Journal of Biomaterials and Nanobiotechnology
Vol.3 No.3(2012), Article ID:20590,6 pages DOI:10.4236/jbnb.2012.33037
Cytotoxicity of Pristine C60 Fullerene on Baby Hamster Kidney Cells in Solution
![]()
School of Public Health, Shandong University, Jinan, China.
Email: *chem@sdu.edu.cn
Received May 23rd, 2012; revised June 25th, 2012; accepted July 2nd, 2012
Keywords: C60; Cell; Cytotoxicity; Fullerene; Toxicity
ABSTRACT
Research on biological effects of C60 and its derivatives is one of the hotspots in the area of biological effects of nanomaterials. Compared with surface-modified C60 derivatives, reports on the biological effects of unmodified pristine C60 are relatively less. This work aimed to investigate the interaction between baby hamster kidney cells and pristine C60 in solution. The C60 suspension was prepared using solvent exchange method and characterized by UV spectro-photometry, electronic transmission microscopy and dynamic light scattering techniques. The baby hamster kidney cells were incubated with different concentrations of C60 suspensions, and light microscopy, cell counting kit 8 assay, and acridine orange staining were used to observe the cell growth and morphology. The results showed that C60 could inhibit the cell growth and induce cell apoptosis with a dose-effect relationship. C60 might enter cells and the possible way it enters cells were also proposed.
1. Introduction
With the rapid development of nanoscience and nanotechnology, research on biological effects of nanomaterials has become a hotspot in the fields of nanoscience in recent years, among which the cellular biological effects of nanomaterials are the basis for comprehending their biological effects (positive and negative). As one of the common nanomaterials, due to their unique structure and physical and chemical properties, C60 fullerene and its derivatives have great potential applications in physical, chemistry, materials, life and pharmaceutical sciences [1-3]. Previous researches have proved that C60 and its derivatives could exhibit cytotoxicity to some cell lines, inhibiting cell growth and causing cell apoptosis [4-9], while under certain conditions it can protect cells as free radical scavenger [10]. The biological properties of nanomaterials are not only simple extracellular interactions, but also more likely the results of intracellular interactions, therefore, study on the effect of nanomaterials on cell structure, intracellular localization of nanomaterials and the ways into cells has great value for comprehending the demonstrated biological characteristics and the potential biomedical applications of nanomaterials. As for C60 and its derivatives, there have several speculations about their ways into cells, passive diffusion [11, 12], endocytosis [13,14] and receptor-mediated endocytosis [15]. They mainly deposited in the cytoplasm, nucleus, lysosome [14,16], cell membrane [17] and mitochondria [15,17].
However, due to the complicated affecting factors, such as diversity of the toxicity evaluation methods, cell lines models, the different methods of preparation and purification of nanomaterials and other possible reasons, the results are lack of comparability and still remain unclear and controversial, and more direct evidence is needed. Moreover, C60 is a prerequisite for the synthesis of its derivatives, and people are easily accessible to it during the production and/or research process. But since C60 is insoluble in water, the related research on the cellular biological effects of fullerenes mainly concentrated on the water-soluable C60 derivatives, while relatively little attention was given to water-insoluble C60. The description of prepared C60 suspension in some previous reports about pristine C60 cytotoxicity was not clear enough, and researches on whether or not C60 could enter cells and the possible ways were relatively less, and the results remains unclear and controversy.
In this paper, in order to better understand C60 cytotoxicity, baby hamster kidney (BHK-21) cells served as subject, the interaction between C60 and BHK-21 cells was investigated. The C60 suspension was prepared by solvent exchange method and characterized, and the light microscopy, cell counting kit 8 (CCK-8) analysis and acridine orange staining were utilized to observe the cell growth and changes of cell viability and morphology. Experimental results showed that C60 could exhibit certain toxicity to BHK-21 cells, and the possible interaction mechanism between them was also discussed.
2. Materials and Methods
2.1. Materials
C60 (99.9%) was purchased from Henan Puyang Yongxin Technology Co., Ltd. (China). Toluene (Chromatographic grade) was purchased from Tianjin Kermel Chemical Reagent Development Center (China). Baby hamster kidney (BHK-21) cells were purchased from Shanghai Institute of Biochemistry and Cell Biology (China). High glucose Dulbecco’s Modified Eagle Medium (DMEM) medium was purchased from Hyclone (USA). Penicillin/Streptomycin solution (10,000 U penicillin/10 mg/mL streptomycin), Trypsin (with EDTA) and RPMI- 1640 medium (with 2.05 mM L-Glutamine) were purchased from Hyclone (Life Technologies, USA). Fetal calf serum was purchased from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Zhejiang, China). 6- and 96-wel cell culture plates were purchased from Corning Life Sciences (USA). Cell Counting Kit-8 was purchased from Dojindo Laboratories (Kumamoto, Japan). Acridine orange was purchased from Ameresco (USA). Phosphate buffer solution (PBS) premixed reagents (pH 7.2 - 7.4) were obtained from Zhongshan Goldenbridge Biotechnology Co., LTD. (Beijing, China). Eth-anol (95%, A.R.) was purchased from Fuyu Fine Chemical Industry Co., Ltd. (Tianjin, China). All solutions were prepared with 18.3 MΩ distilled water (Millipore, USA).
2.2. Instruments
CO2 incubator (Forma Scientific, USA) was used for the cell culture. Inverted microscope (Olympus IX70 112, Japan) was used to observe the cell morphology. UV- 1101 spectrophotometer (Shimadzu, Japan), H 7000 transmission electron microscopy (Hitachi, Japan) and BI- 200SM/BI-9000 laser light scattering system (Brookhaven, USA) were used to characterize the C60 suspension. Microplate spectrophotometer (Thermo labsystems, USA) was used to measure the optical density of CCK-8 to estimate cell viability.
2.3. Preparation of C60 Suspensions
20 mL C60 was completely dissolved in 5 mL toluene and then mixed with 50 mL distilled water. The mixture was dispersed by ultrasound for 5 h and then allowed to stand for 30 minutes. The lower layer was separated and sterilized at 121˚C for 30 min. The C60 suspension was stored in a 4˚C refrigerator.
2.4. Cell Culture and CCK-8 Assay
BHK-21 cells were cultured in High glucose DMEM medium supplemented with 10% fetal calf serum and 1% Penicillin/Streptomycin solution, at 37˚C in 5% CO2 humidified incubator. The medium was exchanged every other day and the cells were passaged every 4 days. Different concentrations of C60 suspension were added into the 6- or 96-well cell culture plates and BHK-21 cells without C60 were used as control. The cell growth and apoptosis were observed by light microscopy to evaluate the cytotoxicity of different C60 suspensions. Cell Counting Kit-8 was applied to evaluate the effect of C60 on BHK-21 cells viability after 48 h exposure. 100 µL/5000 cells for each well of 96-well plates were inoculated for the measurement. The cell viability was calculated by the following formula: cell viability (%) = (optical density (OD) of the treated cells – OD without cells)/(OD of nontreated cells – OD without cells).
2.5. Acridine Orange Staining
The BHK-21 cells were seeded to a 6-well plate preplaced cover slip on the bottom of each well and then incubated with different concentrations of C60 suspension for 48 h. Aspirate the culture medium, add 1 mL PBS to each well and then rinse the wells twice. Aspirate the PBS solutions and add 1 mL 95% ethanol to each well. 15 min later, aspirate ethanol and let the wells air dry. Add sufficient amount of 0.01% acridine orange to the cover slips, stain 10 min and then rinse the wells with 1 mL PBS twice. Place a drop of PBS in the center of the microscope slide, take the treated cover slip over the slide and then observe the cells with a blue-violet excitation filter.
3. Results
3.1. The UV-Vis Spectrum of C60 Suspension
The UV-vis spectroscopy is commonly used for characterize nanomaterials. The C60 suspension was diluted and scanned in the range of 230 - 500 nm, and the result was shown in Figure 1.
As can be seen from Figure 1, the C60 suspension (diluted solution) had absorption in the range of 320 - 400 nm with a peak wavelength of 340 nm, which represented the characteristic absorption of C60. The concentration of prepared C60 suspension was quantitated by spectrophotometry at 340 nm and its concentration was 0.24 mg/mL.
3.2. The Size Distribution of nC60 Nanoparticles in C60 Suspension
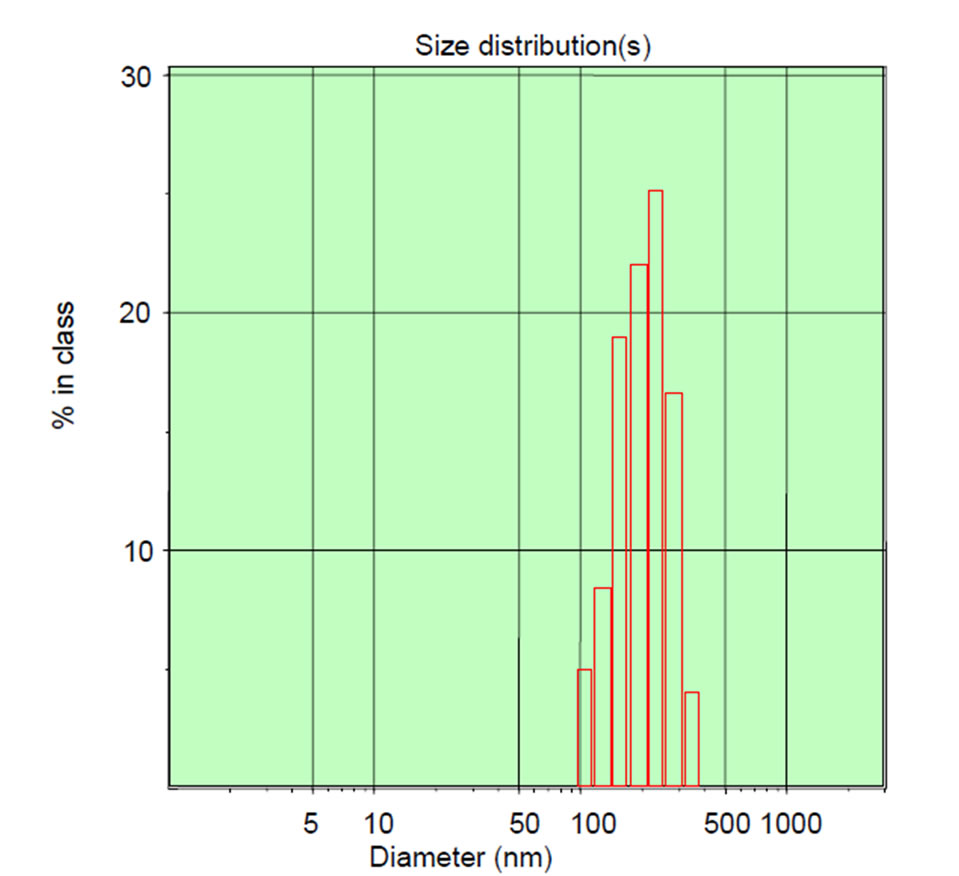
Dynamic light scattering (DLS) is a technique for measuring the hydrodynamic size distribution of molecules
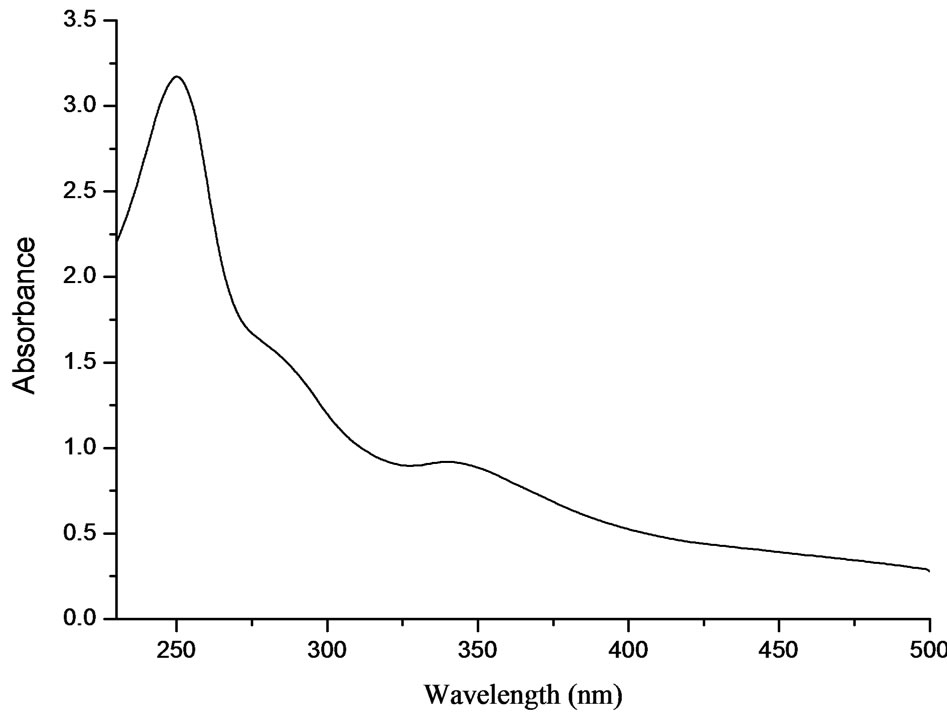
Figure 1. UV-vis spectroscopy of the nano-C60 aggregated suspension (diluted).
and submicron particles, while TEM can directly offer the shape and size information of submicron particles in dry state. Due to the different principles, the size distributions of the same sample by the two methods are often different. In this work, the size distribution of nC60 nanoparticles in prepared C60 suspension was measured by DLS and TEM techniques and the results were shown in Figure 2.
From the DLS measurement, it can be seen that the nC60 nanoparticle were mainly distributed in the range of 105 - 290 nm with an average particle size of 175.8 nm. From the TEM images, most of the nC60 nanoparticles were gathered and roughly round shaped with a size distribution of 50 - 120 nm (Figure 2(b) (up)). Some nC60 nanoparticles had small size distributions, about 5 - 10 nm and even smaller (Figure 2(b) (down)). The above results indicated that pristine C60 was in the form of rough round particles with a wide size distribution.
3.3. Changes of BHK-21 Cells Morphology after Exposure to nC60 Nanoparticles
In order to understand the effects of C60 on BHK-21 cell growth and morphology, different amounts of C60 suspension were added to the cell cultures. After 48 h incubation, the cell growth and changes of cell morphology were observed by electron microscope and the results were shown in Figure 3.
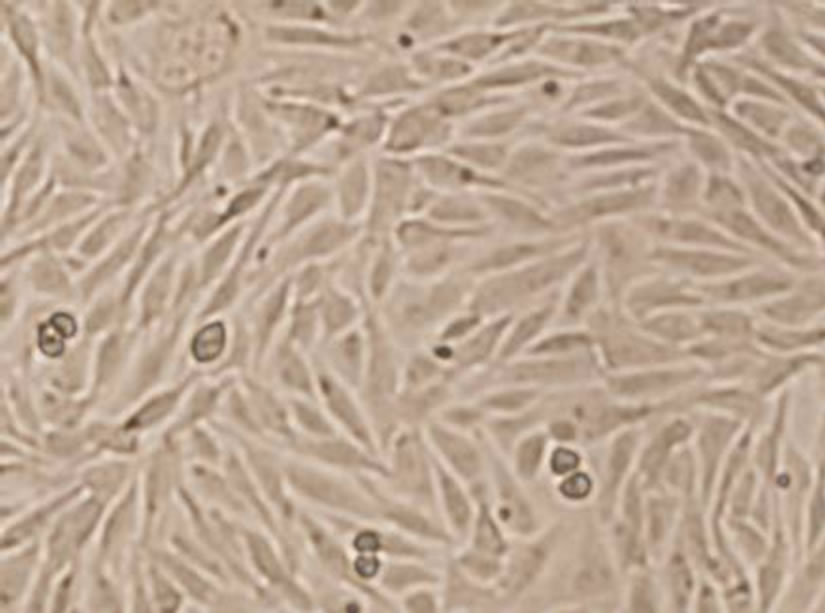
From Figure 3, the BHK-21 cells of control group showed ovoid and polygonal in mosaic arrangement (Figure 3(a)). No significant changes of cell morphology were observed when exposed to 0.09 mg/mL C60 suspension, but necrosis of some cells ocurred and the cell density decreased slightly. When the concentration of C60 suspension was increased to 0.12 mg/mL, the number of dead cells further increased and the cell density obvious decreased, and a few cells shrank. The results indicated
 (a)
(a)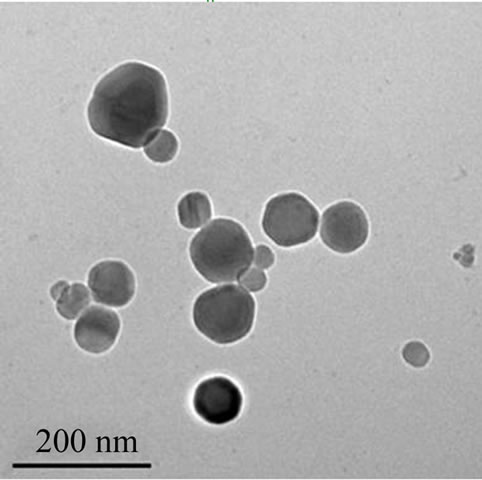 (b)
(b)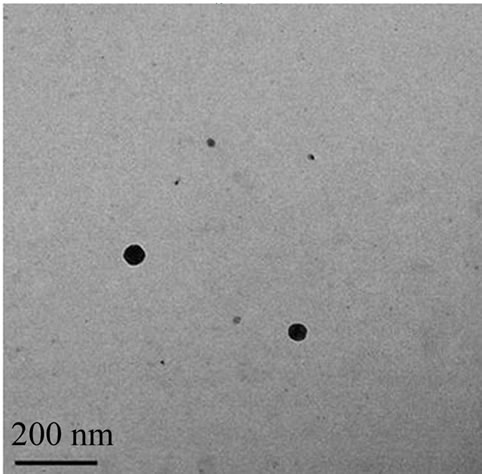 (c)
(c)
Figure 2. The size distribution of nC60 particles. By DLS technique (a); By TEM (b), (c).
that C60 can affect the growth of BHK-21 cells to some extent and cause apoptosis with a dose-response relationship.
3.4. The Cell Viability Measured by Cell Counting Kit-8 (CCK-8)
The CCK-8 assay is an upgrade alternative for the evaluation of cell viability and/or proliferation. Compared with the regular 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-diphenytetrazoliumromide (MTT) assay, it has the advantages of higher sensitivity, better solubility and stability
 (a)
(a)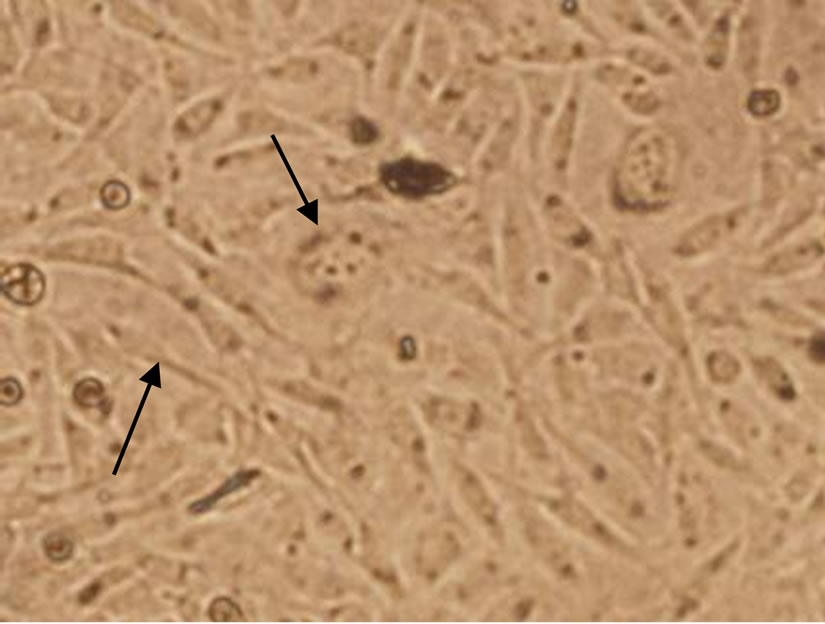 (b)
(b)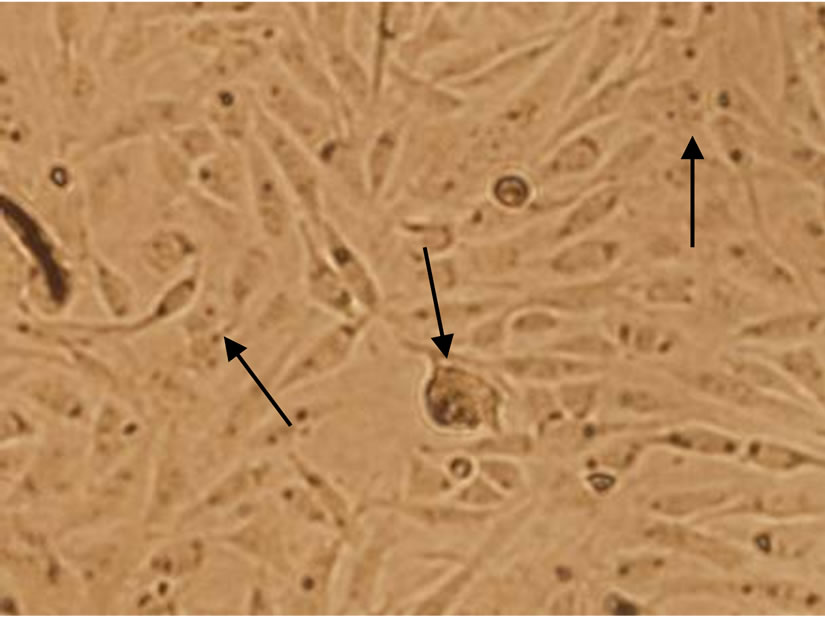 (c)
(c)
Figure 3. Changes of BHK-21 cells morphology after exposure to nC60 (Cells with shrank or apoptosis were indicated by arrows. Concentrations of C60 suspensions: (a) 0 mg/mL; (b) 0.09 mg/mL; (c) 0.12 mg/mL).
and can avoid the occasional false-positive phenomenon. In this work, after 48 h incubation with different concentrations of C60 suspension, the changes of cell viability were shown in Figure 4.
From Figure 4, C60 exhibited certain inhibition to BHK-21 cells with a dose-response relationship. With the increasing concentration of C60 suspension, the cell viability decreased. When the concentration of C60 was increased to 0.32 mg/mL, the cell viability was reduced
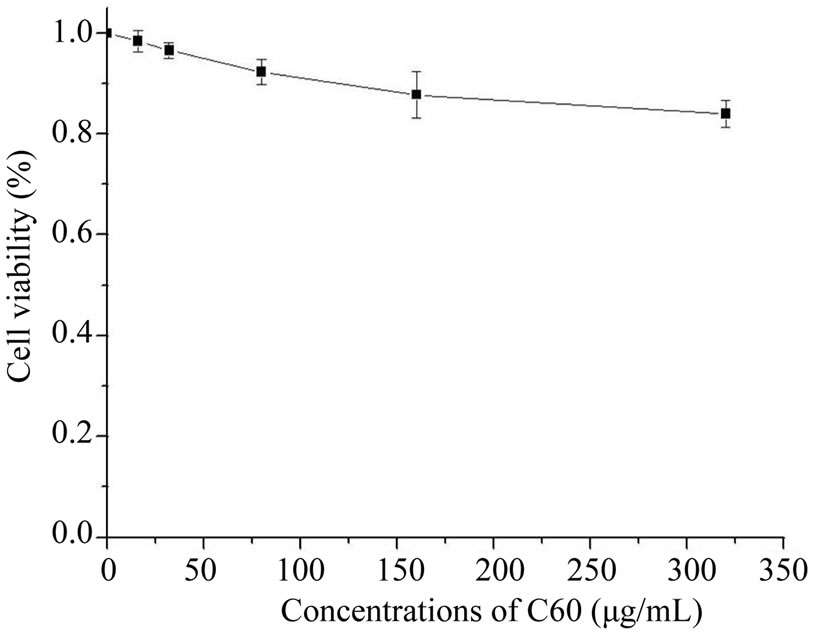
Figure 4. The cell viability at different concentrations of C60 suspension.
to about 84% of the control group. The results indicated that C60 exhibited certain toxicity to BHK-21 cells and caused the decrease of cell viability.
3.5. Acridine Orange Staining of BHK-21 Cells
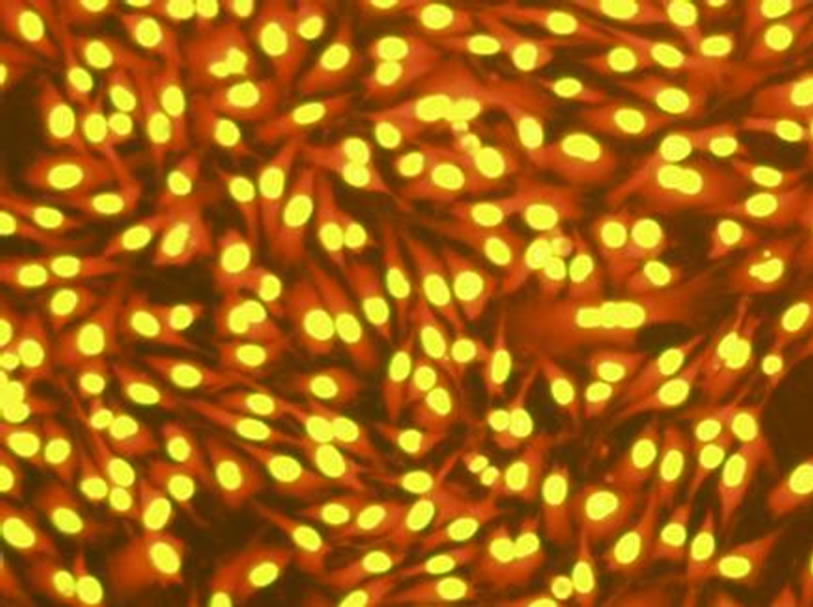
Acridine orange staining (AO) is a commonly used morphological method for the detection of cell apoptosis. Acridine orange can stain both live and dead cells, which can permeate cells and bind DNA and RNA to give two fluorescences of green (or yellow) and red (or orange). According to the changes of cell morphology after staining, we could get some information of cells apoptosis caused by exogenous substances. In this work, after incubated with different concentrations of C60 for 48 h, the fluorescence staining in BHK-21 cells were shown in Figure 5.
As it can be seen from Figure 5, after acridine orange staining, the nucleus of BHK-21 cells emitted bright yellow fluorescence and the cytoplasm emitted bright orange fluorescence. The cell morphology of control group (Figure 5(a)) was integrity, and the nuclei were round or elliptical. When 0.09 mg/mL C60 suspension was added to the cell culture, the number of cells which were capable of emitting fluorescent decreased, and a few fluorescent fragments appeared around the cells. When the concentration of C60 suspension was added to 0.12 mg/mL, the nuclear chromatin condensation could be observed, and more fluorescent fragments appeared. The results indicated the cell membranes and nuclei were destroyed by C60 with a dose-response relationship and the DNAs leaked. It was prompted that C60 might penetrate the cells and even nuclei.
4. Discussion
In this work, from Figure 2 it can be seen C60 was in the form of nC60 nanoparticles with a relatively wide distribution, which indicated that the interaction between C60
 (a)
(a)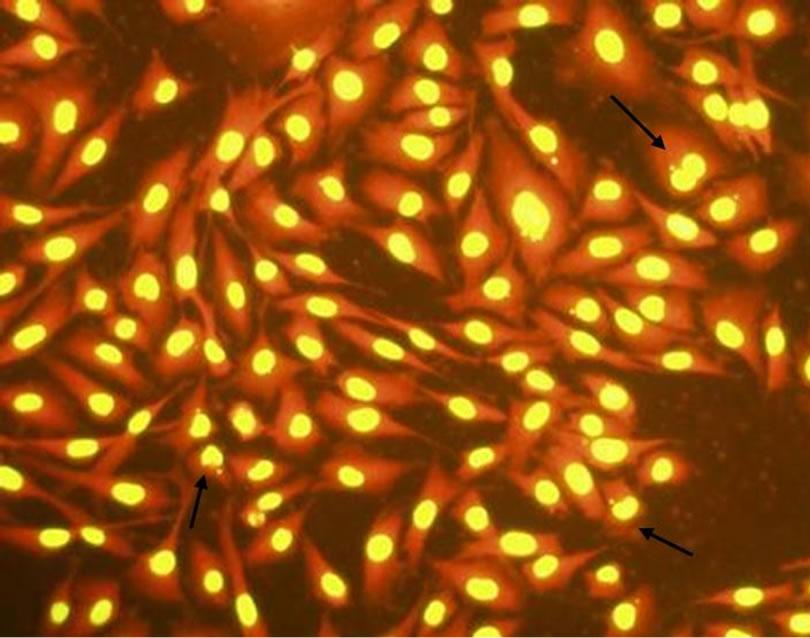 (b)
(b)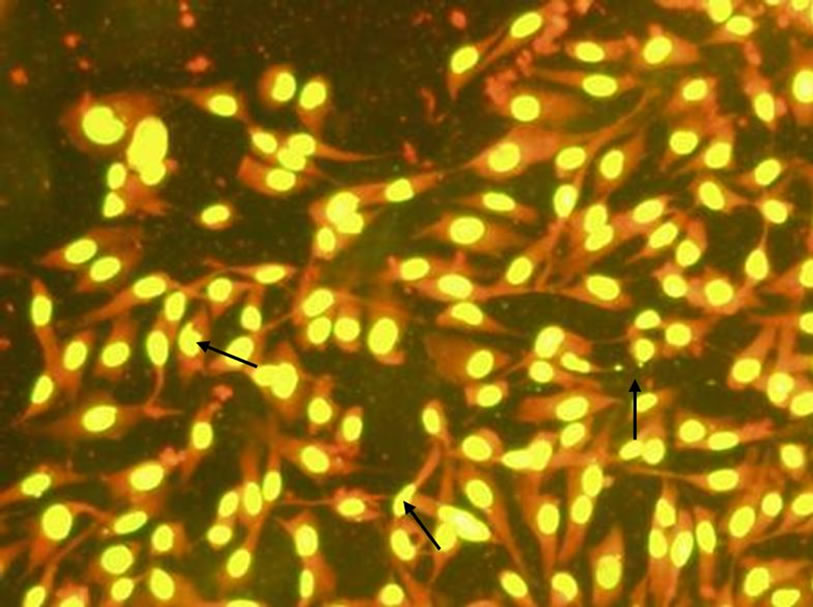 (c)
(c)
Figure 5. Changes of BHK-21 cells morphology (dyed by acridine orange) after exposure to C60 suspension (Concentrations of C60 suspensions: (a) 0 mg/mL; (b) 0.09 mg/mL; (c) 0.12 mg/mL).
and cells and the ways they enter cells were different from conventional molecular and/or ions. It has been proposed that the cell-uptake of nanoparticles mainly depended on their sizes [18]. For larger particles, it was difficult to enter cells by passive diffusion. Most of the nC60 nanoparticles prepared in this experiment were distributed in the range of 50 - 120 nm, while a few of them were 5 - 10 nm or even more. According to the size-dependent theory, there might be two ways for the celluptake of C60 nanoparticles, mainly including energydependent endocytosis and energy-independent passive diffusion. C60 nanoparticles entered cells across cell membranes and caused their rupture. How C60 nanoparticles interact with cell membranes in the process of entering cells needs for further deep study.
In short, a relatively higher concentration of C60 suspension could be obtained by solvent exchange method, and it can show certain toxicity to BHK-21 cells with a dose-response relationship. At higher concentrations, C60 can inhibit the growth of BHK-21 cells, decrease the cell viability and induce cell apoptosis. In the process of cell apoptosis, the chromatin condensation occurred and the cell membrane and nucleus were destroyed, which indicating C60 might exhibit toxicity to BHK-21 cells by disrupting the cell membranes and further enter nuclei. Due to the limits of experimental methods, this work concentrated on the cell damage induced by extracellular concentrations of C60 suspension, the toxicology of C60 was obtained mainly through speculating from experimental results, and direct observation was lacked. Further studies, including more types of cells and different preparation methods of C60 suspension, more research indicators and methods are needed to clarify the C60 and its derivative cellular biological effects.
5. Acknowledgements
The authors acknowledge the considerable support of China Postdoctoral Science Foundation funded project (20090461214) and Innovation Fund for the Post-Doctoral Program of Shandong Province (200902015).
REFERENCES
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl and R. E. Smalley, “C60: Buckminsterfullerene,” Nature, Vol. 318, 1985, pp. 162-163. doi:10.1038/318162a0
- N. Tsao, T. Y. Luh, C. K. Chou, T. Y. Chang, J. J. Wu, C. C. Liu, et al., “In Vitro Action of Carboxyfullerene,” Journal of Antimicrobial Chemotherapy, Vol. 49, No. 4, 2002, pp. 641-649. doi:10.1093/jac/49.4.641
- P. Innocenzi and G. Brusatin, “Fullerene-Based OrganicInorganic Nanocomposites and Their Applications,” Chemistry of Materials, Vol. 13, No. 10, 2001, pp. 3126-3139. doi:10.1021/cm0110223
- C. M. Sayes, J. D. Fortner, W. Guo, D. Y. Lyon, A. M. Boyd, K. D. Ausman, Y. J. Tao, B. Sitharaman, L. J. Wilson, J. B. Hughes, J. L. West and V. L. Colvin, “The Differential Cytotoxicity of Water-Soluble Fullerenes,” Nano Letters, Vol. 4, No. 10, 2004, pp. 1881-1887. doi:10.1021/nl0489586
- A. Isakovic, Z. Markovic, N. Nikolic, B. TodorovicMarkovic, S. Vranjes-Djuric, et al., “Inactivation of Nanocrystalline C60 Cytotoxicity by Gamma-Irradiation,” Biomaterials, Vol. 27, No. 29, 2006, pp. 5049-5058. doi:10.1016/j.biomaterials.2006.05.047
- J. W. Arbogast, A. P. Darmanyan, C. S. Foote, et al., “Photophysical Properties of C60,” Physical Chemistry, Vol. 95, No. 1, 1991, pp. 11-12. doi:10.1021/j100154a006
- P. Møller, N. R. Jacobsen, J. K. Folkmann, P. H. Danielsen, L. Mikkelsen, J. G. Hemmingsen, L. K. Vesterdal, L. Forchhammer, H. Wallin and S. Loft, “Role of Oxidative Damage in Toxicity of Particulates,” Free Radical Research, Vol. 44, 2010, pp. 1-46. doi:10.3109/10715760903300691
- B. Han, M. N. Karim, “Cytotoxicity of Aggregated Fullerene C60 Particles on CHO and MDCK Cells,” Scanning, Vol. 30, No. 2, 2008, pp. 213-220. doi:10.1002/sca.20081
- L. É. Vesnina, T. V. Mamontova, M. V. Mikitiuk, N. L. Kutsenko, L. A. Kutsenko, N. A. Bobrova, L. V. Berkalo and I. P. Kaĭdashev, “Effect of Fullerene C60 on Functional Activity of Phagocytic Cells,” Eksp Klin Farmakol, Vol. 74, No. 6, 2011, pp. 26-29.
- H. W. An, “C60 Fullerene Nanoparticles Protect Neural Cells from Oxidative Damage Caused by the Betaamyloid Peptide,” Free Radical Biology and Medicine, Vol. 49, 2010, p. S176. doi:10.1016/j.freeradbiomed.2010.10.499
- D. Bedrov, G. D. Smith, H. Davande, et al., “Passive Transport of C60 Fullerenes through a Lipid Membrane: A Molecular Dynamics Simulation Study,” The Journal of Physical Chemistry B, Vol. 112, No. 7, 2008, pp. 2078- 2084. doi:10.1021/jp075149c
- R. Qiao, A. P. Roberts, A. S. Mount, S. J. Klaine and K. P. Chun, “Translocation of c60 and Its Derivatives across a Lipid Bilayer,” Nano Letters, Vol. 7, No. 3, 2007, pp. 614 -619. doi:10.1021/nl062515f
- W. Li, C. Y. Chen, C. Ye, T. T. Wei, Y. L. Zhao, et al., “The Translocation of Fullerenic Nanoparticles into Lysosome via the Pathway of Clathrin-Mediated Endocytosis,” Nanotechnology, Vol. 19, No. 14, 2008, Article ID 145102. doi:10.1088/0957-4484/19/14/145102
- A. E. Porter, K. Muller, J. Skepper, P. Midgley and M. Welland, “Uptake of C60 by Human Monocyte Macrophages, Its Localization and Implications for Toxicity: Studied by High Resolution Electron Microscopy and Electron Tomography,” Acta Biomaterialia, Vol. 2, No. 4, 2006, pp. 409-419. doi:10.1016/j.actbio.2006.02.006
- S. S. Ali, J. I. Hardt, K. L. Quick, et al., “A Biologically Effective Fullerene (C60) Derivative with Superoxide Dismutase Mimetic Properties,” Free Radical Biology and Medicine, Vol. 37, No. 8, 2004, pp. 1191-1202. doi:10.1016/j.freeradbiomed.2004.07.002
- A. E. Porter, M. Gass, K. Muller, J. N. Skepper, P. Midgley and M. Welland, “Visualizing the uptake of c60 to the Cytoplasm and Nucleus of Human Monocyte-Derived Macrophage Cells Using Energy-Filtered Transmission Electron Microscopy and Electron Tomography,” Environmental Science & Technology, Vol. 41, No. 8, 2007, pp. 3012-3017. doi:10.1021/es062541f
- S. Foley, C. Crowley, M. Smaihi, et al., “Cellular Localisation of a Water-Soluble Fullerene Derivative,” Biochemical and Biophysical Research Communications, Vol. 294, No. 1, 2002, pp. 116-119. doi:10.1016/S0006-291X(02)00445-X
- J. Rejman, V. Oberle, I. S. Zuhorn, D. Hoekstra, “SizeDependent Internalization of Particles via the Pathways of Clathrinand Caveolae-Mediated Endocytosis,” Biochemical Journal, Vol. 377, 2004, pp. 159-169. doi:10.1042/BJ20031253
NOTES
*Corresponding author.

