Journal of Biomaterials and Nanobiotechnology
Vol.4 No.2(2013), Article ID:29867,14 pages DOI:10.4236/jbnb.2013.42021
Engineering Chitosan Using α, (-Dicarboxylic Acids—An Approach to Improve the Mechanical Strength and Thermal Stability
![]()
1Microbiology Division, CSIR-Central Leather Research Institute, Chennai, India; 2AU-KBC Research Centre, MIT, Anna University, Chennai, India.
Email: *gnanamani3@gmail.com
Copyright © 2013 G. Sailakshmi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 19th, 2013; revised March 5th, 2013; accepted April 7th, 2013
Keywords: Biomaterial; Chitosan; Cytocompatibility; Dicarboxylic Acid; Mechanical Property
ABSTRACT
The current scenario in tissue engineering research demands materials of requisite properties, viz., high porosity, mechanical stability, thermal stability, biocompatibility and biodegradability for clinical applications. However, bringing these properties in single biomaterial is a challenging task, which needs intensive research on suitable cross-linking agents. In the present study, 3D scaffold was prepared with above said properties using chitosan and oxalic (O), malonic (M), succinic (S), glutaric (G), adipic (A), pimelic (P), suberic (SU), azelaic (AZ) and sebacic (SE) acid (OMSGAP-SAS) individually as a non covalent cross-linkers as well as the solvent for chitosan. Assessment on degree of cross-linking, mechanical strength, FT-IR analysis, morphological observation, thermal stability, binding interactions (molecular docking), in vitro biocompatibility and its efficacy as a wound dressing material were performed. Results revealed the degree of cross-linking for OMSGAP-SAS engineered chitosan were in the range between ≈55% - 65% and the biomaterial demonstrated thermal stability more than 300˚C and also exhibited ≥3 - 4 fold increase in mechanical strength compared to chitosan alone. The bioinformatics studies evidently proved the chemistry behind the interaction of OMSGAP-SAS with chitosan. OMSGAP-SAS played dual role to develop the chitosan biomaterial with above said properties, thus matching the requirements needed for various applications.
1. Introduction
Dicarboxylic acids (DCA) are versatile chemical intermediates with different chain length, generally represented as HOOC-(R)n-COOH. These chemical compounds are used as raw materials for the preparation of perfumes, polymers, adhesives and macrolide antibiotics for many years and are well known polymer building block [1]. DCA came to research focus because of its reaction capability with amine (-NH2) and hydroxyl (-OH) group containing compounds through amide (-CONH2) and ester linkage (-COOR) as evidenced in polyamides and polyesters preparations [2-5]. However, the said reaction requires high temperature (>100˚C), which limits its application for the preparations. Alternatively, activation of -COOH group through carbodiimide and/N-hydroxysuccinimide found suitable for the reactions at ambient temperature, results in the formation of active ester (Oacylisourea) as a highly reactive intermediate, which reacts with -NH2 and -OH groups at room temperature. However, the pH dependency, the need for additional steps for purification of the products, the uncontrolled formation of side products, hydrolysis and rearrangement reactions are the major limiting factors identified in this process [6-8]. Hence, DCAs find limited usage in biomaterial/ biopolymer preparations. However, the versatile nature of DCA, if exploited properly, it will solve most of the problems associated with the preparation of biomaterial.
Generally, biomaterial are prepared from bio-macromolecules in the presence of glutaraldehyde [9], and it can easily react with -NH2 group at room temperature and stabilized the bio-macromolecules with the formation of imine (-C=N) linkage. However, recently, for the biomaterials of high mechanical strength and biocompatibility, it has been identified that glutaraldehyde is not a suitable cross-linker or stabilizer because of the instability of the Schiff’s base [10]. Hence, an intensive search for alternative to glutaraldehyde for biomaterial preparations has been initiated at a global level. Related to this, we made an attempt on preparation of chitosan based biomaterial by exploiting the versatile nature of DCAs.
Although chitosan based biomaterials are well recognized for profound biomedical applications, the selection and use of cross-linkers restrict the usage. Apart from various formulations (cream), biomaterials of 2D and 3D forms find applications in various biomedical fields (Tissue engineering: culturing cells, drug delivery and also as a wound dressing material) [11]. These observations suggest the necessity of suitable cross-linkers to transform the chitosan macromolecule to a porous, high strength and biocompatible material for tissue engineering research.
We found, DCA is the best option with the presumption that it may not (i) require high temperature to initiate the reaction, (ii) no need to activate the -COOH groups and (iii) may completely avoid the use of solvents.
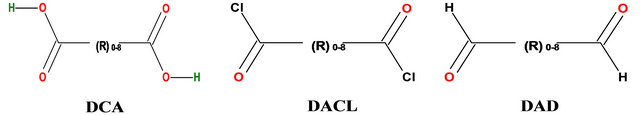
It has been understood that in the series of DCAs, viz., OMSGAP-SAS, were chosen for the present study and the first four (Oxalic, malonic, succinic and glutaric acid) DCAs are soluble in water, whereas, adipic and pimelic are sparingly soluble in water and suberic, azelaic and sebacic acids are completely insoluble in water at room temperature. Though, the carboxyl derivatives of diacid chlorides and dialdehydes are already studied and explored for protein coupling [12] because of the functional groups (-COCl & -CHO) reactivity with the amine group through covalent bond formation (amide and imine linkages), the acid derivatives may also interact with proteins, however, as summarized in the first paragraph, the mesomeric effect of -COOH groups of DCA, requires, high temperature and/activation, and thus restricts the use of DCAs for the preparation of biopolymer materials. However, alike diacid chlorides and dialdehydes, DCAs also can interact with the -NH2 group of chitosan at any temperature without activation of -COOH group of DCA. The present study explores the possibility of said interaction of DCA with chitosan polysaccharide in detail.
In brief, chitosan is usually dissolved in acetic acid because of proton exchange between -NH2 group of chitosan and -COOH group of acetic acid as shown below:
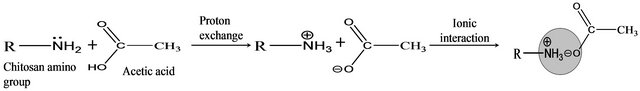
Further, it has been presumed that the bi-functionality of DCA plays a dual role while interacting with the chitosan molecule. The mutual crosstalk between DCAs and chitosan in water medium transforms chitosan polysaccharide to a homogenous solution (similar to the dissolution of chitosan in acetic acid), and the prevalent ionic interactions offered higher strength to the biomaterial. Thus, the present study explores the hypothesis behind the dual role of DCAs with chitosan and the non-toxic nature of DCAs also as an attractive feature to involve DCAs for the preparation of biomaterial. Both dry labs (molecular docking study) and wet lab (preparation of 3D biomaterial and animal models) studies were carried out to substantiate the presumption and our attempt proves that DCA is really an elite compound and suitable for the preparation of biomaterial.
2. Experimental Details
2.1. Materials
Chitosan from shrimp shells (≥75% deacetylated), Oxalic (O), malonic (M), succinic (S), glutaric (G), adipic (A), pimelic (P), suberic (SU), azelaic (AZ) and sebacic acid (SE) and Picrylsulfonic acid [2,4,6-Trinitrobenzene sulfonic acid (TNBS)], were obtained from Sigma-Aldrich (USA). 3-[4,5-Dimethylthiazol-2-yl]-2,5-dephenyltetrazolium bromide (MTT), dexamethasone was purchased from Hi-Media (India). All the other reagents were of analytical reagent grade and used without further purification.
2.2. Engineering Chitosan Scaffold Using DCA
About 1% of chitosan powder was dispersed in water, and to that DCA was added and stirred vigorously. The homogenous solution obtained from the above said process was subjected to centrifugation to remove any unreacted molecules and a clear solution obtained upon centrifugation at 5000 RPM for 10 min was used for the preparation of 3D chitosan biomaterial. For the preparation of 3D scaffold materials, the solution was poured in Tarson (India) vial of an inner diameter of 4.5 cm and frozen at −4˚C for 2 h, −20˚C for 12 h and −80˚C for another 12 h. The frozen samples were lyophilized for 48 h at a vacuum of 7.5 militorr (1 Pa) and a condenser temperature of −70˚C (PENQU CLASSIC PLUS, Lark, India). Followed by the preparations, the samples were subjected to neutralization with repeated washings with 0.05 N NaOH/ ethanol mixtures, neutralized and then lyophilized. The concentration of DCA was varied between 0.05% - 0.5% (w/v) respective of the chosen series of DCAs (OMSGAP-SAS). The biopolymer material obtained from this procedure was designated as DCA-CCH (DCA crosslinked chitosan).
2.3. SEM Analysis of DCACCH
Followed by the preparation, the physical texture and the morphology of the scaffolds of DCA cross-linked chitosan was assessed using physical touch and scanning electron micrograph. SEM micrograph analysis was made using F E I Quanta FEG 200 - High Resolution Scanning Electron Microscope instrument under high voltage at 20 kV.
2.4. FT-IR Analysis of DCA-Engineered Chitosan Scaffolds (DCACCH)
Functional group analysis (FT-IR) for OMSGAP-SAS, chitosan and all DCACCH scaffolds were made by Spectrum One (Perkin-Elmer Co., USA model) FT-IR instrument. All spectra were recorded with the resolution of 4 cm−1 in the range of 400 - 4000 cm−1 with 20 scans.
2.5. Estimation of Percentage of Cross-Linking Degree (TNBS Assay)
Degree of cross-linking was quantified using TNBS assay [13]. In brief, native and all DCA cross-linked scaffold materials were cut into small pieces of size 4.5 mm. Six milligrams of cut pieces were immersed in 2 ml solution [1 ml of 4% (w/v) di-sodium hydrogen orthophosphate and 1 ml of 0.5% (v/v) TNBS], and incubated at 40˚C for 2 h. Termination of reaction was by the addition of 3 ml of 6 M (v/v) HCl and the incubation was continued at 60˚C for 90 min. The percentage of cross-linking was calculated from the difference in the absorbance divided by the absorbance of the native material and then multiplied with 100. The absorbance of the resulting solution was measured at 345 nm using UV-Visible spectrophotometer.
2.6. Mechanical Properties of All DCACCH Scaffolds
Mechanical properties, viz., ultimate tensile strength and percentage of elongation of the dried scaffolds were measured using Universal Testing Machine (INSTRON model 1405) at a crosshead speed of 5 mm min−1 at 25˚C and 65% relative humidity. Length and width of the dumbbell shaped test samples were maintained at 20 and 5 mm respectively. All the mechanical tests were performed with dried samples and were examined in triplicate way.
2.7. Thermo Gravimetric Analysis (TGA)
Thermal decomposition analysis of DCA, native chitosan and all DCACCH scaffolds were carried out under nitrogen flow (40 & 60 ml·min−1) with ramp 20˚C·min−1 using TGA Q 50 (V20.6 build 31) instrument.
2.8. Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) analysis for DCA, native chitosan and all DCACCH scaffolds were analyzed using a differential scanning calorimeter, model -DSC Q 200 (V 23.10 Build 79) with standard mode at nitrogen (50 ml min−1) atmosphere with ramp 10˚C·min−1.
2.9. Docking and Binding Energy Calculations of DCA-Engineered Chitosan Scaffold Material
Docking analysis was carried out to know the binding residues and interactions between biomolecule and chemical compound. In the present study, in addition to docking and binding energy calculations for DCA engineered chitosan (DCACCH), calculations were also attempted for diacid chlorides (DACL) and dialdehydes (DAD) with chitosan for better understanding and substantiation. Structures of di-carboxylic acids (DCA), diacid chlorides (DACL) and dialdehydes (DAD) were generated using ACD/ChemSketch [14] and structure of Chitosan was obtained from PubChem. AutoDock 4.2 [15] force field includes an updated charge-based desolvation term, improvements in the directionality of hydrogen bonds, and several improved models of the unbound state. Current protocol categorized under semiflexible docking in that chitosan was kept as rigid and ligands being docked was kept as flexible; Kollman united atom charges, salvation parameters and polar hydrogen were added to the chitosan PDB file of protein and for ligands, Gasteiger charges were assigned and then non polar hydrogen was merged before docking simulation. Total numbers of rigid roots were defined using automatically with amide bond kept as non-rotatable. The possible dihedral in the ligand is allowed to rotate freely using Auto-Tors.
2.10. Biocompatibility of DCA-Engineered Chitosan (DCACCH) Scaffold—An in Vitro Assessment
Biocompatibility in terms of cytotoxicity (MTT assay) and cell proliferation of the prepared scaffolds were analyzed using NIH 3T3 fibroblast cell line. Cells were grown in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% (v/v) foetal bovine serum and 1% antibiotic and were incubated at 37˚C in 5% CO2 humidified atmosphere. Polystyrene 96 well culture plates (Tarson, India) were coated with chitosan alone and DCACCH scaffolds. The plates were then dried under laminar airflow hood followed by UV sterilization. The cells were seeded at the density of 0.5 × 106 per well and incubated at 37˚C in a humidified atmosphere containing 5% CO2. At scheduled time points of 24, 48, and 72 h, the supernatant of each well was replaced with MTT diluted in serum-free medium, and incubated at 37˚C for 4 h. After removing the MTT solution, acid isopropanol (0.04 N HCl in isopropanol) was added to each well and then left at room temperature for a few minutes to ensure all crystals are dissolved. Finally, absorbance was measured at 570 nm using UV spectrophotometer [16]. Each experiment was performed at least three times. The test samples were run as triplicate.
2.11. Cell Morphology of NIH 3T3 Cells in DCA-Engineered Chitosan (DCACCH) Scaffolds
DCACCH scaffolds (2 × 2 × 1 cm) were placed in 6 well culture plates (Tarson, India) and ETO (Ethylene Oxide) sterilized. Culture media were added to the scaffolds for overnight. NIH 3T3 fibroblast cells were seeded onto the scaffolds at a density of 5 × 104 cells and incubated in an atmosphere of 5% CO2 at 37˚C. The medium was changed every 24 h. Morphology of the cells was examined after 12 days according to the following procedure. The cellsscaffold constructs were fixed in 2.5% glutaraldehyde and dehydrated through a graded ethanol series [17]. The dried cells-scaffold were coated with gold (E-1010 Ion sputter, HITACHI) and examined under SEM (S-3400 N Scanning Electron Microscope, HITACHI).
2.12. Evaluation of Wound Healing Efficacy of DCA-Engineered Chitosan (DCACCH) Scaffold
In order to assess the efficacy of DCACCH as wound cover, animal model study was taken up with prior approval from the ethical committee; with vide approval No.466/01a/CPCSEA for open wound model studies.
Male Albino rats (Wistar strain) of weight ranging over 125 - 150 g were used for the present study. They were housed individually in standardized environmental conditions, feed with pellet rodent diet and water ad libitum. Animals were randomly grouped into three groups with six animals per group. Group-1 (Control—wound covered with cotton soaked in Phosphate buffer saline); Group-II (Wound covered with scaffold prepared from chitosan alone); Group-III (Wound covered with scaffold of DCACCH). An incision of 4 cm (2 cm)2 was created on the dorsal part of all the rats with the help of scissors and surgical blade, and the wound area was sterilized with surgical spirit. Respective dressing scaffolds were applied to animals and closed with gauze. All the rats were received regular dressing changes at every alternative day.
2.13. Rate of Wound Contraction Area
Wound contraction was measured as a percentage reduction in wound size at every 4-day interval and photographed. Progressive decrease in the wound size was monitored periodically by tracing the boundary, and the area was accessed graphically.
2.14. Histological Examination
Granulation tissue was collected at scheduled intervals of healing period and fixed at 10% neutral buffered formalin and then embedded in paraffin. Followed by sectioning, staining was carried out with hematoxylin and eosin (H & E), viewed under a Nikon Eclipse 80i Microscope at 4× magnification and photographed.
2.15. Measurement of Skin Tensile Strength
After complete healing of the wound, skin of healing area was excised and subjected to tensile strength measurements using Universal Testing Machine (INSTRON model 1405) at a crosshead speed of 100 mm·min−1 at 25˚C and 65% relative humidity. Length and width of the dumbbell shaped test samples were maintained at 20 and 5 mm respectively.
3. Results and Discussion
Chitosan, a natural polymer finds immense application in various fields. The realization of its application in biomedical research offered a variety of biomaterials. Despite the intensive research on the transformation of chitosan to novel biomaterial, still search for suitable crosslinkers to have high mechanical strength chitosan based scaffold material is going on. In addition, the insolubility of chitosan in water is the major drawback in the transformation studies. In order to obviate these problems, in the present study, we made to attempt on exploiting the dual role of di-carboxylic acids for the preparation of chitosan based scaffold material. We found, engineering the chitosan with DCAs was a simple technique, and the resultant material has appreciable mechanical properties and also biocompatible. The results obtained from the experimental parts are discussed below with supporting literatures.
Engineering the Chitosan Macromolecules Using DCA: Understanding the Dual Role of DCA
As described in the Introduction, there is a possibility of proton exchange between chitosan and DCA and because of the said proton exchange, chitosan get dissolved in the presence of DCA in water, and the following schematic representations illustrates (Scheme 1) the scenery of proton exchange between DCA and chitosan and emphasizes the strong ionic interactions. Because of the said ionic interactions, chitosan, the natural polymer was completely dissolved in water in the presence of DCA and transformed to the DCACCH scaffold material. Ionic and hydrogen bonding interaction between -NH2 group of chitosan and -COOH molecules were already in reports [18- 21].
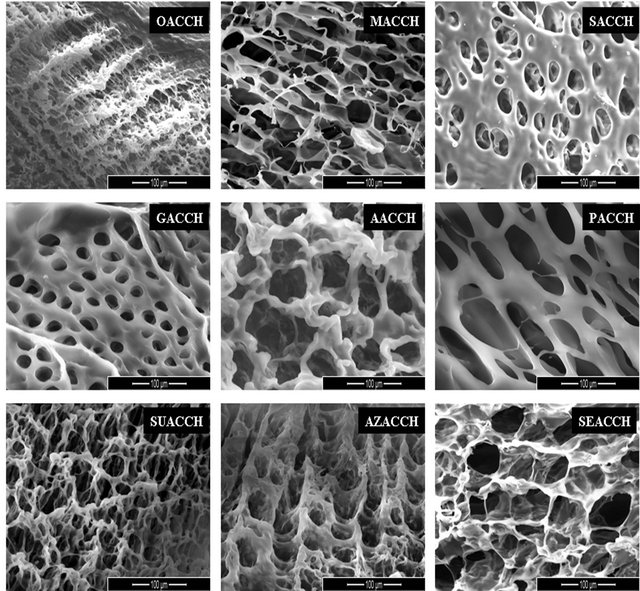
Figure 1(a) demonstrates SEM images of DCA engineered chitosan based scaffolds.
The images depict that the scaffolds were porous in nature, and the porosity increases with an increase in chain length of the DCAs. The increased porosity was well observed with suberic (SU), azelaic (AZ) and sebacic (SE) acid. It was obvious that most of the volume of the membranes was taken up by the interconnecting pore space. The high porosity suggests the suitability of this biopolymer for biomedical applications, including serving as absorption sponges and matrices for cell proliferation. Results obtained from determination of percentage
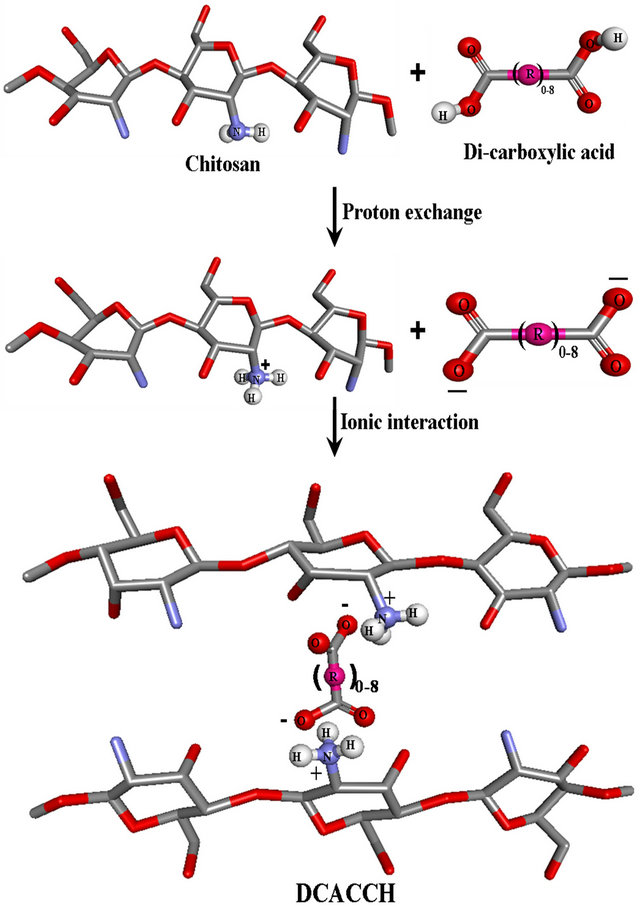
Scheme 1. Proton exchange between chitosan and dicarboxylic acid.
of porosity (experimental details and results are not shown), further authenticate the porous nature of the engineered material. The percentage of porosity was in the range between 94.22% - 96.35% respective to the increasing carbon chain length of dicarboxylic acids chosen for the study.
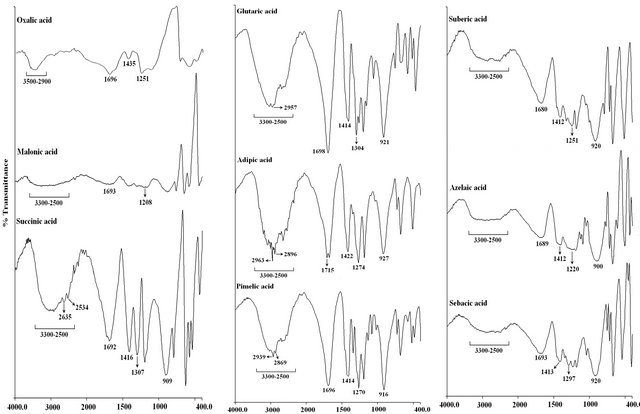
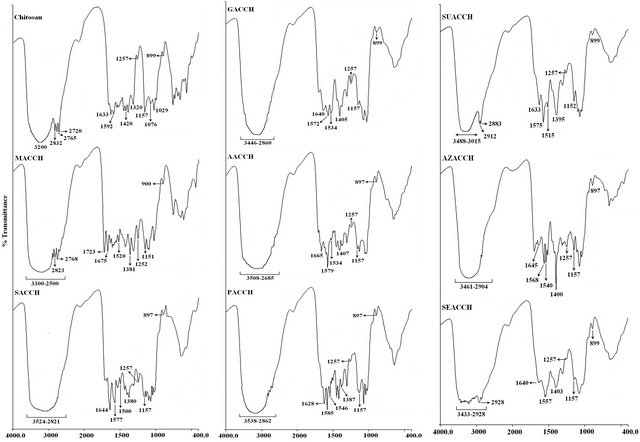
FT-IR studies were conducted to monitor the functional group modifications in engineered chitosan polysaccharide upon interaction with DCA. Figure 1(b) (I) and (ii) illustrates the FT-IR spectral details of OMSGAP-SAS, chitosan and all DCACCH scaffold. Table 1 demonstrates the FT-IR peak assignments of chitosan. In DCACCH spectrum, few significant changes were observed when compared to native chitosan. A broad, strong absorption in the region of 3538 - 2500 cm−1 was resulting from superimposed -OH and -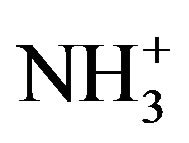 stretching band. Absorptions in the range of 1723 - 1628 and 1675 - 1557 cm−1 correspond to the presence of asymmetric N-H (-
stretching band. Absorptions in the range of 1723 - 1628 and 1675 - 1557 cm−1 correspond to the presence of asymmetric N-H (- ) bend and asymmetric -COO− stretching respectively. Peak observed in the range of 1546 - 1500 and 1407 - 1380 cm−1 was due to symmetric N-H (-
) bend and asymmetric -COO− stretching respectively. Peak observed in the range of 1546 - 1500 and 1407 - 1380 cm−1 was due to symmetric N-H (-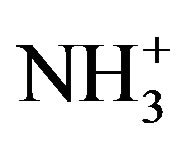 ) bend and symmetric -COO− stretching respectively. Other absorption peaks around 1257, 1157 and 897 cm−1 observed in SACCH spectrum were similar to the native chitosan spectrum which exhibits that there was no change in the main backbone of chitosan structure [22].
) bend and symmetric -COO− stretching respectively. Other absorption peaks around 1257, 1157 and 897 cm−1 observed in SACCH spectrum were similar to the native chitosan spectrum which exhibits that there was no change in the main backbone of chitosan structure [22].
FT-IR spectral analyses confirm the interaction of DCAs with chitosan polysaccharide. However, in order to quantify the interactions, TNBS assay (cross-linking degree analysis) was performed, and the results suggested that increase in concentration of DCA (0.05% - 0.5%), increases the degree of cross-linking up to 0.4% concentration. But, no significant difference was observed on the degree of cross-linking with 0.2%, 0.3% and 0.4% of DCA concentrations. However, about 60% - 65% cross linking was observed with 0.2% DCA with chitosan.
With regard to mechanical property of the scaffold materials (DCACCH), the mechanical property is a fundamental property for any biomaterial in application point of view. From the results, we observed that the mechanical properties (percentage elongation at break and young’s modulus) of the resulting scaffolds were increased by an increase in chain length of DCA (Table 2). Assessment on the role of concentration of each individual DCA on mechanical properties of engineered chitosan scaffold revealed, the maximum properties were observed at 0.2% concentration of DCAs and an additional increase in DCA concentration results in the reduction in mechanical properties (results not shown). An appreciable increase in mechanical properties of DCACCH suggested that the chosen DCAs interacts with chitosan in such a way and TNBS assay substantiate the interaction accordingly.
(a)
Wavenumbers (cm−1) Wavenumbers (cm−1) Wavenumbers (cm−1)
(i)
Wavenumbers (cm−1) Wavenumbers (cm−1) Wavenumbers (cm−1)
(ii)
(b)
Figure 1. (a) SEM Micrographs of all DCA engineered chitosan biopolymers (OACCH—Oxalic acid cross-linked chitosan; MACCH—Malonic acid cross-linked chitosan; SACCH—Succinic acid cross-linked chitosan; GACCH—Glutaric acid crosslinked chitosan; AACCH—Adipic acid cross-linked chitosan; PACCH—Pimelic acid cross-linked chitosan; SUACCH— Suberic acid cross-linked chitosan; AZACCH—Azelaic acid cross-linked chitosan; SEACCH—Sebacic acid cross-linked chitosan); (b) (i) FT-IR spectrum of carboxyl acid derivatives of oxalic, malonic, succinic, glutaric, adipic, pimelic, suberic, azelaic and sebacic; (b) (ii) FT-IR spectrum of all DCA engineered chitosan biopolymer materials (MACCH—Malonic acid cross-linked chitosan; SACCH—Succinic acid cross-linked chitosan; GACCH—Glutaric acid cross-linked chitosan; AACCH —Adipic acid cross-linked chitosan; PACCH—Pimelic acid cross-linked chitosan; SUACCH—Suberic acid cross-linked chitosan; AZACCH—Azelaic acid cross-linked chitosan; SEACCH—Sebacic acid cross-linked chitosan).
Table 1. FT-IR analysis of chitosan.
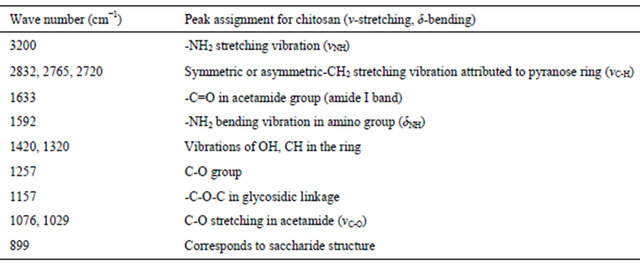
Table 2. Assessment of mechanical properties of native chitosan and DCA engineered chitosan scaffolds.
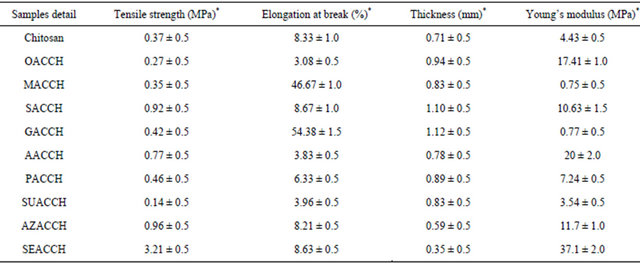
*mean ± SD values.
Results of thermal analysis of the experimental samples DCA, chitosan and DCACCH were displayed in Table 3(a). It has been observed that incorporation of DCA with chitosan tends to shift the thermal region of chitosan (uncross-linked) to higher temperature, and such a shift was attributed to an increase in thermal stability.
Differential scanning calorimetry (DSC) studies were performed to understand the behavior of DCACCH on application of thermal energy. Exothermic peak values from the thermograms of DCA, chitosan and all DCACCH were illustrated in Table 3(b). The higher transition temperature suggests, DCACCH had higher stability at hightemperature environment. The thermal stability also influences the durability of the scaffolds.
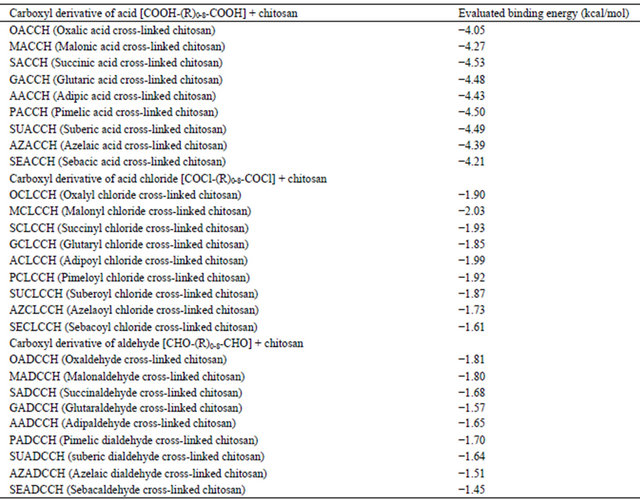
Though all these observations explicitly demonstrated the cross-linking ability of DCA with chitosan, however, understanding the mode of cross-linking between these two components through molecular docking studies using bioinformatics tool software (Autodock 4.2) may add value to the findings. As described in the introduction, the carboxyl derivatives of diacid chlorides and dialedhydes interacted with -NH2 group of protein/polysaccharides through covalent linkage. Theoretically, because of the covalent interactions, there will be only meager intermolecular hydrogen bonding interactions were expected in diacid chlorides and/dialehydes with -NH2 group. But, in the present study, with simple acid derivatives (DCAs) we can’t expect the covalent linkage as described in the previous paragraphs. The interactions observed in the present study between chitosan and DCA could be noncovalent interaction. Further, to understand the possible intermolecular hydrogen bonding interactions between the -NH2 group of chitosan and -COOH group of DCA, we follow the molecular docking studies. In addition, comparisons on binding energy were also made from carboxyl derivatives of diacid chlorides, the dialdehydes with chitosan to assess the intermolecular hydrogen bonding interaction. This can be explained as; in DCA, there is 2 H-bond donor site (green color) and 4 H-bond acceptor site (red color), however, DACL displayed 0 (Zero) H-bond donor site and 2 H-bond acceptor site (red color) and DAD displayed 0 (Zero) H-bond donor site and 2 H-bond acceptor site (red color). Because of the available hydrogen bond donor and acceptor site, the intermolecular interaction through hydrogen bonding occurs with chitosan. The dotted lines in Figure 2(a) illustrated the possible intermolecular hydrogen bond for all DCAs with chitosan.
Autodock is an automated procedure for predicting the interaction of ligands with bio-macromolecular targets. Hundred runs were given for docking. The best binding energy values for all DCA, DACL and DAD docked Chitosan biopolymers were depicted in Table 4. Results proved that chitosan could be cross-linked with DCA with ionic interaction and also through multiple intermolecular hydrogen bonding. From this table, it has been understood that the carboxyl derivatives of diacid chlorides, and dialdehydes demonstrated less binding energy compared to the acid derivatives (OMSGAP-SAS) and these observations further authenticate the presence of intermolecular hydrogen bonding interactions. In addition, with increasing the aliphatic chain length in DCA, an increase in binding energy value was observed, however, with insignificant difference.
Though we can be able to interact with DCAs with chitosan for the preparation of scaffold material with appreciable properties, additional studies on biocompatibility are required to transform the DCA engineered mateTable 3. (a) Thermal analysis of DCA (OMSGAP-SAS) and all DCA engineered chitosan scaffolds under N2 air atmosphere; (b) Differential scanning calorimetry analysis of DCA (OMSGAP-SAS) and all DCA engineered chitosan scaffolds under N2, air atmosphere.
 (a)
(a)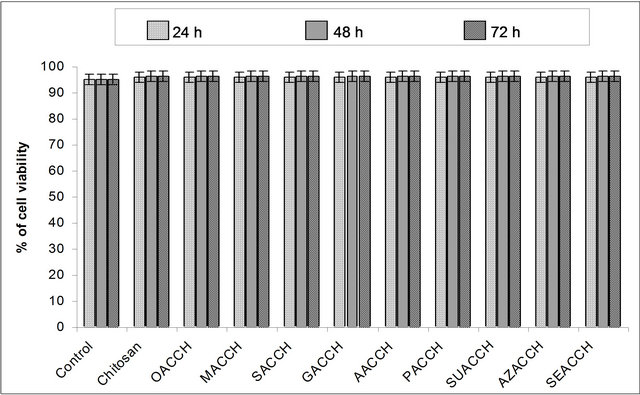 (b)
(b)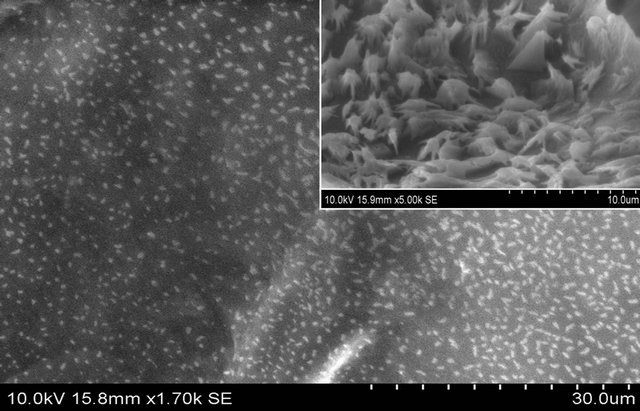 (c)
(c)
Figure 2. (a) Multiple hydrogen bonding interactions between the -NH2 group of chitosan and -COOH group of DCAs (OA— Oxalic acid; MA—Malonic acid; SA—Succinic acid; GA—Glutaric acid; AA—Adipic acid; PA—Pimelic acid; SUA— Suberic acid; AZA—Azelaic acid; SEA—Sebacic acid); (b) NIH 3T3 fibroblast cell cytotoxicity assay performed for the DCA engineered chitosan biopolymer materials; Control, Chitosan, OACCH, MACCH, SACCH, GACCH, AACCH, PACCH, SUACCH, AZACCH, SEACCH measured at 24, 48 and 72 hours (Values are the mean ± SD values of triplicate analysis); (c) Attachment of fibroblast cells on the DCA engineered chitosan scaffold biopolymer material (SEACCH).
Table 4. The best binding energy values of all carboxyl derivatives of DCA (di-carboxylic acid), DACL (diacid chlorides) and DAD (dialdehydes) engineered chitosan scaffolds.
rial in various applications. Results on cytotoxicity assay (MTT) as shown in Figure 2(b), reveals that the engineered material is not toxic to the cells.
The cell attachment studies demonstrated, cells are attached to the porous structure of the engineered material as shown in SEM images (Figure 2(c)). Though cell attachment studies were performed for all DCACCH scaffolds, SEM image of Sebacic acid engineered chitosan was shown as a representative image, which, illustrated the presence of fibroblast cells with typical spindle shape after being cultured for a prolonged time and suggests that the cells were infiltrated to the scaffold.
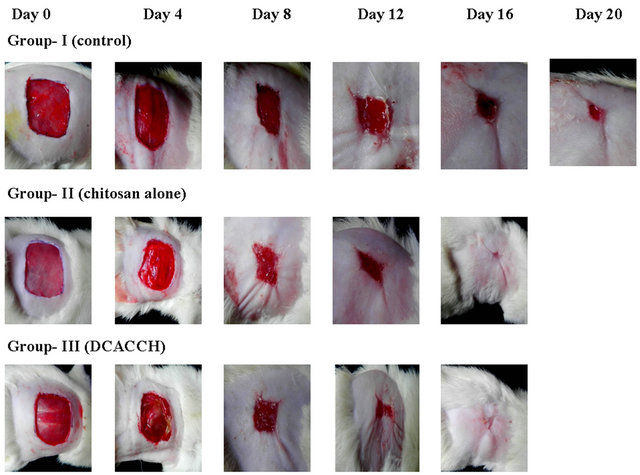
Followed by the observations on MTT and cell attachment studies, the suitability of the DCA engineered scaffolds for biomedical applications was further subjected to test as a wound dressing material (open wound) using animal models (Wistar strain). Figure 3(a) illustrates the progressive wound closure of three different experimental groups.
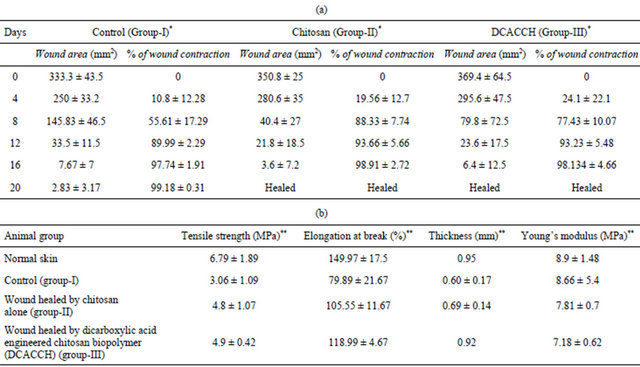
The control group (Group-I) took more than 20 days for complete closure, whereas, complete wound closure was evidenced within 16 days for Group-II (chitosan alone) and Group-III (DCA engineered biopolymer) animals. Similarly, wound contraction measurements (Table 5(a)) also showed accelerated wound closure in both Group-II and Group-III animals compared to Group-I. The results discussed over here were the representative results obtained for sebacic acid engineered chitosan scaffold.
Further, histological examination of the skin after 16 days of the experimental period showed that the healing of wound took progressive steps and remodeled with well structured epidermis and dermis along with regenerated hair follicles with dense collagen fibers compared to
 (a)
(a)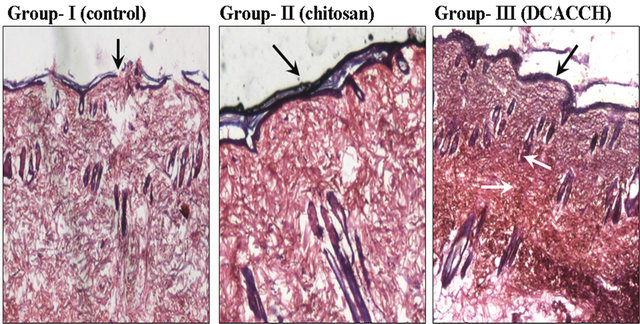 (b)
(b)
Figure 3. (a) Open wound closer upon treatment with chitosan and DCACCH (DCA engineered chitosan) scaffold biopolymer materials; (b) The H & E staining of 16th day granulation tissue sections (4×) (The black & white arrow mark indicates the remodeled epidermis, dermis and regenerated hair follicles).
Table 5. (a) Wound contraction measurements in experimental groups of animals; (b) Mechanical profile of the skins of experimental groups (Group-I, II and III) after healing of wounds in comparison with normal skin of rat.
*mean ± SD values, DCACCH—DCA engineered chitosan scaffold material; **mean ± SD values.
control Figure 3(b).
Additional experiments on testing of tensile strength of the healed skin of all the experimental groups demonstrated that the tensile strength of both Group-II and Group-III were comparatively higher than the control groups and more or less equal to the normal animal skin (Table 5(b)).
These observations suggest, DCA engineered chitosan accelerated the healing of wound in such a way that the skin retrieved its properties back within a short time period.
4. Conclusion
In the present study, an attempt was made on to exploit the dual role of α, w-di-carboxylic (OMSGAP-SAS; Oxalic, Malonic, Succinic, Glutaric, Adipic, Pimelic, Suberic, Azelaic and Sebacic) acids for the transformation of chitosan polysaccharide to high mechanical strength and highly porous scaffold material for advanced tissue engineering research. The study explicitly explored the simple intermolecular proton exchange between dicerboxylic acid and chitosan in water, dissolute the chitosan and built the non-covalent interactions (ionic interaction and multiple intermolecular hydrogen bonding) between -NH2 group of chitosan and -COOH group of DCAs, transforms chitosan to a high strength porous scaffold material. The interaction was assessed using various experimental tools and authenticated with docking study. The reason behind the interaction was explained with schematic diagrams. Necessary comparisons on binding energy calculations for chitosan interactions with carboxyl derivatives of diacid chlorides and dialdehydes were included for better understanding. Animal model wound healing studies further explored, DCA engineered chitosan scaffold acts as a wound dressing material, and however, the engineered material can be exploited for various biomedical applications because of the high mechanical, biocompatible and porous characteristics. Ultimately, the study provides a new avenue for the preparation of chitosan scaffold with said properties for tissue engineering research using DCA, without incorporating any activation agents and solvents. The process of preparation was simple and can be executed even at room temperature. Among the di-carboxylic acid series taken for the study, our choice for tissue engineering research in the future will be with sebacic acid engineered chitosan material because of the porosity, flexibility and biocompatibility.
5. Acknowledgements
One of the authors Mr. Tapas Mitra acknowledges CSIR, New Delhi for the financial assistance provided in the form of CSIR-SRF. All the authors acknowledge Dr. Swaraj Sinha, AU-KBC Centre, MIT, Anna University, for his kind help in performing cell lines studies.
REFERENCES
- S. Huf, S. K. Gener, T. Hirth, S. Rupp and S. Zibek, “Biotechnological Synthesis of Long-Chain Dicarboxylic Acids as Building Blocks for Polymers,” European Journal of Lipid Science and Technology, Vol. 113, No. 5, 2011, pp. 548-561. doi:10.1002/ejlt.201000112
- Y. Wang, G. A. Ameer, B. J. Sheppard and R. Langer, “A Tough Biodegradable Elastomer,” Nature Biotechnology, Vol. 20, No. 6, 2002, pp. 602-606. doi:10.1038/nbt0602-602
- E. Valeur and M. Bradley, “Amide Bond Formation: Beyond the Myth of Coupling Reagents,” Chemical Society Reviews, Vol. 38, No. 2, 2009, pp. 606-631. doi:10.1039/b701677h
- S. M. Papkov, R. Langer and A. J. Domb, “Synthesis of Aliphatic Polyesters by Polycondensation Using Inorganic Acid as Catalyst,” Polymers for Advanced Technologies, Vol. 22, No. 5, 2009, pp. 502-511. doi:10.1002/pat.1541
- D. G. Barrett, W. Luo and M. N. Yousaf, “Aliphatic Polyester Elastomers Derived from Erythritol and α, ω-diacids,” Polymer Chemistry, Vol. 1, 2010, pp. 296-302. doi:10.1039/b9py00226j
- L. H. Olde Damink, P. J. Dijkstra, M. J. Van Luyn, P. B. Van Wachem, P. Nieuwenhuis and J. Feijen, “CrossLinking of Dermal Sheep Collagen Using a Water-Soluble Carbodiimide,” Biomaterials, Vol. 17, No. 8,1996, pp. 765-773. doi:10.1016/0142-9612(96)81413-X
- F. Everaerts, M. Torrianni, M. Hendriks and J. Feijen, “Biomechanical Properties of Carbodiimide Crosslinked Collagen: Influence of the Formation of Ester Crosslinks,” Journal of Biomedical Materials Research Part A, Vol. 85, No. 2, 2008, pp. 547-555. doi:10.1002/jbm.a.31524
- K. Nam, T. Kimura and A. Kishida, “Controlling Coupling Reaction of EDC and NHS for Preparation of Collagen Gels Using Ethanol/Water Co-Solvents,” Macromolecular Bioscience, Vol. 8, No. 1, 2008, pp. 32-37. doi:10.1002/mabi.200700206
- C. Tual, E. Espuche, M. Escoubes and A. J. Domard, “Transport Properties of Chitosan Membranes: Influence of Crosslinking,” Journal of Polymer Science Part B: Polymer Physics, Vol. 38, No. 11, 2000, pp. 1521-1529. doi:10.1002/(SICI)1099-0488(20000601)38:11<1521::AID-POLB120>3.0.CO;2-#
- C. D. Meyer, C. S. Joiner and J. F. Stoddart, “TemplateDirected Synthesis Employing Reversible Imine Bond Formation,” Chemical Society Reviews, Vol. 36, No. 11, 2007, pp. 1697-1844. doi:10.1039/b513441m
- P. K. Dutta, J. Dutta and V. S. Tripathi, ”Chitin and Chitosan: Chemistry, Properties and Applications,” Journal of Scientific and Industrial Research, Vol. 63, No. 1, 2004, pp. 20-31.
- S. S. Wong, “Chemistry of Protein Conjugation and Crosslinking,” CRC Press, Boca Raton, 1991.
- W. A. Bubnis and C. M. Ofner, “The Determination of ε-Amino Groups in Soluble and Poorly Soluble Proteinaceous Materials by a Spectrophotometric Method Using Trinitrobenzenesulfonic Acid,” Analytical Biochemistry, Vol. 207, No. 1, 1992, pp. 129-133. doi:10.1016/0003-2697(92)90513-7
- ACD/ChemSketch Version 12, Advanced Chemistry Development, Inc., Toronto, 2009.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, “Autodock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility,” Journal of Computational Chemistry, Vol. 30, No. 16, 2009, pp. 2785-2791. doi:10.1002/jcc.21256
- T. Mossmann, “Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays,” Journal of Immunological Methods, Vol. 65, No. 1-2, 1983, pp. 55-63. doi:10.1016/0022-1759(83)90303-4
- S. E. Kim, Y. W. Cho, E. J. Kang, I. C. Kwon, E. B. Lee, J. H. Kim, H. Chung and S. Y. Jeong, “Three-Dimensional Porous Collagen/Chitosan Complex Sponge for Tissue Engineering,” Fibers and Polymers, Vol. 2, No. 2, 2001, pp. 64-70. doi:10.1007/BF02875260
- Q. X. Li, B. Z. Song, Z. Q. Yang and H. L. Fan, “Electrolytic Conductivity Behaviors and Solution Conformations of Chitosan in Different Acid Solutions,” Carbohydrate Polymers, Vol. 63, No. 2, 2006, pp. 272-282. doi:10.1016/j.carbpol.2005.09.024
- N. B. Milosavljevic, L. M. Kljajevic, I. G. Popovic, J. M. Filipovic and M. T. K. Krusic, “Chitosan, Itaconic Acid and Poly (Vinyl Alcohol) Hybrid Polymer Networks of High Degree of Swelling and Good Mechanical Strength,” Polymer International, Vol. 59, No. 5, 2010, pp. 686-694.
- R. Vijayaraghavan, B. C. Thompson, D. R. MacFarlane, K. Ramadhar, M. Surianarayanan, S. Aishwarya and P. K. Sehgal, “Biocompatibility of Choline Salts as Crosslinking Agents for Collagen Based Biomaterials,” Chemical Communication, Vol. 46, No. 2, 2010, pp. 294-296. doi:10.1039/b910601d
- P. H. Chen, Y. H. Hwang, T. Y. Kuo, F. H. Liu, J. Y. Lai and H. J. Hsieh, “Improvement in the Properties of Chitosan Membranes Using Natural Organic Acid Solutions as Solvents for Chitosan Dissolution,” Journal of Medical and Biological Engineering, Vol. 27, No. 1, 2007, pp. 23- 28.
- D. L. Pavia, G. M. Lampman and G. S. Kriz, “Introduction to Spectroscopy,” In: J. Vondeling and S. Kiselica, Eds., Infrared Spectroscopy, Harcourt College Pub, Orlando, 2001.
NOTES
*Corresponding author.

