Agricultural Sciences
Vol.3 No.4(2012), Article ID:20171,7 pages DOI:10.4236/as.2012.34060
Plant growth promoting H2-oxidizing bacteria as seed inoculants for cereal crops
![]()
Department of Biology, Saint Mary’s University, Halifax, Canada; *Corresponding author: zhongmin.dong@smu.ca
Received 17 March 2012; revised 22 April 2012; accepted 18 May 2012
Keywords: Legumes; Crop Rotation; Uptake Hydrogenase; ACC Deaminase; PGPR; Biofertilizer
ABSTRACT
The long-term success of hydrogenase uptake negative legume-rhizobia associations, in spite of their apparent inefficiency, may be explained by the positive effects of H2 release to soil. A primary benefit of H2 release to soil is the stimulation of H2-oxidizing, plant growth promoting rhizobacteria (PGPR) [1]. Two such previously isolated strains were tested as seed inoculants for barley and spring wheat; there were significant differences between treatments and controls in tiller and grain head production, supported by data from greenhouse trials. T-RFLP analysis of barley soil samples, supported by DNA sequencing data, successfully distinguished both species inoculated. Successful re-isolation indicates that these isolates can reproduce themselves in soils and can be used as effective inoculants with peat as the standard carrier. This study showed that we are able to achieve some of the beneficial effects of crop rotation without the need to implement actual crop rotation.
1. INTRODUCTION
Many chemical fertilizers and pesticides used for crop production have adverse effects on both human and environmental health [2]. Studies have shown that using biofertilizers for maintaining soil productivity and promoting plant growth would reduce the usage of inorganic fertilizers and their harmful effects [3]. The search for bacterial inoculants as effective biofertilizers is therefore an important step to developing new means of sustainable agriculture.
There is a wide variety of plant growth promoting rhizobacteria (PGPR) which associate with plants and may promote plant growth either directly or indirectly [3]. For example, some PGPR directly regulate the availability of nutrients such as nitrogen and phosphorus [4], while others may indirectly foster plant growth through stimulation or suppression of specific plant hormones, such as ethylene [5,6]. Suppression of ethylene promotes nodulation in most legumes [7,8] and increases root elongation of non-leguminous plants [9], thereby enhancing nutrient access and promoting growth. Some PGPR are capable of phytopathogen suppression by directly competing with pathogens for space and resources [10], whereas other PGPR produce antifungal compounds [11].
The exchange of nutrients between plants and their symbiotic rhizobia plays an important role in legume plant growth [12]. Nitrogen fixation has been considered the main reason for the rotation benefit involving legume plants. However, studies suggest the leftover nitrogen may contribute only about 25% of the benefits caused by crop rotation [13].
H2 is an obligate energy rich by-product of nitrogen fixation that occurs in legume nodules [14]. Approximately 5% of a plants daily net photosynthetic energy gain is lost to the production of H2 [1]. Some rhizobia possess genes coding for an uptake hydrogenase (Hup) enzyme, which enables H2 to be oxidized by the bacteria [15]. Therefore, Hup+ rhizobia associations are much more efficient, as there is less energy lost to the surrounding soil. However, many symbioses, especially those used in agriculture, lack this uptake hydrogenase (Hup–), and the H2 produced by the nitrogenase diffuses out of the nodule into the soil [16-18]. This H2 loss from nodules to soil is traditionally considered a disadvantage in Hup– versus Hup+ symbiosis. However, the release of H2 from nodules alters soil biology and may indirectly contribute to plant growth [1,19].
The amount of H2 released into the soil from Hup– nodules is dependent on the rate of nitrogen fixation by nitrogen fixing bacteria (Table 1). Released H2 diffuses into the surrounding soil [1] and is taken up by nearby H2-oxidizing bacteria [20,21]. The presence of H2 stimulates the soil H2-oxidizing bacterial community [22,23]. Previous work has shown that H2 treated soil can promote plant growth and that the plant growth promoting agent in the H2-treated soil is bacterial in nature [24].

Table 1. Annual H2 production by soybean and alfalfa.
H2-oxidizing bacteria may foster plant growth promotion through various mechanisms [22], and their presence is beneficial to leguminous and non-leguminous plants [19,23]. Thus, the growth enhancement in the soils of Hup– plants seemingly offsets the greater energy efficiency of the Hup+ associations [19]. It should therefore be possible to use H2-oxidizing PGPR as biofertilizer inoculants to promote the beneficial effects of crop rotation in non-leguminous crops.
H2-oxidizing bacteria isolated from H2-treated soil and soil adjacent to Hup– nodules were used in the present study. JM63 (Variovorax paradoxus) and JM162a (Flavobacterium johnsoniae) have exhibited plant growth promoting properties in that they increase root elongation by decreasing ethylene levels in the host plant [23]. Root elongation benefits plants by making soil nutrients more accessible. JM63 and JM162a were used in this study to inoculate barley (Hordeum vulgare) and spring wheat (Triticum aestivum), two non-legumous crop species. Both field and greenhouse conditions were used to determine if the benefits of these H2-oxidizing PGPR to plant growth and yield are comparable to crop rotation. The study also aimed to verify that the inoculants can be reisolated from the soil surrounding the plants after growth.
2. MATERIALS AND METHODS
2.1. Preparation of Inoculants
Two isolates, JM62 (Variovorax paradoxus) and JM- 162a (Flavobacterium johnsoniae), were used to inoculate 250 ml of LB broth and were incubated at 37˚C for 3 days with 120 rpm shaking. The OD600 of the inoculants were determined and used to calculate the concentration of bacteria in each sample. Cells were pelleted by centrifuging at 4˚C and 8000 g for 10 minutes, and then washed with 5% saline to remove excess broth. Sterile peat was then mixed with pelleted cells (2.0 × 109 cells g–1 of sterile peat) with a 39% - 40% moisture content. A total of 3 inoculants were prepared: JM63, JM162a and a 1:1 (w/w) mixture of JM63/JM162. Sterile peat was used as a control inoculant. A total of 5 treatments were used for each seed type in all trials: JM63, JM162a, JM63/ JM162a mixed, sterile peat and non-inoculated seeds.
2.2. Truro Field Experiments
Barley (Hordeum vulgar cv. Chapais) growth trials were performed in Truro, Nova Scotia in 2007. Before planting, all plots were treated with N fertilizer at a rate of 50 kg/ha. Barley seeds were mixed with peat inoculants at a 10:1 (w/w) ratio. Four replicates of each treatment (JM63, JM162a and JM63/JM162a mixed, sterile peat, and non-inoculated) were placed in a random complete block design (RCBD) at the Plumdale Facility at the Nova Scotia Agricultural College (NSAC) (Plumdale #4) in Truro, Nova Scotia. Twenty plots (4.5 m × 1.5 m) were used with a seeding density of 300 seeds m2 with eight rows per plot. A week prior to barley harvesting, tillers were counted for 10 plants from each treatment; at this point, soil samples (0 - 7.5 cm depth) were taken from the fields and barley grain heads were also counted. Rows were randomly chosen and grain heads were counted for the length of one meter in the row; five rows were counted per treatment.
2.3. Minto Field Trials
Barley (Hordeum vulgar cv. Copeland) and Spring Wheat (Triticum aestivum cv. Harvest) growth trials were performed in Minto, Manitoba, in 2009. Before planting, all plots were treated with Buctril M and Axial to control weeds. Plots were also treated with Pivot to control disease. In the fall prior to seeding, plots were treated with N fertilizer (82-0-0) at 90 kg N/ha. Plots were also treated with P fertilizer (11-51-0) at a rate of 28 kg P2O5/ha during seeding. All samples were planted in a randomized complete block design (1.5 m × 7.5 m) with six replicates. Seeds were planted at a depth of 3 cm with a row spacing of 20 cm. Seeds were mixed with inoculants to equal a rate of 1.25 g inoculant/m2. Barley and spring wheat were seeded with a cone seeder at a rate of 70 kg/ha. Tiller numbers were counted 45 days after seeding. Head density was counted 67 and 68 days after seeding for barley and spring wheat, respectively. Mature height was measured 78 days after seeding. All plots were harvested 106 and 112 days after seeding for barley and spring wheat, respectively. Grain moisture was adjusted for constant moisture content (14.5%) in all treatments.
2.4. Taber Field Trials
Barley (Hordeum vulgar cv. Ponoka) and Spring Wheat (Triticum aestivum var. 5700) growth trials were also performed in Taber, Alberta in 2009. Before planting all plots were treated with Buctril M and Achieve to control weeds. All samples were planted in a randomized complete block design (1.5 m × 6 m) with six replicates. Seeds were planted at a depth of 2.5 cm with a row spacing of 20 cm. Seeds were mixed with inoculants to equal a rate of 12.5 g inoculant/plot. Barley and Spring Wheat were seeded with a cone seeder at a rate of 70 kg/ha. Tiller numbers were counted 49 days after seeding. Head density was counted after head emergence was complete. Plant height was measured at maturity. All plots were harvested 124 days after seeding. Grain moisture was adjusted for a constant moisture content for all treatments of 14.5% and 14.8% for barley and spring wheat, respectively.
2.5. Greenhouse Barley Trials
Soil collected from NSAC in the fall of 2008 was used to prepare 20 1-gallon pots for greenhouse trials in 2008 and 2009 at Saint Mary’s University. Sieved soil was mixed with silica sand in a 2:1 (v/v) ratio. In 2008, four pots were used for each of the five treatments (JM63, JM162a, JM63/JM162a mixed, sterile peat and no inoculant). Three barley seeds (Hordeum vulgar var. Chapais) were planted in each pot for a total of 12 replicates for each treatment. Plants were grown under supplemental lighting in a temperature range of 24˚C to 32˚C for an 18-hour photoperiod; they were watered regularly with a nutrient solution. The 1000X stock solution contained 34.98 g·L–1 KH2PO4; 9.93 g·L–1 K2HPO4; 87.48 g·L–1 K2SO4; 59.89 g·L–1 MgSO4·7H2O; 50.01 g·L–1 MgCl2·6H2O; 109.97 g·L–1 CaCl2·2H2O; 10.27 g·L–1 FeCl3·6H2O; 1.69 g·L–1 MnSO4; 0.250 g·L–1 CuSO4·5H2O; 0.287 g·L–1 ZnSO4·7H2O; 1.92 g·L–1 H3BO3; 0.121 g·L–1 Na2MoO4·2H2O; 0.056 g·L–1 CoSO4·7H2O and 50.55 g·L–1 KNO3. Tiller numbers were counted seven weeks after planting. In 2009 the trials were repeated using the same protocol. Soil from the previous trial was used again to grow seeds with the same inoculants.
2.6. Re-Isolation of H2-Oxidizing Bacteria
Soil from both Truro field and Saint Mary’s greenhouse trials were serially diluted in sterile water and inoculated onto MSA plates. Plates were kept at room temperature in H2 enriched air for two weeks. H2 enriched air was used in isolating bacteria because H2 oxidation was a characteristic of both JM63 and JM162a [23]. Inoculated plates were re-plated for further use and isolation of colonies. Single colonies were isolated for sequencing.
2.7. Sequencing of Re-Isolated Strains
Single colonies were picked and used directly for DNA in 16s rDNA PCR reactions. The 49-µL PCR mix contained 40.6 µL sterile dH2O, 5.0 µL 10X ThermoPol reaction buffer (New England Biolabs Ltd., Pickering, ON), 1.0 µL 2mM dNTP (New England Biolabs Ltd., Pickering, ON), 1.0 µL BSF8/20 forward primer (5’- AGAGTTTGATCATGGCTCAG-3’), 1.0 µL BSR- 1541/ 20 reverse primer (5’-AAGGAGGTGATCCAGCCGCA- 3’) (Applied Biosystems, Foster City, CA) and 0.4 µL 5U/µL Taq polymerase (New England Biolabs Ltd., Pickering, ON). Soil bacterial DNA from barley trials was extracted using the PowerSoil Soil DNA Isolation Kit (MoBio Labs Inc., Carlsbad, CA). 1.0 µL of template DNA was added to the PCR reaction. PCR reactions were run using a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA) as follows: Three minutes at 94˚C; 35 cycles of 94˚C for one minute, 55˚C for 45 seconds and 72˚C for 45 seconds, one cycle of ten minutes at 72˚C and finally a 4˚C hold. PCR products (~1500 bp) were verified by gel electrophoresis (1% agarose; 40 V; 250 mA). The resulting PCR products were purified and then sequenced to determine their identity.
2.8. TRFLP Analysis of Barley Soil Samples
Soil bacterial DNA from barley trials was extracted using the PowerSoil Soil DNA Isolation Kit (MoBio Labs Inc., Carlsbad, CA). 1.0 µL of template DNA was added to the 16 s (500 bp) rRNA PCR reactions as previously described, except a fluorescently labeled 6-FAM forward primer (phosphoramidite fluorochrome 5-carboxy fluorescein)-BSF8/20 (5’-AGAGTTTGATCATGGCTCAG-3’) (Applied Biosystems, Foster City, CA) was used with the reverse primer BSR534/18 (5’-CAGCAGCCGCGGTAATAC-3’). The PCR protocol was the same as previously mentioned. PCR products (~500 bp) were purified using a QIAquick PCR Purification Kit (QIAGEN Inc., Mississauga, ON) then verified by gel electrophoresis (1% agarose gel; 40 V; 250 mA).
Four restriction enzymes were used to digest the purified DNA: BstUI, HinfI, HaeIII and MspI. DNA digestion reactions were completed as follows: 31.0 µL of sterile dH2O; 5.0 µL 10X Buffer #2 (New England Biolabs Ltd., Pickering, ON); 10.0 µL 16S PCR product and 3.0 µL of either BstUI, HindfI, HaeIII or MspI (all enzymes from New England Biolabs Ltd., Pickering, ON) for a total of 49 µL per reaction. Samples digested with HinfI, HaeIII and MspI were incubated in a 37˚C water bath, and samples digested with BstUI were incubated at 60˚C. The reactions were terminated using the QIAquick Nucleotide Removal Kit (Qiagen, Canada) and samples were sent for TRFLP analyses.
3. RESULTS
Field and greenhouse trials showed significant differences between treatments and controls in tiller numbers, grain head production and yield. TRFLP analysis of barley soil samples, supported by DNA sequencing data, successfully distinguished both JM63 and JM162a in treated soils. Successful re-isolation indicates that these isolates can reproduce themselves in soils and can be used as effective inoculants with peat as the standard carrier.
3.1. Truro Field Trials
There was a significant increase in tiller numbers for plants inoculated with JM63, JM162a and JM63/JM162a mixed compared to non-inoculated and non-treated controls (Table 2). There was however, no significant difference in yield between plots. It was noted that lodging of plants occurred due to a storm in the week before harvesting which made it difficult to accurately determine yield.
3.2. Greenhouse Trials
In the 2008 greenhouse trial there was a significant increase in tiller numbers for plants inoculated with JM63, JM162a and JM63/JM162a mixed, compared to noninoculated and non-treated controls (Table 2). JM63 also had significantly higher tiller numbers than JM162a and JM63/JM162a mixed. There was also a significant increase in plant height in JM63, JM162a and JM63/ JM162a mixed compared to non-inoculated and nontreated controls (data not shown).
In the 2009 greenhouse trial there was a significant increase found in tiller numbers for plants inoculated with JM63, JM162a and JM63/JM162a mixed, compared to controls. There was also a significant increase in plant height in JM63 and JM162a compared to non-inoculated and non-treated controls (data not shown).
3.3. Minto Field Trials
In the Minto barley field trials, plots inoculated with JM162a had the highest tiller number and head densityfollowed by JM63/JM162a mixed and JM63; controls had the lowest tiller numbers and head density (Table 2). There was no significant increase in yield for any treatments but plots inoculated with JM162a and JM63/ JM162a showed increased performance.
Spring wheat plots inoculated with JM162a and JM63/ JM162a combined had the highest tiller number and head density, followed JM63; controls had the lowest tiller numbers and head density. There was no significant difference in yield between treatments and controls.
3.4. Taber Field Trials
In the Taber barley trials there were significantly higher tiller numbers in plots treated with JM63, JM162a and JM63/JM162a mixed compared to non-inoculated and non-treated controls (Table 2). All treatments also showed higher head density than controls, however, there was no significant difference between inoculated treatments. There was a significant increase in yield for plots treated with JM63, JM162a and JM63/JM162a mixed compared to non-inoculated and non-treated controls. JM63/JM162a mixed provided the highest yield of all treatments (Figure 1).
The Taber spring wheat trials showed significantly higher tiller numbers in plots treated with JM63, JM162a and JM63/JM162a mixed compared to controls. Again, all treatments also showed higher head density than controls, however, there was no significant difference between inoculated treatments. There was a significant increase in yield for plots treated with JM63, JM162a and JM63/JM162a mixed compared to controls. JM63/ JM162a mixed provided the highest yield of all treatments (Figure 1).
3.5. TRFLP and Re-Isolation
TRFLP results from soil collected from Truro field trials showed the continued presence of inoculants used throughout the growth trials (data not shown). Closest matching isolates from barley plots inoculated with
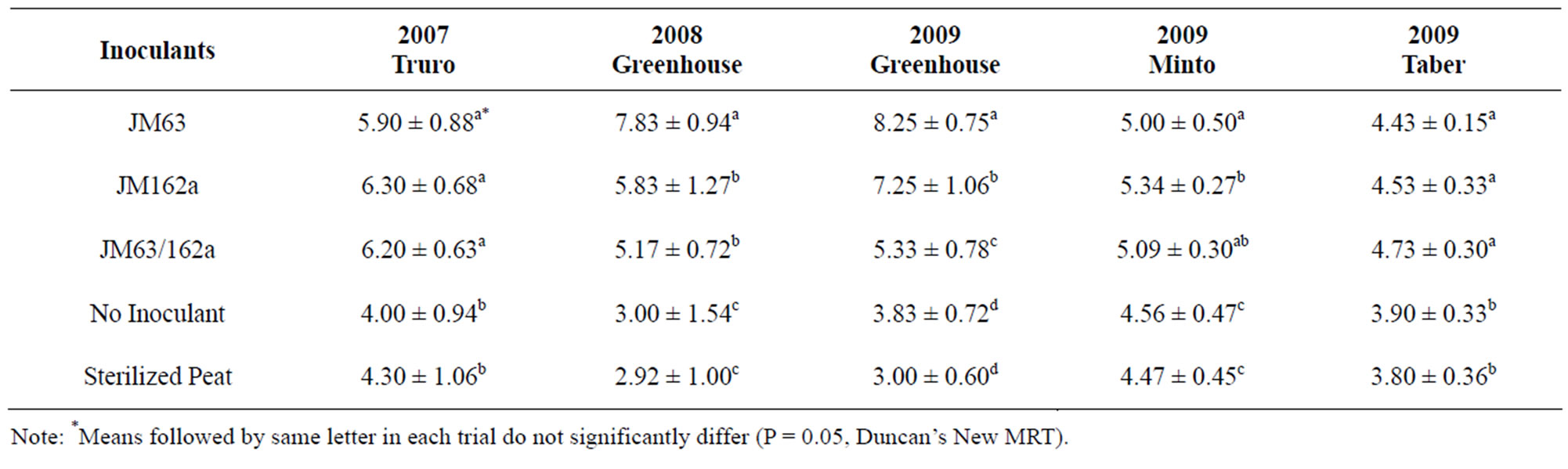
Table 2. Barley tiller numbers per plant, 7 weeks after planting.
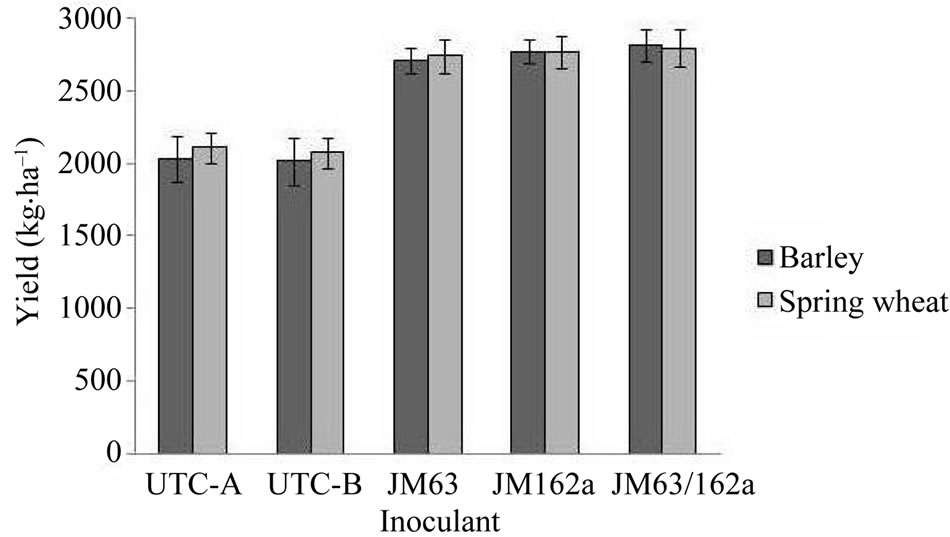
Figure 1. Yield (kg·ha–1) of barley and spring wheat grown in Taber, 2009.
JM162a showed up to a 98% sequence match with the 16s rRNA partial sequence from JM162a (Flavobacterium johnsoniae) (DQ256490). Closest matching isolates from barley plots inoculated with JM63 showed up to a 95% sequence match to the 16s rRNA partial sequence from JM63 (Variovorax paradoxus) (DQ256487). Neither JM63 nor JM162a were detected in the control soils.
4. DISCUSSION
The fixation of nitrogen within legume nodules does not explain the full benefit to cereal crops grown in rotation with legumes [27]. The H2 produced as a by-product of nitrogen fixation and released into the soil from Hup– nodules accounts for an energy loss of up to 5% of the plants daily net photosynthetic gain [28]; yet these Hup– associations persist in agriculture and in nature. H2 oxidation is a well documented response to H2 exposure in soils such as that experienced in the rhizosphere of H2 releasing legume nodules [21]. This response is easily duplicated in the lab using a controlled source of H2 gas, and previous studies have documented the numerous effects that exposure to H2 has on the soil bacteria, which include changes to the soil bacterial community structure and changes in gas exchange [1,29].
Previous studies have shown that crops rotated with legumes with H2 releasing (Hup–) nodules leads to a promotion in yield in barley [30]. Furthermore, inoculating plants with H2 treated soil has also shown a promotion in tiller numbers in barley and spring wheat [19]. In the present study, all trials showed an increase in tiller numbers for barley that was inoculated with JM63, JM162a and JM63/JM162a mixed. In the Taber and Minto field trials there was also a significant increase in tiller numbers for spring wheat with all three inoculants. Previous work has shown that inoculating barley and spring wheat with H2 treated soil led to an increase in tillering of up to 42% and 48%, respectively, compared to air treated control soils [19]. Tiller numbers are a good indicator of plant growth promotion, as tillering can be a major determinant in grain yield [31,32]. This persistent increase in tiller numbers is a good indicator of the viability of JM63 and JM162a as commercial plant growth promoting inoculants.
In the Taber field trial there was also a significant increase in yield for both barley and spring wheat inoculated with JM63, JM162a and JM63/JM162a mixed compared to non-inoculated controls. The yield increase in barley when using the inoculants JM63 and JM162a seen here is similar to that seen in a previous study where barley was grown in rotation with Hup+ and Hup– legumes [28]. Re-isolation of both JM63 and JM162a from the inoculated soils shows that the presence of these bacteria persist beyond the initial inoculation; and it follows that their plant growth promoting effects should persist as well. The inoculants were re-isolated in a H2 enriched environment, as H2 oxidation was a defining characteristic of these PGPRs. Many H2 oxidizing bacteria have been isolated from soils treated with H2 and many of these have displayed ACC deaminase activity which is known to inhibit ethylene production and promote plant growth [23]; both JM63 and JM162a displayed ACC deaminase activity. 1-aminocyclopropane-1- carboxylate (ACC) is a precursor in the production of ethylene, a known plant growth inhibitor. ACC deaminase reduces the ACC concentrations in plant roots which lowers ethylene production in the roots and, in turn, promotes root elongation [33,34]. Improved root elongation would inherently lead to improved plant growth by allowing better access to nutrients in the soil. The presence and expression of ACC deaminase in various bacterial species has been shown to offer plants resistance to phytopathogens [35], high salt concentrations [36], and flooding [37].
It has become evident that H2 fertilization from legume crops is a major contributor to the benefits experienced in crop rotation. The results of this study should help to explain some of the plant growth promoting effects of H2 fertilization. JM63 and JM162a showed consistent increases in tiller numbers for both barley and spring wheat and showed the potential for increasing yield in both. It is evident that these two species are responsible, in part, for the plant growth promoting effects of H2 release from Hup– nodule associations in legumes. With further investigation in inoculation methods and field conditions, both JM63 and JM162a have the potential to act as viable commercial inoculants for promoting plant growth in agricultural crops.
5. ACKNOWLEDGEMENTS
We thank the Natural Sciences and Engineering Research Council (NSERC) for the Discovery Grant to Zhongmin Dong. This research was supported by the Growing Canadian Agri-Innovations program’s Canadian Agri-Science Clusters Initiative, and the Saskatchewan Pulse Growers. We would also like to thank Dr. Claude Caldwell for his assistance with the Truro field trials.
REFERENCES
- Dong, Z. and Layzell, D.B. (2001) H2 oxidation, O2 uptake and CO2 fixation in hydrogen treated soils. Plant and Soil, 229, 1-12. doi:10.1023/A:1004810017490
- Welbaum, G.E., Sturz, A.V., Dong, Z. and Nowak, J. (2004) Managing soil micro-organisms to improve productivity of agro-ecosystems. Critical Reviews in Plant Science, 23, 175-193. doi:10.1080/07352680490433295
- Vessey, J.K. (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil, 255, 571-586. doi:10.1023/A:1026037216893
- Dey, R., Pal, K.K., Bhatt, D.M. and Chauhan, S.M. (2004) Growth promotion and yield of peanut (Arachis hypogea L.) by application of plant growth-promoting rhizobacteria. Microbiological Resesearch, 1, 371-394. doi:10.1016/j.micres.2004.08.004
- Esitken, A., Pirlak, L., Turan, M. and Sahin, F. (2006) Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Scientia Horticulturae, 110, 324-327. doi:10.1016/j.scienta.2006.07.023
- Farag, M.A., Ryu, G.M., Sumner, L.W. and Paré, P.W. (2006) GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth and induced systemic resistance in plants. Phytochemistry, 67, 2262-2268. doi:10.1016/j.phytochem.2006.07.021
- Hunter, W.J. (1993) Ethylene production by root nodules and effect of ethylene on nodulation in glycine max. Applied and Environmental Microbiology, 59, 1947-1950.
- Nascimento, F., Brigido, C. and Glick, B.R. (2012) Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant and Soil, 353, 221-230. doi:10.1007/s11104-011-1025-2
- Shah, S., Li, J., Moffatt, B.A. and Glick, B.R. (1998) Isolation and characterization of ACC deaminase genes from two different plant growth-promoting rhizobacteria. Canadian Journal of Microbiology, 44, 833-843. doi:10.1139/w98-074
- Matos, A., Kerkhof, L. and Garland, J.L. (2005) Effects of microbial community diversity on the survival of pseudomonas aeruginosa in the wheat rhizosphere. Microbial Ecology, 49, 257-264. doi:10.1007/s00248-004-0179-3
- Ping, L. and Boland, W. (2004) Signals from the underground: bacterial volatiles promote growth in Arabidopsis. Trends in Plant Science, 9, 263-266. doi:10.1016/j.tplants.2004.04.008
- Postgate J. (1998) Nitrogen fixation. 3rd edition, Cambridge UP, Cambridge.
- Bullock, D.G. (1992) Crop rotation. Critical Reviews in Plant Sciences, 11, 309-326.
- Schubert, K.R. and Evans, H.J. (1976) Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proceedings of the National Academy of Sciences of the United States of America, 73, 1207-1211. doi:10.1073/pnas.73.4.1207
- Dixon, R.O.D. (1972) Hydrogenase in legume root nodule bacteroids: occurrence and properties. Achives of Microbiology, 85, 193-201. doi:10.1007/BF00408844
- Ruiz-Argüeso, T., Maier, R.J. and Evans, H.J. (1979) Hydrogen evolution from alfalfa and clover nodules and hydrogen uptake by free-living Rhizobium meliloti. Applied and Environmental Microbiology, 37, 582-587.
- Uratsu, S.L., Keyser, H.H., Weber, D.F. and Lim, S.T. (1982) Hydrogen uptake (HUP) activity of Rhizobium japonicum from major US soybean production areas. Crop Science, 22, 600-602. doi:10.2135/cropsci1982.0011183X002200030040x
- Baginsky, C., Brito, B., Imperial, J., Ruiz-Argüeso, T. and Palacios, J.M. (2005). Symbiotic hydrogenase activity in Bradyrhizobium sp. (Vigna) increases nitrogen content in Vigna unguiculata plants. Applied Environmental Microbiology, 71, 7536-7538. doi:10.1128/AEM.71.11.7536-7538.2005
- Dong, Z., Wu, L., Kettlewell, B., Caldwell, C.D. and Layzell, D.B. (2003) Hydrogen fertilization of soils—is this a benefit of legumes in rotation? Plant Cell and Environment, 26, 1875-1879. doi:10.1046/j.1365-3040.2003.01103.x
- Conrad, R. and Seiler, W. (1979) The role of H2 bacteria during the decomposition of H2 by soil. FEMS Microbiology Letters, 6, 143-145. doi:10.1111/j.1574-6968.1979.tb04296.x
- La Favre, J.S. and Focht, D.D. (1983) Conservation in soil of H2 liberated from N2 fixation by Hup– nodules. Applied and Environmental Microbiology, 46, 304-311.
- Zhang, Y. (2006). Mechanisms of isolated hydrogenoxidizing bacteria in plant growth promotion and effects of hydrogen metabolism on rhizobacterial community structure. Master’s Thesis, Saint Mary’s University, Halifax.
- Maimaiti, J., Zhang, Y., Yang, J., Cen, Y.-P., Layzell, D.B., Peoples, M. and Dong, Z. (2007) Isolation and characterization of hydrogen-oxidizing bacteria induced following exposure of soil to hydrogen gas and their impact on plant growth. Environmental Microbiology, 9, 435- 444. doi:10.1111/j.1462-2920.2006.01155.x
- Irvine, P., Smith, M. and Dong, Z. (2004) Hydrogen fertilizer: bacteria or fungi? Acta Horticulturae, 631, 239- 242.
- LaRue, T.A. and Patterson, T.G. (1981) How much do legumes fix? Advances in Agronomy, 34, 15-38. doi:10.1016/S0065-2113(08)60883-4
- Hunt, S. and Layzell, D.B. (1993) Gas exchange of legume nodules and the regulation of nitrogenase activity. Annual Review of Plant Physiology and Plant Molecular Biology, 44, 483-511. doi:10.1146/annurev.pp.44.060193.002411
- Hesterman, O.B., Sheaffer, C.C., Barnes, D.K., Lueschen, W.E. and Ford, J.H. (1986) Alfalfa dry matter and nitrogen production and fertilizer nitrogen response in legume-corn rotations. Agronomy Journal, 78, 19-23. doi:10.2134/agronj1986.00021962007800010005x
- Dong, Z. and Layzell, D.B. (2002) Why do legumes nodules evolve hydrogen gas? The 13th International Congress on Nitrogen Fixation, Hamilton, 2-7 July 2001, 331- 335.
- Stein, S., Selesi, D., Schilling, R., Pattis, I., Schmid, M. and Hartmann, A. (2005) Microbial activity and bacterial composition of H2-treated soils with net CO2 fixation. Soil Biology & Biochemistry, 37, 1938-1945. doi:10.1016/j.soilbio.2005.02.035
- Dean, C., Sun, W., Dong, Z. and Caldwell, C.D. (2006) Soybean nodule hydrogen metabolism affects soil hydrogen uptake and growth of rotation crops. Canadian Journal of Plant Science, 86, 1355-1359. doi:10.4141/P06-082
- Garcia del Moral, L.F., Ramos, J.M. and Recalde, L. (1984) Tillering dynamics of winter barley as influenced by cultivar and nitrogen fertilizer: A field study. Crop Science, 24, 179-181. doi:10.2135/cropsci1984.0011183X002400010042x
- Garcia del Moral, M.B. and Garcia del Moral, L.F. (1995) Tiller production and yield in relation to grain yield in winter and spring barley. Field Crops Research, 44, 85- 93. doi:10.1016/0378-4290(95)00072-0
- Glick, B.R., Penrose, D.M. and Li, J. (1998) A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. Journal of Theoretical Biology, 190, 63-68. doi:10.1006/jtbi.1997.0532
- Yang, S.F. and Hoffman, N.E. (1984) Ethylene biosynthesis and its regulation in higher plants. Annual Reviews of Plant Physiology, 35, 155-189. doi:10.1146/annurev.pp.35.060184.001103
- Wang, C., Knill, E., Glick, B.R. and Defago, G. (2000) Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHAO and its gacA derivatives CHA96 on their growth-promoting and disease-suppressive capabilities. Canadian Journal of Microbiology, 46, 898-907.
- Mayak, S., Tirosh, T. and Glick, B.R. (2004) Plant growth promoting bacteria that confer resistance in tomato to salt stress. Plant Physiology and Biochemistry, 42, 565-572. doi:10.1016/j.plaphy.2004.05.009
- Grichko, V.P. and Glick, B.R. (2001) Amelioration of flooding stress by ACC deaminase-containing plant growthpromoting bacteria. Plant Physiology and Biochemistry, 39, 11-17. doi:10.1016/S0981-9428(00)01212-2

