were (4.43 ± 2.17) × 10–3, (9.17 ± 3.04) × 10–5 and (5.72 ± 2.51) × 10–3 mol·dm–3 respectively.
 (5)
(5)
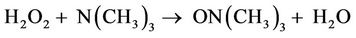 (6)
(6)
Moreover, for a limited number of the salivary supernatant samples examined (n = 3), we also found that the amino acid methionine (-S-CH3 group singlet resonance, δ = 2.13 ppm) was also consumed, a process which generated its primary oxidation product methionine sulphoxide (with a characteristic -SO-CH3 group singlet signal located at δ = 2.725 ppm) as previously described [21] and fully consistent with Equation (7). For these 3 samples, the mean ±SEM methionine concentration was 1.60 ± 0.44 × 10–4 mol·dm–3, and the mean percentage decrease in its 2.13 ppm -S(CH3) group signal was 93% ± 4% (p < 0.005 for n = 3 specimens).
 (7)
(7)
Treatment of human salivary supernatant specimens with a combination of the H2O2-containing bleaching gel supernatant (derived from syringe 1) with the diluted bleaching accelerant solution (from syringe 2) gave rise to similar modifications to the 1H NMR profile of this biofluid [Figures 2(d) and 3(d)], specifically the complete oxidative consumption of pyruvate, accompanied by smaller reductions in the intensities of the lactate signals and increases in that of formate, together with even smaller decreases in that of the methionine-S-CH3 resonance, although the latter observation was again limited to the small number of samples in which it was 1H NMR-observable (n = 3).
As expected, addition of the tooth bleaching aminoalcohol accelerant alone to each salivary supernatant sample did not give rise to the 1H NMR spectral modifications which were observed in those treated with either the H2O2-containing bleaching gel supernatant or a combination of an equivalent volume of this solution (76 µl) with an additional 38 µl of the 9.50% (v/v) solution of the tooth-whitening accelerant (apart, of course, from the appearance of signals ascribable to the added accelerant and an acetate contaminant also present in its solution). A Typical example of the spectra acquired from these samples is shown in Figures 2(b) and 3(b).
However, a sharp singlet resonance located at ca. 2.06 ppm was markedly and reproducibly diminished in intensity on treatment of each saliva sample with the bleaching activator solution [Figures 2(b) and 3(b)], and this observation was also notable in samples that were treated with a combination of this activator solution and the H2O2 gel-derived supernatant [Figure 1(d)], but not in those to which the H2O2-containing supernatant was added alone [Figures 2(c) and 3(c)]. As noted above, this particular signal is ascribable to the acetamido group (-NHCOCH3) protons of low-molecular-mass N-acetylsugars or N-acetyl amino acids. This observation is not simply explicable, but if this resonance is assignable to an N-acteyl amino acid, the decrease in its intensity may arise from a condensation reaction between the carboxylate group (s) of these biomolecules (including the side-chain carboxylate group of N-acetylglutamate if this species is responsible for the signal) and the free amino group of the bleaching accelerant added, although this reaction is unlikely to proceed at the 37˚C equilibration temperature; however, it may be assisted by one or more salivary enzymes.
Moreover, also noteworthy were elevations in the electronically-integrated intensities of a resonance assignable to the malodorous biomolecule dimethylamine (singlet, δ = 2.78 ppm), an observation which is presumably attributable to the displacement and hence mobilisation of this species (which is protonated and hence positively-charged at salivary pH values which are close to neutrality) from negatively-charged binding sites present in salivary macromolecules by a relatively large excess of this added positively-charged amino-alcohol accelerating agent. Such binding-sites could include the carboxylate groups of aspartate or glutamate residues in salivary proteins, and/or that of glucuronyl residues in salivary polysaccharides or glysoaminoglycans such as soft tissue-derived hyaluronate.
3.3. Chemical Model Studies of the Reaction of Pyruvate with H2O2 Present in the Tooth-Whitening Gel
Aqueous 5.00 × 10–3 mol·dm–3 standard solutions of pyruvate (n = 3) were treated with a 76 µl volume of the supernatant solution derived from the H2O2-containing tooth-whitening gel (Section 2) in order to provide further information regarding the nature and mechanism of this agent’s ability to oxidise this α-keto acid anion in human saliva. 1H NMR analysis revealed that reaction of pyruvate (initially 5.00 × 10–3 mol·dm–3) with this bleaching agent gave rise to an essentially stoichiometric transformation of the α-keto acid anion substrate to acetate and CO2. Indeed, >54% of the pyruvate was oxidatively decarboxylated to acetate and CO2 under the experimenttal conditions employed, and after making allowances for the presence of a small amount of pyruvate as pyruvate hydrate ( ) in aqueous solution, and asming that the reaction goes to completion, the expected level of acetate production from Equation (1) was 55.9%.
) in aqueous solution, and asming that the reaction goes to completion, the expected level of acetate production from Equation (1) was 55.9%.
Pyruvate hydrate has a singlet resonance (δ = 1.50 ppm) of relatively low intensity which was also present in all 1H NMR spectra acquired on aqueous pyruvate solutions. This resonance was also completely removed from the spectrum subsequent to treatment with the H2O2- containing product evaluated here.
Plots of the ratio of the concentration of acetate generated to that of the initial (pre-H2O2-treated) pyruvate plus pyruvate hydrate concentrations versus the added tooth-whitening product supernatant volume or corresponding (predicted) added H2O2 concentration ([H2O2]) were linear with a zero intercept (Figure 4), data demonstrateing that this technique serves as an excellent method for the determination of this agent in such commerciallyavailable tooth-whitening products. Indeed, the correlation coefficient (r value) obtained was 0.9988. The relationship between this ratio and added H2O2 concentration is described by equation 8, where [acetate]0, [pyruvate]0 and [pyruvate hydrate]0 represent the initial concentrations of acetate, pyruvate and pyruvate hydrate respecttively [the initial concentration of pyruvate hydrate was also determined by the electronic integration of its singlet resonance (δ = 1.50 ppm) and normalisation of its intensity to that of the TSP internal standard of known concentration]. The [acetate]0 value (the initial, untreated pyruvate solution acetate concentration) was corrected for a contribution from small amounts of an acetate contaminant present in the H2O2 bleaching gel supernatant solution employed for these studies (i.e. this contribution was dependent on the volume of supernatant added).
 (8)
(8)
The estimated percentage H2O2 content of the gel present in syringe 1 of the tooth-whitening formulation investigated was found to be 12.37% ± 0.35% (w/w) (mean ±SEM) for n = 5 repeat determinations, a value in close agreement with that of its specified value [12.50% (w/w)].
4. Discussion
4.1. H2O2-Scavenging Capacity of Salivary Biomolecules
Multicomponent 1H NMR investigations of the oxidising
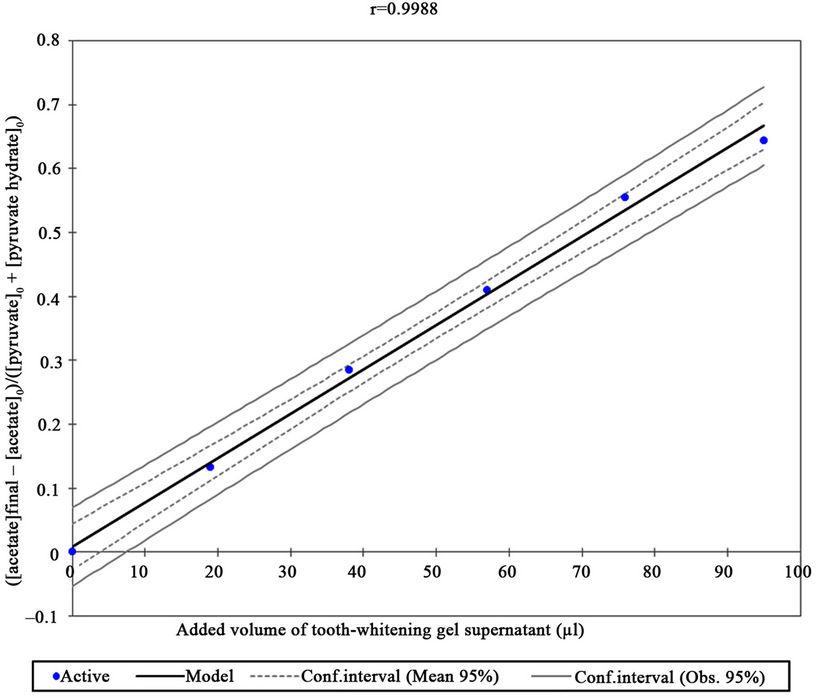
Figure 4. Plot of the 1H NMR-determined ratio of acetate concentration generated to that of initial pyruvate. Specifically, [Acetate]/[Pyruvate]0, as represented by the right-hand side of equation 8 (i.e. ([acetate]final – [acetate]0)/([pyruvate]0 + [pyruvate hydrate]0)was plotted against microliter (µl) volumes of added supernatant derived from the H2O2-containing toothwhitening gel formulation. The added supernatant volumes were 0, 19, 38, 57, 76 and 95 µl, and the resonance intensities and corresponding estimated H2O2 concentrations were adjusted to allow for the small level of dilution arising from the added H2O2 solution. The 95% confidence intervals shown represent those for the mean values and for the regression estimates, the latter the estimate of ([acetate]final – [acetate]0)/([pyruvate]0 + [pyruvate hydrate]0), i.e. H2O2 concentration, for each added volume of tooth-whitening gel supernatant.
ability of the tooth-whitening formulation investigated here towards salivary biomolecules demonstrated that critical salivary electron-donors are readily consumed by H2O2 therein. Indeed, it is clear that any H2O2 which “escapes” from the tooth-whitening site to saliva (or alternative oral fluids), or that which inadvertently comes into contact with this oral fluid, will be effectively consumed by salivary electron-donors which therefore serve to protect soft tissues against any sensitivity or damage inducible by this tooth-whitening agent or any reactive oxygen radical species derivable therefrom. These further oxidants include hydroxyl radical (ŸOH) arising from Fenton-type reactions [22] involving any low-molecularmass redox-active transition metal ions available in human saliva and serving as catalytic sources, mono-deprotonated H2O2 ( ), which is more bleaching-active than its protonated precursor [23], (although is only generated in significant amounts at alkaline pH values since H2O2’s first pKa value is 11.65), and perhaps also superoxide anion (
), which is more bleaching-active than its protonated precursor [23], (although is only generated in significant amounts at alkaline pH values since H2O2’s first pKa value is 11.65), and perhaps also superoxide anion ( ) generated from the single-electron oxidation of H2O2 and/or
) generated from the single-electron oxidation of H2O2 and/or .
.
Indeed, the salivary concentrations of pyruvate (a two electron donor) range from (0.10 - 10.60) × 10–3 mol·dm–3 [17], and the mean level of thiols (a single electron supplier) therein is 3.6 × 10–5 mol·dm–3 [24]. After making appropriate allowances for thermodynamic equilibria, and the rate of each reaction involved under physiological conditions, data acquired here indicates that further reductants present in human saliva (e.g., urate, ascorbate, thiocyanate anion and the amino acids cysteine and methionine), those at relatively low concentrations, will also be at least partially effective with regard to the neutralisation of any adverse effects exertable by H2O2 during tooth-whitening episodes conducted with this particular product.
However, it should be noted that selected single electron-donors present in human saliva such as thiols and ascorbate also have the capacity to reduce higher oxidation state metal ions to their lower ones [for example, reduction of Fe(III) to Fe(II)] in accordance with Equation (2), so that the latter can then take part in Fenton or pseudo-Fenton reaction systems which generate the aggressively-reactive ŸOH radical (Equation (3)).
All of the above salivary metabolites serve to act as biomolecular protectants against any adverse sensitivity reactions and/or toxic effects exertable by the leakage of H2O2 to soft tissue areas which, without an adequate form of control, are accessible by this particular biofluid.
Interestingly, methionine is known to be an essential precursor required for the production of volatile sulphur compounds (VSCs) by Gram-negative anaerobic bacteria in the oral environment; i.e., it plays a major role in the development of halitosis. Moreover, malodorous TMA has been implicated as a major factor involved in recalcitrant oral malodour. However, their involvement in these conditions will not be discussed further here.
4.2. Administration and Effectiveness of Tooth-Whitening Products
Currently, there are four methods for the administration of such tooth-whitening agents, specifically 1) that administered by a dental clinician, a process which can involve the employment of high H2O2 concentrations, which commonly range from 35% - 40% (w/w)—this treatment is frequently supplemented with the use of a light and/or heat source, 2) that supervised by a dental clinician in which a customised bleaching tray containing high levels of CP, often ranging from 35% - 45% (w/w), is placed into the mouth of the patient for periods of up to 1.00 hr in the clinician’s surgery, 3) a home-use tooth-whitening kit provided by a dentist (otherwise known as “Home” or “Nightguard” bleaching) and applied by the patient [usually a 10% - 22% (w/w) CP formulation] present in a customised application tray, and 4) products which are purchasable by consumers in pharmacies and other retail outlets (known as “over-the-counter” products); these often contain H2O2 or CP [up to an EU legal limit of 0.10% (w/w or w/v)], or peroxoborates or peroxosulphates, but more recently this range of products has expanded to include those which contain chlorine dioxide ( )-generating systems, especially those which involve the acidulation of aqueous chlorite solutions. The product investigated here can be directly applied in the dental surgery [under 1) and 2) above], but is predominantly for “at-home” use as in 3) above.
)-generating systems, especially those which involve the acidulation of aqueous chlorite solutions. The product investigated here can be directly applied in the dental surgery [under 1) and 2) above], but is predominantly for “at-home” use as in 3) above.
Both a series of small clinical investigations and case studies have provided evidence that a 10% (w/w) CPcontaining gel formulation applied in a bleaching tray overnight, (i.e. the “Nightguard” vital bleaching technique), gave rise to a predictable level of tooth whitening [25-32], as did H2O2-containing strips [33] and a “Powerbleaching” technique employing 35% (w/v) H2O2 coupled with or without activation by UV light and/or heating stages [34,35].
4.3. Deleterious Tooth-Sensitivity Effects of H2O2-Containing Tooth-Whitening Products
Tooth sensitivity is a common deleterious effect of external tooth-whitening processes [36], and particularly noteworthy in this context is the observation that with the use of products containing 10% (w/v) CP, 15% - 65% of patients reported an enhanced level of tooth sensitivity [37,38]. Moreover, increased incidences of tooth sensitivity (from 67% - 78%) have been noted following toothwhitening episodes with H2O2 coupled with a thermal enhancement process [39].
It is well known that tooth sensitivity usually lasts for up to four days post-bleaching [39,40]. However, this adverse effect can be prolonged for up to 39 days [37, 38]. Furthermore, a clinical study that involved comparisons of two differing 10% (w/w) CP brands revealed that 55% of a large number of patients investigated found tooth sensitivity and/or gingival irritation, and 20% of those who had experienced such deleterious effects discontinued the treatment in view of their discomfort [37].
Although mechanisms that could account for such tooth sensitivities during or following such external toothwhitening episodes remain speculative, one in vitro study revealed that H2O2 can penetrate enamel and dentine, and also penetrate the pulp chamber [41].
4.4. Advantages of 1H NMR Analysis for Evaluations of the Fate of H2O2 in the Oral Environment
High-resolution, high-field 1H NMR spectroscopy is a technique which offers many advantages over alternative time-consuming, labour-intensive analytical methods for evaluating the fate of H2O2 (or alternative peroxo-adduct tooth-whitening agents) in oral fluids since 1) it permits the rapid, non-invasive and simultaneous examination of a very wide range of components present in biofluids, and 2) it generally requires little or no knowledge of sample composition prior to analysis. Furthermore, chemical shift values, coupling patterns and coupling constants of resonances present in the 1H NMR spectra of such multicomponent systems provide much valuable information regarding the molecular nature of both endogenous and exogenous agents therein.
5. Conclusions
In conclusion, high-resolution 1H NMR analysis is a technique of much utility regarding multicomponent assessments of the interactions of active agents present in commercially-available tooth-whitening products with human salivary biomolecules, and the oxidative decarboxylation of salivary pyruvate, together with the oxidation of lactate, trimethylamine, methionine and carbohydrates in general, by H2O2 present in a tooth-whitening formulation as demonstrated here, serves as an important fundamental example of this which is clearly of some relevance to the ability of human saliva to offer protecttion against any adverse effects arising from the leakage of this oxidant onto sensitive oral environments such as gums and further soft tissue environments.
REFERENCES
- B. A. Matis, M. A. Cochran and G. Eckert, “Review of the Effectiveness of Various Tooth-Whitening Systems,” Operative Dentistry, Vol. 34, No. 2, 2009, pp. 230-235. doi:10.2341/08-74
- C. J. Tredwin, S. Naik, N. J. Lewis and C. Scully, “Hydrogen Peroxide Tooth-Whitening (Bleaching) Products: Review of Adverse Effects and Safety Issues,” British Dental Journal, Vol. 200, No. 7, 2006, pp. 371-376. doi:10.1038/sj.bdj.4813423
- M. Grootveld, A. Sheerin, M. Atherton, A. D. Millar, E. J. Lynch, D. R. Blake and D. P. Naughton, “Applications of High Resolution NMR Analysis to the Study of Inflammatory Diseases at the Molecular Level,” In: R. J. H. Clark and R. Hester, Eds., Biomedical Applications of NMR Spectroscopy, Advances in Spectroscopy, John Wiley and Sons Ltd., Chichester, Vol. 25, 1996, pp. 295-327.
- J. N. S. Evans, “Biomolecular NMR Spectroscopy,” Biophysical Journal, Vol. 70, No. 6, 1996, pp. 2996-2997. doi:10.1016/S0006-3495(96)79871-5
- J. K. Nicholson, P. J. Foxall, M. Spraul, R. D. Farrant and J. C. Lindon, “750 MHz 1H and 1H-13C NMR Spectroscopy of Human Blood Plasma,” Analytical Chemistry, Vol. 67, No. 5, 1995, pp. 793-811. doi:10.1021/ac00101a004
- F. Savorani, M. Kristensen, F. H. Larsen, A. Astrup and S. B. Englesen, “High Throughput Prediction of Chylomicron Triglycerides in Human Plasma by Nuclear Magnetic Resonance and Chemometrics,” Nutrition and Metabolism, Vol. 7, No. 43, 2010, pp. 1-8.
- S. Zhang, C. Zheng, I. R. Lanza, N. K. Sreekumaran, D. Raftery and O. Vitek, “Interdependence of Signal Processing and Analysis of Urine 1H NMR Spectra for Metabolic Profiling,” Analytical Chemistry, Vol. 81, No. 15, 2009, pp. 6080-6088. doi:10.1021/ac900424c
- G. E. J. Willem, C. Schoonen, P. A. M. Kloks, J.-P. H. T. M. Ploemen, M. J. Smit, P. G. Zandberg, G. J. J. Horbach, J.-R. Mellema, C. Thijssen-van Zuylen, A. C. Tas, J. H. J. van Nesselrooij and J. T. W. E. Vogels, “Uniform Procedure of 1H NMR Analysis of Rat Urine and Toxicometabonomics Part II: Comparison of NMR Profiles for Classification of Hepatotoxicity,” Toxicological Sciences, Vol. 98, No. 1, 2007, pp. 286-297. doi:10.1093/toxsci/kfm077
- D. Naughton, M. Whelan, E. C. Smith, R. B. Williams, D. R. Blake and M. Grootveld, “An Investigation of the Abnormal Metabolic Status of Synovial Fluid from Patients with Rheumatoid Arthritis by High Field Proton Nuclear Magnetic Resonance Spectroscopy,” FEBS Letters, Vol. 317, No. 1-2, 1993, pp. 135-138. doi:10.1016/0014-5793(93)81508-W
- M. Grootveld and C. J. Silwood, “1H NMR Analysis as a Diagnostic Probe for Human Saliva,” Biochemical and Biophysical Research Communications, Vol. 329, No. 1, 2005, pp. 1-5. doi:10.1016/j.bbrc.2005.01.112
- G. N. Jenkins, “Saliva. The Physiology and Biochemistry of the Mouth,” 4th Edition, Blackwell Scientific, Oxford, 1978.
- C. Lentner, Ed., “Geigy Scientific Tables,” Vol. 1, 8th Edition, Ciba-Geigy, Basle, 1981.
- W. P. Aue, E. Bartholdi and R. R. Ernst, “Two Dimensional Spectroscopy. Application to Nuclear Magnetic Resonance,” Journal of Chemical Physics, Vol. 64, No. 5, 1976, pp. 2229-2247. doi:10.1063/1.432450
- F. C. Gibson and C. A. Genco, “The Genus Porphyromonas,” In: M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer and E. Stackebrandt, Eds., The Prokaryotes Volume 7: Proteobacteria: Delta, Epsilon Subclass, Part 6, Springer, Singapore City, 2006, pp. 428-454.
- E. L. Hendrickson, Q. W. Xia, T. S. Wand, R. J. Lamont and M. Hackett, “Pathway Analysis for Intracellular Porphyromonas gingivalis Using a Strain ATCC 33277 Specific Database,” BioMed Central Microbiology, Vol. 9, 2009, p. 185.
- J. D. Bell, J. C. C. Brown, J. K. Nicholson and P. J. Sadler, “Assignment of Resonances for ‘Acute-Phase’ Glycoproteins in High Resolution Proton NMR Spectra of Human Blood Plasma,” FEBS Letters, Vol. 215, No. 2, 1987, pp. 311-315. doi:10.1016/0014-5793(87)80168-0
- C. J. L. Silwood, E. Lynch, A. W. D. Claxson and M. C. Grootveld. “1H and 13C NMR Spectroscopic Analysis of Human Saliva,” Journal of Dental Research, Vol. 81, No. 6, 2002, pp. 422-427. doi:10.1177/154405910208100613
- R. A. D. Williams and J. C. Elliot, “Basic and Applied Dental Biochemistry,” Churchill Livingstone, Edinburgh, 1979.
- J. R. Turnlund, C. L. Keen and R. G. Smith, “Copper Status and Urinary and Salivary Copper in Young Men at Three Levels of Dietary Copper,” American Journal of Clinical Nutrition, Vol. 51, No. 4, 1990, pp. 658-664.
- S. Dreizen, H. A. Spies Jr. and T. D. Spies, “The Copper and Cobalt Levels of Human Saliva and Dental Caries Activity,” Journal of Dental Research, Vol. 31, No. 1, 1952, pp. 137-142. doi:10.1177/00220345520310011001
- E. Lynch, A. Sheerin, C. J. Silwood and M. Grootveld, “Multicomponent Evaluations of the Oxidising Actions and Status of a Peroxoborate-Containing Tooth-Whitening System in Whole Human Saliva using High Resolution Proton NMR Spectroscopy,” Journal of Inorganic Biochemistry, Vol. 73, No. 1-2, 1999, pp. 65-84. doi:10.1016/S0162-0134(98)10092-2
- B. Halliwell and M. Grootveld, “The Measurement of Free Radical Reactions in Humans,” FEBS Letters, Vol. 231, No. 1, 1987, pp. 9-14. doi:10.1016/0014-5793(87)81455-2
- E. Lynch, A. Sheerin, M. Atherton, J. Hawkes, P. Haycock and M. C. Grootveld, “Molecular Mechanisms Associated with the Bleaching Actions of CommerciallyAvailable Whitening Oral Health Care Products,” Journal of the Irish Dental Association, Vol. 41, No. 4, 1996, pp. 94-102.
- J. Grigor and A. J. Roberts, “Reduction in the Levels of Oral Malodor Precursors by Hydrogen Peroxide: In-Vitro and In-Vivo Assessments,” Journal of Clinical Dentistry, Vol. 3, No. 4, 1992, pp. 111-115.
- V. B. Haywood and H. O. Heymann, “Nightguard Vital Bleaching,” Quintessence International, Vol. 20, No. 3, 1989, pp. 173-176.
- J. Howard, “Patient-Applied Tooth Whiteners,” Journal of the American Dental Association, Vol. 132, No. 2, 1992, pp. 57-60.
- J. W. Reinhardt, S. C. Eivins, E. J. Swift and G. E. Denehy, “Clinical Study of Nightguard Vital Bleaching,” Quintessence International, Vol. 24, 1993, pp. 379-384.
- G. M. Kowitz, S. A. Nathoo and K. N. Rustogi, “Clinical Comparison of Colgate Platinum Tooth Whitening System and Rembrant Gel Plus,” Compendium of Continuing Dental Education, Vol. 15, No. 17, 1994, pp. S646-S651.
- C. M. Russell, G. L. Dickinson, M. H. Johnson, et al., “Dentist-Supervised Home Bleaching with Ten Per Cent Carbamide Peroxide Gel: A Six Month Study,” Journal of Esthetic and Restorative Dentistry, Vol. 8, No. 4, 1996, pp. 177-182. doi:10.1111/j.1708-8240.1996.tb00422.x
- B. A. Matis, M. A. Cochran, G. Eckert and T. J. Carlson, “The Efficacy and Safety of a 10% Carbamide Peroxide Bleaching Gel,” Quintessence International, Vol. 29, No. 7, 1998, pp. 555-563.
- H. O. Heymann, E. J. Swift, S. C. Bayne, et al. “Clinical Evaluation of Two Carbamide Peroxide Tooth Whitening Agents,” Compendium of Continuing Dental Education, Vol. 19, No. 4, 1998, pp. 359-362.
- A. J. McCaslin, V. B. Haywood, B. J. G. Potter, L. Dickenson and C. M. Russell, “Assessing Dentin Colour Changes from Nightguard Vital Bleaching,” Journal of the American Dental Association,” Vol. 130, No. 10, 1999, pp. 1485-1490.
- R. W. Gerlach and X. Zhou, “Vital Bleaching with Whitening Strips: Summary of Clinical Research on Effectiveness and Tolerability,” Journal of Contemporary Dental Practice, Vol. 2, No. 3, 2001, pp. 1-15.
- V. B. Haywood, “History, Safety and Effectiveness of Current Bleaching Techniques and Application of the Nightguard Vital Bleaching Technique,” Quintessence International, Vol. 27, 1992, pp. 471-488.
- M. Sulieman, M. Addy and J. S. Rees, “Development and Evaluation of a New Method in Vitro to Study the Effectiveness of Tooth Bleaching,” Journal of Dentistry, Vol. 31, 2003, pp. 415-422. doi:10.1016/S0300-5712(03)00069-1
- L. Tam, “The Safety of Home Bleaching Techniques,” Journal of the Canadian Dental Association, Vol. 65, No. 8, 1999, pp. 453-455.
- R. H. Leonard, V. B. Haywood and C. Phillips, “Risk Factors for Developing Tooth Sensitivity and Gingival Irritation Associated with Nightguard Vital Bleaching,” Quintessence International, Vol. 28, No. 8, 1997, pp. 527-534.
- L. Tam, “Clinical Trial of Three 10% Carbamide Peroxide Bleaching Products,” Journal of the Canadian Dental Association, Vol. 65, No. 4, 1999, pp. 201-205.
- S. C. Cohen and C. Chase, “Human Pulpal Responses to Bleaching Procedures on Vital Teeth,” Journal of Endodontics, Vol. 5, No. 5, 1979, pp. 134-138. doi:10.1016/S0099-2399(79)80033-3
- J. R. Schulte, D. B. Morrissette, E. J. Gasior and M. V. Czajewski, “The Effects of Bleaching Application Time on the Dental Pulp,” Journal of the American Dental Association, Vol. 125, No. 10, 1994, pp. 1330-1335.
- W. Thitinanthapan, P. Satamanont and N. Vongsavan, “In Vitro Penetration of the Pulp Chamber by Three Brands of Carbamide Peroxide,” Journal of Esthetic and Restorative Dentistry, Vol. 11, No. 5, 1999, pp. 259-264. doi:10.1111/j.1708-8240.1999.tb00407.x
NOTES
*Corresponding author.
1Get2smile Home-Whitening System (Wyten Technology Ltd., UK).
2S2Power Thermal Diffuser (Wyten Technology Ltd., UK).

