Open Journal of Modelling and Simulation
Vol.05 No.01(2017), Article ID:73541,15 pages
10.4236/ojmsi.2017.51008
Global Stability of a SVEIR Epidemic Model: Application to Poliomyelitis Transmission Dynamics
L. N. Nkamba1,2*, J. M. Ntaganda3, H. Abboubakar2,4,5, J. C. Kamgang5,6, Lorenzo Castelli7
1Department of Mathematics, Higher Teacher Training College, University of Yaoundé I, Yaoundé, Cameroon
2Laboratoire d’Analyse, Simulation et Essai (LASE), Ngaoundéré, Cameroon
3Department of Mathematics, School of Science, College of Science and Technology, University of Rwanda, Butare, Rwanda
4Laboratoire de Mathématiques Expérimentales (LAMEX), Ngaoundere, Cameroon
5Department of Computer Engineering, University of Ngaoundéré, UIT, Ngaoundéré, Cameroon
6Department of Mathematics and Computer Science, ENSAI-University of Ngaoundere, Ngaoundéré, Cameroon
7DIA-University of Trieste, Trieste, Italy
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 12, 2016; Accepted: January 15, 2017; Published: January 18, 2017
ABSTRACT
The lack of treatment for poliomyelitis doing that only means of preventing is immunization with live oral polio vaccine (OPV) or/and inactivated polio vaccine (IPV). Poliomyelitis is a very contagious viral infection caused by poliovirus. Children are principally attacked. In this paper, we assess the impact of vaccination in the control of spread of poliomyelitis via a deterministic SVEIR (Susceptible-Vaccinated- Latent-Infectious-Removed) model of infectious disease transmission, where vaccinated individuals are also susceptible, although to a lesser degree. Using Lyapunov- Lasalle methods, we prove the global asymptotic stability of the unique endemic equilibrium whenever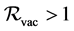 . Numerical simulations, using poliomyelitis data from Cameroon, are conducted to approve analytic results and to show the importance of vaccinate coverage in the control of disease spread.
. Numerical simulations, using poliomyelitis data from Cameroon, are conducted to approve analytic results and to show the importance of vaccinate coverage in the control of disease spread.
Keywords:
Deterministic SVEIR Model, Poliomyelitis, Imperfect Vaccine, Direct Lyapunov Method, Equilibrium States, Global Stability

1. Introduction
In the 70s, having noticed that five million children died every year further to an avoidable disease by the vaccination like poliomyelitis, the WHO introduced the Global Immunization Vision and Strategy (GIVS). Poliomyelitis has been eliminated in the most of countries, but recently we observe the upsurge of infectious in some countries [1] . Since October 2013, Cameroon is classified by the WHO as the exporting country of the poliovirus [2] . Poliomyelitis is an acute and sometimes devastating viral disease very contagious caused by poliovirus. Human is the only natural host for poliovirus [3] . Children are principally attacked. Poliovirus is predominantly transmitted via mother and food contaminated. In the most of case, infection is asymptomatic but the persons infected can transmit disease via their feces [4] . When a susceptible is exposed to infection by a virulent poliovirus, we can observe few days or few weeks three types of responses (minor illness, aseptic meningitis, and paralytic poliovirus). In case of minor illness, after 3 - 5 days, symptoms can be slight, fever, tiredness, headache, sore throat and vomiting. In the minor illness, the patient recovers in a few days 24 to 72 hours. In the case of non paralytic poliomyelitis in addition in some of minor illness signs and symptoms includes stiffness and pain in the back of neck. In the past days of illness, healing will rapid and complete. In the paralytic poliomyelitis, the predominant damage is flaccid paralysis resulting from lower motor neurons damage. The maximal recovery usually occurs after 6 months, but residuals paralysis lasts much longer. There does not exist a specific treatment for poliomyelitis although improved sanitation and hygiene help to limit the spread of poliovirus. The only specific means of preventing polio is immunization with live polio vaccine (OPV) or/and inactivated polio vaccine (IPV) [5] [6] [7] [8] .
As part of the necessary multi-disciplinary research approach, mathematical models have been extensively used to provide a framework for understanding of poliomyelitis transmission dynamics and the best strategies to control the spread of infection in the human population. In the literature, considerable work can be found on the mathema- tical modeling of poliomyelitis [9] - [18] . Some of these works refer to vaccination as polio control mechanism [9] [12] [17] [18] , using a standard SEIR model [19] .
Some SVEIR models are used to assess the potential impact of an imperfect SARS vaccine like SARS vaccine [20] , Hepatitis B vaccine [21] , Tuberculosis vaccine [22] , HIV vaccine [23] [24] , to mention only these four diseases. From a mathematical point of view, to show the global asymptotic stability of equilibrium points in general, and especially, the global asymptotic stability of the endemic equilibrium, is not an easy task. This requires, in most cases, the use of several different techniques, such as the theory of compound matrix [25] [26] , the comparison theorem [27] , or the use of Lyapunov functions associated with the Lassalle invariance principle [28] , to name a few techniques commonly used by authors. For example, in [20] , the authors used compound matrix techniques to show the global stability of the endemic equilibrium under some constraints on the parameters of the system. Huiming Wei et al. [29] proposed an SVEIR model with time delay, and analyzed the dynamic behavior under pulse vaccination. Using comparison theorem, they showed that the infection-free periodic solution is globally attractive. Yu Jiang et al. [30] modified that model by adding saturation incidence, and used too the comparison theorem to show the global stability of “infection-free” periodic solution.
In this paper, we study the impact of vaccination in the control of poliomyelitis spread via an SVEIR model of infectious disease transmission. Individuals are classified as one of susceptible , vaccinated
, vaccinated , exposed
, exposed , infectious
, infectious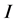 , or recovered
, or recovered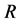 . The model is based on a standard SEIR model [19] , but allows that susceptible individuals may be given an imperfect vaccine that reduces their susceptibility to the disease. Since we consider a leaky vaccine, the V-compartment of vaccinated individuals is considered as a susceptible compartment, and thus we are dealing with a differential susceptibility system with bilinear mass action as in Hyman and Li [31] . However, we include one-way flow between these two compartments due to vaccination making the model studied here distinct from the model in [31] . For the case where the basic reproduction number is less than one, the global stability of the disease-free equilibrium has been shown by Gumel et al. in 2006 [20] . However, the global dynamics when the basic reproduction number is greater than one have not been resolved before. By allowing different death rates for each of the compartments, the model studied in this paper is slight generalization of the model studied in [20] . Using Lyapunov-LaSalle methods, we fully resolve the global dynamics of the model for the full parameter space. We demonstrate that the model exhibits threshold behavior with a globally stable disease-free equilibrium if the basic reproduction number is less than unity and a globally stable endemic equilibrium if the basic reproduction number is greater than unity. Thus, we also fully resolve the global dynamics for the model studied in [20] .
. The model is based on a standard SEIR model [19] , but allows that susceptible individuals may be given an imperfect vaccine that reduces their susceptibility to the disease. Since we consider a leaky vaccine, the V-compartment of vaccinated individuals is considered as a susceptible compartment, and thus we are dealing with a differential susceptibility system with bilinear mass action as in Hyman and Li [31] . However, we include one-way flow between these two compartments due to vaccination making the model studied here distinct from the model in [31] . For the case where the basic reproduction number is less than one, the global stability of the disease-free equilibrium has been shown by Gumel et al. in 2006 [20] . However, the global dynamics when the basic reproduction number is greater than one have not been resolved before. By allowing different death rates for each of the compartments, the model studied in this paper is slight generalization of the model studied in [20] . Using Lyapunov-LaSalle methods, we fully resolve the global dynamics of the model for the full parameter space. We demonstrate that the model exhibits threshold behavior with a globally stable disease-free equilibrium if the basic reproduction number is less than unity and a globally stable endemic equilibrium if the basic reproduction number is greater than unity. Thus, we also fully resolve the global dynamics for the model studied in [20] .
In order to study the stability of a positive endemic equilibrium state, we use Lyapunov’s direct method and LaSalle’s Invariance Principle with a Lyapunov function of the form:
 (1)
(1)
where  are constants,
are constants, 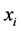 is the population of ith compartment and
is the population of ith compartment and 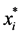 is the equilibrium level. Lyapunov functions of this type have also proven to be useful for Lotka-Voltera predator-preys systems [32] , and it appears that they can be useful for more complex compartmental epidemic models as well [33] [34] .
is the equilibrium level. Lyapunov functions of this type have also proven to be useful for Lotka-Voltera predator-preys systems [32] , and it appears that they can be useful for more complex compartmental epidemic models as well [33] [34] .
The main aim of the present paper is to show that our model has a unique endemic equilibrium which is globally asymptotically stable.
This SVEIR model could be used to assess the potential impact of an extended vaccination program (such as for the monovalent serogroup A conjugate MenVacAfric, an anti-meningococcal vaccine introduced in 2011 in Sub-saharan Africa), in order to compare with the impact of a pulse vaccination program.
In the next section, we present our SVEIR epidemic model. Section 3 presents some basic properties like the computation of the basic reproduction ration,  , and such as the existence of the equilibrium points. In Section 4, we study the stability properties of the model and in Section 7, numerical simulations will be done with Cameroon data which deal with the vaccination campaign against polio. An conclusion round the paper.
, and such as the existence of the equilibrium points. In Section 4, we study the stability properties of the model and in Section 7, numerical simulations will be done with Cameroon data which deal with the vaccination campaign against polio. An conclusion round the paper.
2. Model Description
We divide the entire population into 5 sub-populations of epidemiological significance: susceptible, vaccinated, exposed, infective, and removed compartments with respective sizes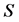 ,
, 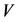 ,
, 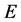 ,
, 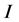 and
and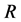 . The latent compartment,
. The latent compartment, 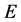 , takes into account the delay between the moment of the infection and the moment when an infected individual becomes infectious. The per capita death rates for susceptible, vaccinated, exposed, infective and recovered individuals are
, takes into account the delay between the moment of the infection and the moment when an infected individual becomes infectious. The per capita death rates for susceptible, vaccinated, exposed, infective and recovered individuals are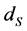 ,
, 





We assume mass action incidence 





The average duration of latency in class 




Our model consists of the following system of ordinary differential equations:

with initial conditions which satisfy
Since 

Figure 1. Schematic of the compartmental model.

with initial conditions which satisfy
3. Basic Properties and Equilibriums
3.1. A Compact Positively Invariant Absorbing Set
In order that the model be well-posed, it is necessary that the state variables





where


Lemma 1. The compact set 
Proof. For each of the variables





Let
Consequently,

Similarly, 


Let




for

This holds for all 

W
Since 

It is easy to see that the model system (3) has a disease-free equilibrium 
Additionally, an endemic equilibrium 
3.2. Basic Reproduction Ratio and Equilibrium
Using the method of the references [36] [37] , the basic reproduction number 

Replacing 


When there is no vaccination (


From Equation (10), we claim the following result.
Proposition 1. 

Proof. It follows from (11) that

Thus, 

from which the result follows. W
The value of 
Theorem 1. If


(See Appendix for proof).
4. Stability Analysis of Equilibriums
4.1. Stability Analysis of the DFE
For local stability of the disease-free equilibrium, we claim the following:
Theorem 2. If

Proof. The Jacobian matrix of model (3) evaluate at the disease-free equilibrium is given by

The eigenvalues of 



The characteristic polynomial of 

It clear that the roots of 




The following result is proven in ( [20] , Theorem 4.1).
Theorem 3. If
If
4.2. Stability Analysis of the Endemic Equilibrium
Our main result is the following theorem.
Theorem 4. If


Proof. Consider the following candidate Lyapunov function

Differentiating 

Since

and,

Since arithmetical mean is greater than geometrical mean, we have the following inequalities

Therefore

It remain to prove that 
set

it’s clear that;
Backing to the above relations, we have the following implications.
If we set

Finally we have,

At the endemic equilibrium, we have

Replacing 

If we compare relation (26) with the last equation of (25), then we have:

Consequently:
Finally

Thus, the largest invariant set contained in 

Then the global stability of 
5. Numerical Simulations
In this section we show via numerical simulations that when 


Parameters Description and Values
Most of parameters values are from Cameroon, like natural rate of mortality. We assume that the natural rates of mortality of susceptible, recovered, exposed are the same. Value of vaccine efficacy, recovery rate and rate of apparition of clinical symptoms are coming from WHO. For vaccination coverage, we take different values in order to explore different situations. The recruitment rate of susceptible humans, 
6. Numerical Results and Interpretations
Figure 2 illustrate the minor illness form of polio. We assume that


In Figure 3, we are in the presence of the meningitis form of polio. Assuming that
Table 1. Description and values of parameters of model (3).
Figure 2. Minor illness
Figure 3. Meningitis form of polio



In Figure 4, we are in the presence of the most severe form of polio: the paralytic form with

We are in front of paralytic polio. We assume
Figure 6, we explored three cases:
1) even if the vaccine is perfect and nobody is vaccinated; the infection is and remains high in the population 

2) The vaccination is made; even if the coverage is low infection decreases and reaches a an equilibrium point 

3) The last and not realistic situation is that infection is eradicated after one year, and when we have perfect vaccine and maximal vaccination coverage 

Figure 4. Paralytic form of polio
Figure 5. Impact of vaccine coverage.
Figure 6. Impact of vaccine efficacy.
7. Conclusions
We highlighted in this article the importance of vaccination in the control of the propagation of the poliomyelitis. We relied on the compartmentalized SVEIR model that characterizes the infectious diseases. We computed

Using data from AHALA (district of Yaound in Cameroon), we simulated the three different forms of polio namely the minor illness, the meningitis form and the paralytic form. In the case of minor illness of polio, we assumed that



Acknowledgements
The first author acknowledges with thanks the High teacher Training College of Yaounde. H. A. would like to thank the Direction of the University Institute of Technology of Ngaoundere for their financial assistance in the context of research missions of September 13, 2016.
Cite this paper
Nkamba, L.N., Nta- ganda, J.M., Abboubakar, H., Kamgang, J.C. and Castelli, L. (2017) Global Stability of a SVEIR Epidemic Model: Application to Po- liomyelitis Transmission Dynamics. Open Journal of Modelling and Simulation, 5, 98- 112. http://dx.doi.org/10.4236/ojmsi.2017.51008
References
- 1. World Health Organiation, Media Centre: Poliomyelitis, Fact Sheet.
http://www.who.int/mediacentre/factsheets/fs114/en/# - 2. Donaldson, L., El Sayed, N., Koplan, J., Nduati, R., Toole, M., Chowdhury, M., et al. (2012) Comité de suivi indépendant de l’initiative mondiale pour léradication de la poliomyélite.
- 3. Centers for Disease Control and Prevention (CDC and Others), Follow-Up on Poliomyelitisunited States, Canada, Netherlands. 1979. MMWR. Morbidity and Mortality Weekly Report, 46, 1195.
- 4. Miller, N.Z. (2004) The Polio Vaccine: A Critical Assessment of Its Arcane History, Efficacy, and Long-Term Health-Related Consequences. Medical Veritas, 1, 239-251.
- 5. Sabin, A.B. (1984) Strategies for Elimination of Poliomyelitis in Different Parts of the World with Use of Oral Poliovirus Vaccine. Review of Infectious Diseases, 6, S391-S396.
- 6. Miller, N.Z. (2004) The Polio Vaccine: A Critical Assessment of Its Arcane History, Efficacy, and Long-Term Health-Related Consequences. Medical Veritas, 1, 239-251.
- 7. Centers for Disease Control and Prevention (CDC) and Others, The Pink book: Epidemiology and Prevention of Vaccine Preventable Diseases.
- 8. A. A. of Pediatrics, et al. (1998) American Academy of Pediatrics. Committee on Infectious Diseases. Severe Invasive Group a Streptococcal Infections: A Subject Review. Pediatrics, 101, 136.
- 9. Browne, C.J., Smith, R.J. and Bourouiba, L. (2015) From Regional Pulse Vaccination to Global Disease Eradication: Insights from a Mathematical Model of Poliomyelitis. Journal of Mathematical Biology, 71, 215-253.
- 10. Tebbens, R., Pallansch, M.A., Kew, O.M., Cáceres, V.M., Sutter, R.W. and Thompson, K.M. (2005) A Dynamic Model of Poliomyelitis Outbreaks: Learning from the Past to Help Inform the Future. American Journal of Epidemiology, 162, 358-372.
https://doi.org/10.1093/aje/kwi206 - 11. Kalkowska, D.A., Tebbens, R., Pallansch, M.A., Cochi, S.L., Wassilak, S.G. and Thompson, K.M. (2015) Modeling Undetected Live Poliovirus Circulation after Apparent Interruption of Transmission: Implications for Surveillance and Vaccination. BMC Infectious Diseases, 15, 66.
https://doi.org/10.1186/s12879-015-0791-5 - 12. Bunimovich-Mendrazitsky, S. and Stone, L. (2005) Modeling Polio as a Disease of Development. Journal of Theoretical Biology, 237, 302-315.
https://doi.org/10.1016/j.jtbi.2005.04.017 - 13. Rahmandad, H., Hu, K., Tebbens, R.D. and Thompson, K. (2011) Development of an Individual-Based Model for Polioviruses: Implications of the Selection of Network Type and Outcome Metrics. Epidemiology and Infection, 139, 836-848.
https://doi.org/10.1017/S0950268810001676 - 14. Gandica, I.D., Ocampo, L.M., Marín, E. and González, M. (2013) Model for the Vaccination against the Poliomyelitis. Journal of Mathematics and System Science, 3, 62-67.
- 15. Thompson, J.E. (2014) A Class Structured Mathematical Model for Polio Virus in Nigeria.
- 16. O’Reilly, K.M., Chauvin, C., Aylward, R.B., Maher, C., Okiror, S., Wolff, C., Nshmirimana, D., Donnelly, C.A. and Grassly, N.C. (2011) A Statistical Model of the International Spread of Wild Poliovirus in Africa Used to Predict And Prevent Outbreaks. PLoS Medicine, 8, e1001109.
https://doi.org/10.1371/journal.pmed.1001109 - 17. Wagner, B.G. and Earn, D.J. (2008) Circulating Vaccine Derived Polio Viruses and Their Impact on Global Polio Eradication. Bulletin of Mathematical Biology, 70, 253-280.
https://doi.org/10.1007/s11538-007-9255-4 - 18. Thompson, K.M., Duintjer, R., Tebbens, J. and Pallansch, M.A. (2006) Evaluation of Response Scenarios to Potential Polio Outbreaks Using Mathematical Models. Risk Analysis, 26, 1541-1556.
https://doi.org/10.1111/j.1539-6924.2006.00843.x - 19. Hethcote, H.W. (2000) The Mathematics of Infectious Diseases. SIAM Review, 42, 599-653.
https://doi.org/10.1137/S0036144500371907 - 20. Gumel, A., McCluskey, C. and Watmough, J. (2006) An Sveir Model for Assessing Potential Impact of an Imperfect Anti-Sars Vaccine. Mathematical Biosciences and Engineering, 3, 485-512.
https://doi.org/10.3934/mbe.2006.3.485 - 21. Zou, L., Ruan, S. and Zhang, W. (2010) An Age-Structured Model for the Transmission Dynamics of Hepatitis B. SIAM Journal on Applied Mathematics, 70, 3121-3139.
https://doi.org/10.1137/090777645 - 22. Castillo-Chavez, C. and Song, B. (2004) Dynamical Models of Tuberculosis and Their Applications. Mathematical Biosciences and Engineering, 1, 361-404.
https://doi.org/10.3934/mbe.2004.1.361 - 23. Gumel, A., McCluskey, C.C. and Van den Driessche, P. (2006) Mathematical Study of a Staged Progression HIV Model with Imperfect Vaccine. Bulletin of Mathematical Biology, 68, 2105-2128.
https://doi.org/10.1007/s11538-006-9095-7 - 24. Sharomi, O., Podder, C., Gumel, A., Elbasha, E. and Watmough, J. (2007) Role of Incidence Function in Vaccine-Induced Backward Bifurcation in Some HIV Models. Mathematical Biosciences, 210, 436-463.
https://doi.org/10.1016/j.mbs.2007.05.012 - 25. Li, M.Y. and Muldowney, J.S. (1995) Global Stability for the Seir Model in Epidemiology. Mathematical Biosciences, 125, 155-164.
https://doi.org/10.1016/0025-5564(95)92756-5 - 26. Muldowney, J.S. (1990) Compound Matrices and Ordinary Differential Equations. Rocky Mountain Journal of Mathematics, 20, 857-872.
https://doi.org/10.1216/rmjm/1181073047 - 27. Lakshmikantham, V. and Leela, S. (1969) Differential and Integral Inequalities. Academic Press, Cambridge.
- 28. LaSalle, J.P. (1976) The Stability of Dynamical Systems. Vol. 25, SIAM.
https://doi.org/10.1137/1.9781611970432 - 29. Wei, H., Jiang, Y., Song, X., Su, G. and Qiu, S. (2009) Global Attractivity and Permanence of a SVEIR Epidemic Model with Pulse Vaccination and Time Delay. Journal of Computational and Applied Mathematics, 229, 302-312.
https://doi.org/10.1016/j.cam.2008.10.046 - 30. Jiang, Y., Wei, H., Song, X., Mei, L., Su, G. and Qiu, S. (2009) Global Attractivity and Permanence of a Delayed SVEIR Epidemic Model with Pulse Vaccination and Saturation Incidence. Applied Mathematics and Computation, 213, 312-321.
https://doi.org/10.1016/j.amc.2009.03.024 - 31. Hyman, J. and Li, J. (2005) Differential Susceptibility Epidemic Models. Journal of Mathematical Biology, 50, 626-644.
https://doi.org/10.1007/s00285-004-0301-7 - 32. Beretta, E. and Takeuchi, Y. (1988) Global Asymptotic Stability of Lotka-Volterra Diffusion Models with Continuous Time Delay. SIAM Journal on Applied Mathematics, 48, 627-651.
https://doi.org/10.1137/0148035 - 33. Fall, A., Iggidr, A., Sallet, G. and Tewa, J.J. (2007) Epidemiological Models and Lyapunov Functions. Mathematical Modelling of Natural Phenomena, 2, 55-73.
https://doi.org/10.1051/mmnp:2008011 - 34. Thieme, H. (2011) Global Stability of the Endemic Equilibrium in Infinite Dimension: Lyapunov Functions and Positive Operators. Journal of Differential Equations, 250, 3772-3801.
https://doi.org/10.1016/j.jde.2011.01.007 - 35. Haddad, W.M., Chellaboina, V. and Hui, Q. (2010) Nonnegative and Compartmental Dynamical Systems. Princeton University Press, Princeton.
- 36. Diekmann, O. and Heesterbeek, J. (2000) Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis, and Interpretation. Wiley, Hoboken.
- 37. Van den Driessche, P. and Watmough, J. (2002) Reproduction Numbers and Sub-Threshold Endemic Equilibria for Compartmental Models of Disease Transmission. Mathematical Biosciences, 180, 29-48.
https://doi.org/10.1016/S0025-5564(02)00108-6 - 38. Bonzi, B., Fall, A., Iggidr, A. and Sallet, G. (2011) Stability of Differential Susceptibility and Infectivity Epidemic Models. Journal of Mathematical Biology, 62, 39-64.
https://doi.org/10.1007/s00285-010-0327-y - 39. Abboubakar, H., Kamgang, J.C., Nkamba, L.N., Tieudjo, D. and Emini, L. (2015) Modeling the Dynamics of Arboviral Diseases with Vaccination Perspective. Biomath, 4, Article ID: 1507241.
https://doi.org/10.11145/j.biomath.2015.07.241 - 40. Abboubakar, H., Kamgang, J.C. and Tieudjo, D. (2016) Backward Bifurcation and Control in Transmission Dynamics of Arboviral Diseases. Mathematical Biosciences, 278, 100-129.
http://www.sciencedirect.com/science/article/pii/S0025556416300633 https://doi.org/10.1016/j.mbs.2016.06.002
Appendix
Proof of Theorem 1
Proof. In order to determine the existence of possible endemic equilibrium, that is, equilibrium with all positive components which we denote by
we have to look for the solution of the algebraic system of equations obtained by equating the right hand sides of system (3) to zero. In this way we obtain the implicit system of equations,

where 

with
and
Note that coefficient 





Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
Or contact ojmsi@scirp.org




















