Food and Nutrition Sciences
Vol.07 No.11(2016), Article ID:70948,19 pages
10.4236/fns.2016.711100
Vitamin D and Bone Health
Alberto Falchetti1,2, Elisa Rossi3,4, Roberta Cosso1,2, A. Buffa3,4, Stefania Corvaglia3,4, Nazzarena Malavolta3,4
¹EndOsMet Division, Branch of Metabolic Bone Diseases, Villa Donatello Private Hospital, Florence, Italy
2Hercolani Center, Bologna, Italy
3St. Orsola-Malpighi Hospital, Bologna, Italy
4Cardio-Thoracic-Vascular Department, Division of Internal Medicine, University of Bologna, Bologna, Italy

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 18, 2016; Accepted: September 25, 2016; Published: September 28, 2016
ABSTRACT
The vitamin D3 molecule, or cholecalciferol, is now considered a hormone that acts on multiple homeostatic fronts, either skeletal or extra-skeletal. After 100 years since from its identification as a protective and curative factor of rickets, during the last decade the interest in vitamin D among scientists and general public increased substantially and the use of vitamin D supplements has increased nearly exponentially in many countries. The aim of the present review is to clarify the mechanisms of action of vitamin D compounds on bone health through well-known concepts identified, and readily available, in the scientific literature. Taking advantage of our multi-year and consolidated experience in the pathophysiology of bone and mineral metabolism, we aim to briefly describe those few “hot” topics that we have perceived not to be easy to understand both for health professionals engaged on metabolic bone disorders and for those that are non-experts in this field and who should approach it. We describe, through basic pathophysiology concepts, the relationships between vitamin D and skeletal health: the main sources of vitamin D in humans, the vitamin D metabolites, evaluation of vitamin D status, tissue distribution of vitamin D, natural factors affecting the half-life of vitamin D3, the effects of cholecalciferol on calcium homeostasis and bone tissue, the relationship between vitamin D3 and bone cells and physical performance in the elderly, the effects of low vitamin D3 levels on bone loss and increased risk of fracture. Although currently different international institutional-academic positions exist about which is the best threshold value of serum vitamin D to be considered as adequate, it is important to bring out and lay a solid foundation supporting at least the crucial role played by it in the pathophysiology of skeletal tissue, despite the difficulties arising from the current lack of the specific molecular basis that clearly explain the balance between bone formation, mineralization and skeletal resorption. Finally, it should be also taken into consideration also the numerous reports, more recently identifiable in PubMed, in which, even in presence of very high levels of circulating values of 25OHD, clinically and biochemically observable toxic effects are not reported.
Keywords:
Vitamin D Metabolites, Vitamin D Metabolism, Vitamin D and Bone Cells, Vitamin D and Sun Exposure, Vitamin D Pathophysiology

1. Introduction
The vitamin D3 (VD3) molecule, or cholecalciferol, has an early origin in the context of evolution of life as the end product of the photochemical conversion of 7-dehydrocho- lesterol by ultraviolet light B (UVB). It is well known that the cholesterol synthesis pathway occurs very early in the life of all eukaryotes, starting from squalene and lanosterol. The synthesis path from 7-dehydrocholesterol to cholecalciferol includes a large number of enzymes involving P450-like structures requiring molecular O2 to oxidize cho- lesterol precursors, and this entire pathway is highly conserved during evolution [1] [2] .
It is likely that the photochemical reaction bringing to VD3 represented a highly efficient protection of life in early marine organisms against DNA damage induced by UVB, a more critical problem a few billion years ago than today because of low O2 and also of low or absent ozone layers of the atmosphere [3] [4] . The dual origin of VD3, by photosynthesis or by dietary intake, was discovered nearly a century ago, and its widespread use in infants and young children efficiently eliminated endemic rickets. However, it took a long time to decipher its metabolism, its spectrum of activities and its mode of action. After 100 years since from its identification as a protective and curative factor of rickets, for a long time remained insufficient evidence to support the concept that VD3 directly stimulates osteoblastic bone formation and mineralization. Paradoxically, it has been much easier to demonstrate that the VD endocrine system stimulates the process of osteoclastic bone resorption than osteoblastic activity and bone mineralization. However, during the last decade the interest in VD among scientists and the general public increased substantially and the use of VD supplements has increased nearly exponentially in many countries. VD3 is now considered in all respects a hormone (seco-steroid, which is a steroid molecule with a “broken” ring) acting on multiple homeostatic fronts, skeletal and extra-skeletal.
In this review, we will not go about into extra-skeletal effects, still to be clearly deciphered, nor the existing disagreements at the international level regarding the serum threshold levels of VD3 to be considered as adequate, although a clear support for the 30 ng/ml, to reduce bone loss and the risk for fractures fragility exist, nor in “boring” patterns of how and how much supplementations of cholecalciferol have to be given to obtain beneficial effects on bone strength in insufficient/deficient subjects. We describe, through “simple” basic pathophysiology concepts, the relationships between VD3 and skeletal health so that even non-experts can break into this area of human health with few but clear concepts.
The aim of the present review is to clarify, in the easiest and understandable way, the mechanisms of action of VD compounds on bone health.
2. Which Are the Main Sources of VD in Humans?
The sun exposure represents the main source for VD3 in humans, accounting for the 95% of its production. Our organism produces VD3 at the skin level, starting from 7- dehydrocholesterol, as above mentioned, when the skin is exposed to the UVB at wavelengths within 282 - 310 nanometers [3] .
With the sun exposure of vertical surfaces (face, arms and legs) doses of UVB on horizontal surfaces (e.g. when lying on the bed-beach), the time needed to synthesize 800 IU depends not only on the type of skin, but also on the season (see below). In fact, during summer, at 12.00 a .m., a sun exposure of face and hands (8% of body surface) of 30 minutes - 1 hour is sufficient, but in winter, at the same time, in the same conditions 20 hours would be needed [5] [6] .
Although the sun source is apparently simple, it is not completely reliable for the increased risk of associated skin aging and skin cancer. Even if the Australian Dermatology Society now recommends sensible sun exposure as a source of VD3, an Australian dermatologists study, performed at the end of the summer, revealed that 87% of subjects had a 25(OH)VD3 lower than 20 ng/mL [7] , that is insufficient. In fact, we have to take also into account that the aging of skin decreases by 4 times the cutaneous synthesis as much as the product at a young age [8] , as also that sunscreen creams may reduce the synthesis of up to 97% [9] [10] .
Food-derived VD forms account for no more than 10% - 20% of VD3 circulating levels and they are reported at Table 1 [11] .
3. VD Metabolites
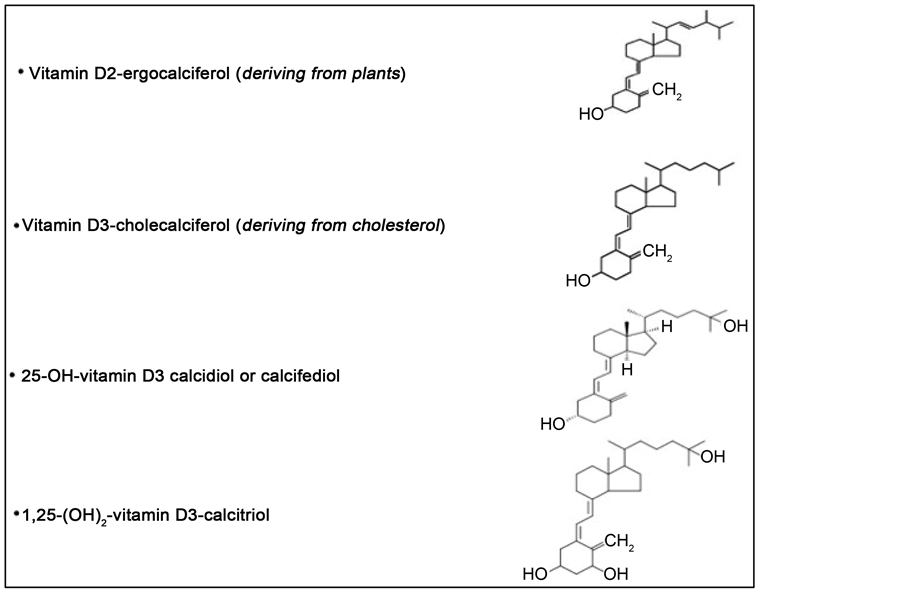
The main VD metabolites are represented by: ergocalciferol (VD2), deriving from UV irradiation of ergosterol in the plants, cholecalciferol (VD3), of animal origin, produced by irradiation of 7-dehydrocholesterol present in the skin, calcidiol or calciferol (the 25 hydroxylated form of D2 and D3), and calcitriol or 1,25-dihydroxylated form of VD3, the latter representing the active VD metabolite (Figure 1).
The VD2 and VD3 forms have been traditionally considered equivalent according to their ability to cure rickets. Most of the steps involved in the metabolism and action of VD2 and VD3 are identical and both forms effectively increase the serum level of 25OHD. Although it seems that nutritional doses of the D2 and D3 are equivalent, high-dose vitamin VD2 seems to be less powerful than VD3 [12] .
At the skin level, the conversion from pre-VD3 metabolite to VD3 is a temperature- dependent reaction at the equilibrium (37˚C).
4. VD3 Metabolism
Circulating VD3 is bound to VD Binding Protein (VDBP). In the liver, VD3 is hydroxylated (CYP2R1 enzyme) in 25-hydroxy-VD3 [25(OH)D], representing the circulating
Table 1. Food sources of vitamin D (modified from [11] ).
Figure 1. Vitamin D: a steroid, liposoluble, pro-hormone. Principal metabolites.
form of VD. Subsequently, 25(OH)D is hydroxylated in the bioactive 1α,25-dihydroxy- VD [1,25(OH)2D] by the 1α-hydroxylase (1α-OHase or CYP27B1) enzyme, predominantly in the kidney, at the proximal tubular level. The renal 1α-OHase is tightly regulated: positively by PTH, hypocalcemia, and hypophosphatemia and negatively by hyperphosphatemia, Fibroblast Growth Factor 23 (FGF-23) and 1,25(OH)2D itself. 1,25(OH)2D exerts its biological functions either via non-genomic or genomic related pathways and exerts endocrine effects of mineral metabolism regulating calcium/ phosphate homeostasis (see below).
Circulating VD3, after UVB exposure, is associated with VDBP and after intestinal absorption is coupled with both VDBP and lipoproteins [13] , while orally, in the diet as VD2 or VD3, the absorption is mainly based on diffusion, dependent, non-saturable, solubilization by bile acids [14] [15] .
5. Evaluation of the VD Status
Determination of serum 25(OH)D is the currently more appropriate method to estimate the status of VD repletion, differently from 1,25(OH)2VD that is significantly influenced by PTH serum levels [16] . According to above reported on VDBP, it has been suggested that “free” and bioavailable rather than total 25(OH)D, determines its biological action, but further evaluation of direct “free” 25(OH)VD assay measurements are required for an its future role in research and clinical practice [17] [18] .
Table 2 describes which are the populations at higher VD deficiency risk (or when it should be suspected) [19] [20] .
Table 2. Who are the people at higher VD deficiency risk (or when it should be suspected)?
6. What Fate of VD3 in the Human Body
After modest UVs exposure, it could be possible to produce and release thousands of IU of VD3, circulating within 24 h, whereas after oral ingestion, the VD3 appears in bloodstream at a more rapid rate, peaking at 12 h [13] [21] [22] . The circulating half-life of the compound from each route is 12 - 24 h [13] . Due to this short half-life, also a big bolus, 50,000 - 100,000 IU, of VD3 is cleared from the circulation within one week, making the VD3 undetectable in the circulation [21] - [24] .
6.1. Tissue Distribution of VD and 25(OH)D
It is based on simple diffusion or endocytosis. Endocytosis requires the tissue-specific megalin-cubilin system (megalin and cubilin are two structurally different interacting endocytic receptors that mediate uptake of extracellular ligands, extensively studied in the kidney, present also on parathyroid cells, where they play a key role in VD homeostasis), whereas simple diffusion is primarily controlled by the dissociation constant of the VD compound form the VDBP.
6.2. Affinity for the α-Globulin VDBP
This parameter is the principle determinant for how long time a metabolite will remain in circulation [25] - [27] . The dissociation constants for VD metabolites bounded to VDBP dictate the concentration of “free” form for the diffusion through the cell membrane and metabolization or modulation of the activity of cells. The “free” circulating concentrations of 1,25(OH)2D is higher than VD3 which in turn is higher than 25(OH)D (matching their relative circulating half-lives).
VD, 25(OH)D and 1,25(OH)2D have significantly different dissociation constants: 25(OH)D is about 10−9 and VD and 1,25(OH)2D is about 10−7. For VD is probably reduced to about 10−8 for its relative in vitro insolubility [28] .
These dissociation constants contribute to the different circulating half-life assets: VD one day, 25(OH)D weeks, and 1,25(OH)2D few hours [29] . It is the “free” metabolite that easily enters the cell by activating the cell response [30] .
VD3 is much more accessible than 25(OH)D for the internalization in every cell except for the cells with the megalin-cubilin system, such as at kidney and parathyroid glands levels (endocrine system of VD) [31] .
6.3. Low Levels in VDBP in African Americans Represent a Mechanism for Compensation?
In a quite recent study, it has been reported that total (bound plus unbound to VDBP) serum 25(OH)D levels were lower in a US African-American population with respect to the white US counterpart, but the concentration of the bioavailable (free circulating form) 25(OH)D was similar between the two populations. This finding may explain why the frequently reduced levels of total 25(OH)D in the African-Americans are not associated to a consequent increased risk for fragility fractures. All this puts in discussion the use of the measurement of total 25(OH)D to really identify the deficiency status of VD in all populations [32] .
6.4. Natural Factors Affecting the Half-Life of VD3
Its half-life is approximately 3 - 6 weeks.
§ Seasonal influences
It is well known that from November to the end of March (nadir) the UVB intensity (290 - 315 nm) is not sufficient for the purpose of adequate skin irradiation. In fact, during winter the synthesis of VD is minimum, independently from the age of individuals. Thus, the seasonal peak is reached at September, then rapid declining (northern latitudes). Moreover, even if in the summer sufficient levels of VD can be reached, they do not guarantee for the winter-early spring months. In the southern latitudes these findings are opposite to the ones of the north hemisphere. Interestingly, together the seasonal variations of VD production and circulating levels, it has been observed also a seasonal-dependent mRNA expression of VD receptor (VDR) at the human peripheral immune system [33] . Finally, the time of day exposure, together the atmospheric conditions (clouds reduce UV energy by 50% and air pollution by 60%) strongly influence the cutaneous synthesis of VD.
§ Geographical influences
Above and below the 33th parallel (Europe and Mediterranean basin), low or no skin synthesis for most of the winter season has been reported [34] . The geographical features of the location (e.g. altitude has a light reflection phenomenon) contribute to alter/facilitate VD production from the skin [35] .
§ Individual influences
VD3 metabolite is strongly liposoluble and a greater fat mass “dilutes” circulating VD levels, with higher risk of deficiency in obese patients. Moreover, the adipose tissue frees small amount of VD compared to the stored amount. All these considerations brought to suggest that obese subjects may “require” a 40% increase in intake vs. non- obese [36] .
Table 3 summarizes the results merging out from a systematic review of the VD status in worldwide populations [37] .
Table 3. A systematic review of VD status in worldwide populations [34] .
6.5. Degradation of 1α, 25(OH)2D
The CYP24A1 gene is induced by 1α,25(OH)2D. The resulting enzyme carries out all the reactions shown to produce the biologically inactive excretion product, calcitroic acid. Presumably, a similar set of reactions takes place with 25(OH)D as the substrate. Clearly, 1,25(OH)2D programs its own destruction through the CYP24A1 enzyme.
1,25(OH)2D inhibits the 1-OHase action and stimulates kidney expression of 25- (OH)D-24-hydroxylase(24-OHase). The induction of 24-OHase degrades 1,25(OH)2D in calcitroic acid, biologically inactive and water soluble.
The discovery that almost all human tissues and cells express the VDR and that many also express CYP27B1 [which converts 25(OH)D into 1,25(OH)2D] has opened new horizons on the multiple non-skeletal functions of this molecule. At the extraskeletal level, unlike the renal enzyme consideration, the 1-alpha-hydroxylase is not regulated by PTH, but by the availability of the substrate, or from the levels of 25(OH)D. In this regard, it can be assumed that a supplementation with VD can play a role in reducing the risk of chronic diseases, including even some cancers (breast, colon, prostate), autoimmune diseases (DM1, multiple sclerosis), infectious and cardiovascular pathologies [38] , although this issue is still far from proven.
7. VDR
Once activated to 1,25(OH)2D, VD binds to its specific receptor (VDR) that belongs to the superfamily of steroid receptors. In reality, there are two types of VDRs. The first identified, localizes in the nucleus and it is able to directly stimulate the transcription of genes, and then ex-novo synthesis of proteins (genomic mechanism). The other receptor is localized on the cell membrane and acts by inducing the formation of second cellular messengers (such as cAMP, diacylglycerol, inositol triphosphate, arachidonic acid) or phosphorylating some cellular proteins. This second mechanism of action is not genomic and is able to rapidly modulate the cellular response to various stimuli [30] .
VDRs are virtually ubiquitous, as proof of their important physiological role, not only in mineral metabolism but also in many other body functions. The affinity of the VDR for the 1,25(OH)2D is a thousand times greater than that for the 25(OH)D or for other metabolites. The VDR distribution in many tissues, explains the myriad of physiological actions of VD.
The 1,25(OH)2D/VDR complex coordinates, directly or indirectly, the transcription of several genes (approximately 2000) whose products are involved in the regulation of glucose metabolism, immunity (innate and acquired), insulin secretion and cardiovascular system and other.
Overall, VDR/VD complex regulates the expression of 2000 genes, many of whose gene promoters contain VDR responsive elements (VDREs). VDR partners, with other transcription factors, particularly the retinoic X receptor (RXR), and co-activators and co-repressors, provide the specificity of target genes. The membrane VDR mediates the most immediate interventions, nongenomic actions [39] .
It is therefore understandable as any improvement in the condition of the VD will affect significantly the expression of genes with a variety of biological functions of over 80 pathways related to cancer, autoimmune and cardiovascular diseases, associated with the VD deficiency [40] .
8. VD3, Calcium Homeostasis and Bone Health
VD3 is regarded as an essential nutrient required for increasing intestinal calcium (Ca) and phosphate (Pi) absorption. As above reported, VD3 is converted by 25-hydroxylase in the liver to 25(OH)D, which is then activated by 1α-hydroxylase in the kidneys to become 1,25(OH)2D. The active VD, 1,25(OH)2D, acts in the intestine to stimulate Ca absorption and maintain Ca balance. Under conditions of VD insufficiency or deficiency, intestinal Ca absorption decreases resulting in a subtle reduction in serum Ca, which causes an increase in the secretion of parathyroid hormone (PTH). Sustained PTH elevation enhances not only activation of VD3 to 1,25(OH)2D, but also bone resorption. As a result, the bone remodeling balance becomes negative under conditions of VD insufficiency or deficiency. Since osteoporosis treatment aims to improve negative remodeling balance in the bone, it is important to make the calcium balance positive. Therefore, VD3 supplementation is recommended whenever patients are given therapeutic drugs for osteoporosis.
8.1. Classical Bone Effects of VD3
They can be simply summarized in promoting: 1) Osteoclast (OCL)-mediated bone resorption; and 2) Osteoblast (OBL)-mediated bone formation.
In fact, VD is recognized by VDR in OBL and stimulates the production of the Receptor Activator of Nuclear factor-Kb Ligand (RANKL) and the differentiation and function of both OBL and OCL. RANKL binds to its receptor, RANK, on the membrane surface of pre-OCL cells inducing their maturation. Consequently, the mature OCL begin the bone resorption contributing to calcium/phosphate homeostasis. With appropriate levels of both ionized calcium and plasma phosphate, the mineralization of the skeleton is favored [41] [42] .
8.2. 1,25(OH)2D and Mineral Homeostasis
All bone cells, including mesenchymal and hematopoietic stem cells, express VDR. The regulatory pathways between OBLs, osteocytes (Ocs), and OCLs include several negative and positive feedback loops, all potentially modulated by 1,25(OH)2D. Such molecular mechanisms include the “local” release of matrix components, such as Transforming Growth Factor-β (TGF-β) and bone morphogenetic proteins (BMPs) influencing OBL activity, as also humoral factors modulating the mineral homeostasis at distant sites, such as FGF23 that has a major role on the renal phosphate handling. The complexity of these loops may explain why any regulatory effect on one specific cell type can influence the other bone cells. Moreover, such a complexity may be helpful to explain the differential effects of 1,25(OH)2D in driving toward anabolic and/or catabolic outcomes. Recently, the identification of microRNAs (miRNAs) set, positively and/or negatively regulated by 1,25(OH)2D in OBLs, and of miRNAs that govern action, synthesis and metabolism of the VD, makes even more difficult the task of tangling this complexity [43] .
As above reported, among more than 2000 genes, 1,25(OH)2D coordinates the expression of functional genes, such as the ones encoding for RANKL, Transient receptor potential cation channel subfamily V member 5 (TRPV5), the sodium-calcium exchanger (NCX-1), the transient receptor potential family member 6 (TRPV6), the S100 calcium-binding protein G (S100g), the plasma membrane Ca(2+)-ATPase (PMCA) isoform 1b (PMCA1b), all highly selective for Ca ions, and other proteins, for the uptake of calcium and phosphate from the diet, recovery and the conservation of calcium and phosphate acting on kidney, “bone remodeling” and skeletal mineralization [44] - [46] .
In a extreme simplified model, it could be stated that initially VD stimulates the synthesis of osteoprotegerin (OPG), the decoy receptor of RANKL produced by OBLs and bone stromal cells, which reduces RANKL expression by OBL and blocking the formation and activation of the OCL [47] , then activates RANKL and inhibits OPG, favoring either the mineralization or the OCLgenesis and bone resorption [48] .
8.3. Relationship between Serum Levels of 25(OH)D, Bone Resorption Markers, and Skeletal Outcomes
Values of 20 ng/ml of circulating 25(OH)D would be sufficient to reduce the bone resorption markers [49] , improve Ca intestinal absorption and BMD, contributing to the reduction of altered mineralization status such as rickets (bone accrual) in pre-pubertal individuals, and osteomalacia (bone maintenance) in adults [50] .
However, the relationship between serum levels of 25(OH)D and the percentage of presence of osteoid tissue demonstrates that circulating levels of VD upper/equal to 30 ng/ml are needed to significantly decrease osteoid amount in bone [51] .
8.4. Large Doses of VD3 Supplements, Bone Strength and Metabolism
The “schizophrenic” behavior of bone strength and turnover, relatively to the dosage of VD supplementation, namely cholecalciferol, may be considered emblematic to “justify” how above reported on the mineralization effects of 1,25(OH)2D.
In fact, in an Australian randomized controlled trial [52] , the use of annual oral boluses of 500,000 IU of VD3 for 3 - 5 years has been associated to an increased risk of falls and fractures in a women community-dwelling population.
Moreover, it has been lately reported that large doses oral boluses of VD3, more than 300,000 IU, may be associated with acute increase of bone turnover markers, such as s-CTX and NTX, which could explain the negative clinical results with intermittent high doses to treat/prevent VD3 deficiency [53] .
In any case, the increase of bone resorption (persistent up to 60 days following bolus administration of VD3) must be considered a side effect that can potentially have adverse effects on skeletal health. For this reason, these results have justified and amplified the doubts on whether to use the mega-oral doses of VD3 (600,000 IU) and also led to investigate the effect of lower doses [54] .
8.5. VD3 and Bone Cells
§ VD3 and OBLs
It affects proliferation, differentiation of OBL and the mineralization process. These effects vary with the treatment time, dosage and the origin of OBL. The effects of VD3 on differentiation and mineralization are mostly stimulating OBLs in human and rat, and inhibitory on murine OBLs.
As above mentioned, several genes and mechanisms have been designed to explain the effects of 1,25(OH)2D on OBL differentiation and bone formation. In addition to the classical pathway of the VDR, OBLs also express a membrane VDR, non genomic pathway, and in vitro studies have shown that OBLs are able to synthesize 25(OH)D [55] .
The differentiation of OBLs is mediated by both local and systemic factors and is a key step in the skeletal development and acquisition of bone mass. 1,25(OH)2D can adjust both positively and negatively the OBL expression of markers of mineralization depending on the stage of differentiation of OBLs and adaptive responses to calcium malabsorption [55] .
§ VD3 and OCLs
It has been already cited that treating OBLs with 1,25(OH)2D stimulates expression of RANKL, which in turn induces OCLgenesis. However, active VD compounds such as calcitriol, 3a-calcidiol, 1α(OH)D, and eldecalcitol [1α, 25-dihydroxy-2B-(3-hydrox- ypropoxy) VD3] represent therapeutic drugs for osteoporosis, as they increase the BMD in the osteoporotic patients. Paradoxically, the increase in BMD is caused by the suppression of bone resorption [47] .
§ VD3 and Ocs
1,25(OH)2D regulates the expression, in Ocs and OBLs, of FGF23, factor important for the homeostasis of phosphate whose activities have significant systemic effects. Besides stimulating FGF23, it has been shown that 1,25(OH)2D can adjust the Phosphate-regulating neutral endopeptidase, X-linked, or PHEX peptidase, an indirect putative regulator of the secretion of FGF23 [A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets (XLH/HYP). The HYP Consortium] [56] . A possible set of other factors could exist in the Ocs. The physiological significance of the VDR and 1αhydroxylase activity in Ocs are only partially understood and this is an area of active investigation. Interactions between 1,25(OH)2D and Ocs are often only apparent in altered mineral homeostasis conditions, and these interactions could have a role for the treatment options in metabolic bone diseases [57] .
9. Relationship between 25(OH)D and Skeletal Muscle Performance in the Elderly. An Indirect Role on Bone Health
It is well known that the lack of VD3, especially if continued in time, bring to disability and atrophy of muscle fibers type 2, with increased space between the muscle fibrils and replacement of muscle tissue with fat cells and fibrous tissue, directly determining an increased risk of falls.
In fact, VD3, influences: 1) differentiation of mesenchymal cells into myoblasts; 2) proliferation of myoblasts; and 3) differentiation of myoblasts into myocytes [58] and the current evidences demonstrate its effects on muscle cells, intracellular calcium, differentiation and composition of contractile proteins [59] . All together, these findings may explain why parameters such as walking speed, distance made in 6-minute walk, and strength of hand grip dramatically improve when 25(OH)D serum levels move from low values towards the 30, and more, ng/ml. Thus, VD deficiency consequently increases the risk for accidental falls in the elderly and, indirectly, for fragility fractures.
10. 25(OH)D Levels, BMD, and Fragility Fractures
Although it has been widely reported the VD insufficiency is prevalent in postmenopausal women and related to low BMD, controversial results still exist on the relationship between serum 25(OH)D levels, BMD, and risk for fragility fractures. Here we make a brief summary on the state of the art about these issues.
10.1. Evidences from Cross-Sectional Studies
A positive association between serum 25(OH)D levels and BMD in post-menopausal women has been reported in many studies in past twenty-five years [60] - [65] .
A Women Health Initiative (WHI) case-control study showed a double probability of risk of hip fracture in subjects with serum 25(OH)D lower than 20 ng/mL (50 nmol/L) [66] . Lately, an Italian study [67] and the US 2010 National Health and Nutrition Examination Survey (NHANES) [68] demonstrated a similar positive relationship, between serum 25(OH)D levels and BMD, also evident in women before menopause.
In 2014, an Italian study on post-menopausal Sicilian women concludes that deficiency/insufficiency of VD3 is common in this population and may be associated with low BMD and increased bone turnover markers [69] .
10.2. Loss of BMD, Increased Risk of Fracture and Low 25(OH)D
An annual loss of approximately 35% of total hip BMD and an increased fracture risk, approximately 2 times, have been reported for low levels, less than 30 ng/ml, of 25(OH)D. Both these parameters worse, reaching approximately 50% and 4 times, respectively, when VD3 insufficiency/deficiency is also associated with lack of sex steroids as happen in post-menopausal women, without HRT, and elderly subjects [70] .
10.3. Conclusions and Take Home Messages on VD3 and Skeleton
Although an universal consensus to define the adequate levels of serum 25(OH)D has not been reached in order to obtain normalization of PTH levels, there are some evidences on the need of optimizing serum 25(OH)D levels for the prevention of bone loss and reducing minimal trauma fractures in postmenopausal women and older men. Moreover, it has to be taken into account that an adequate calcium intake is also necessary to optimize benefit from an improved VD status in insufficient subjects, since an increased calcium intake associates with suppression of PTH levels [71] [72] . Finally, a small benefit either on fracture or bone loss prevention in individuals with inadequate 25(OH)D serum levels, such as institutionalized elderly patients, can be considered but only when VD3 supplements are combined with adequate daily dietary calcium intake or supplements.
VD3 is of fundamental importance to ensure adequate and proper development of the skeleton and subsequent maintenance of its integrity. Among its multiple key functions, it has to be remembered:
- Intestinal absorption of calcium;
- Effect on proper regulation of PTH levels;
- Proper bone mineralization;
- Direct stimulation on the muscle and preventing falls;
- Improve strength and muscle function (elders);
- Increase BMD;
- Reduce the risk of fractures, including hip.
It is always to assess the single individual, his/her family, clinical, pharmacological history, and the general state of bone fragility.
In conclusion, it is important to emphasize that to try to obtain the potential benefits of reaching/maintaining adequate circulating levels of 25(OH)D, the VD3 supplementation must be appropriated and not prescribed with a “rain distribution” approach that does not guarantee this obtainment and contributes both to an its inadequate prescription and to an improper allocation of economic resources to invest in the prevention of metabolic bone diseases.
A clear resolution to these problems can only be achieved by a shared, and recognized, definition of the minimum threshold of adequacy of circulating levels of VD. This, however, appears to be more difficult to solve because of, apart from the need to understand even better the molecular adjustments, tissue- and cell-specific, the metabolism of VD itself; it remains unclear what are the biochemical and clinical parameters for classifying the adequacy concept. Even limiting ourselves only to the aspects of bone health, will we have different clinical parameters to consider depending on the clinical outcomes to be pursued (e.g. bone mineralization rate, increase of BMD, reduced risk of fragility fractures, optimization of the calcium-phosphate management, improved muscle mass and strength, reduction of falls, and so on)?
In the waiting for such responses, it should to be taken into account that currently it is possible to identify categories of individuals at risk of VD deficiency/insufficiency and that the costs of its supplementation are quite content.
Cite this paper
Falchetti, A., Rossi, E., Cosso, R., Buffa, A., Corvaglia, S. and Malavolta, N. (2016) Vitamin D and Bone Health. Food and Nutrition Sciences, 7, 1033-1051. http://dx.doi.org/10.4236/fns.2016.711100
References
- 1. Bloch, K.E. (1983) Sterol Structure and Membrane Function. CRC Critical Reviews in Biochemistry, 14, 47-92.
http://dx.doi.org/10.3109/10409238309102790 - 2. Summons, R.E., Bradley, A.S., Jahnke, L.L. and Waldbauer, J.R. (2006) Steroids, Triterpenoids and Molecular Oxygen. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 361, 951-968.
http://dx.doi.org/10.1098/rstb.2006.1837 - 3. Holick, M.F. (2011) Vitamin D: Evolutionary, Physiological and Health Perspectives. Curr Drug Targets, 12, 4-18.
http://dx.doi.org/10.2174/138945011793591635 - 4. Steenbock, H. and Black, A. (1925) Fat-Soluble Vitamins: XXIII. The Induction of Growth-Promoting and Calcifying Properties in Fats and Their Unsaponifiable Constituents by Exposure to Light. Journal of Biological Chemistry, 64, 263-298.
- 5. Diffey, B. (2008) A Behavioral Model for Estimating Population Exposure to Solar Ultraviolet Radiation. Photochemistry and Photobiology, 84, 371-375.
http://dx.doi.org/10.1111/j.1751-1097.2007.00271.x - 6. Engelsen, O., Brustad, M., Aksnes, L. and Lund, E. (2005) Daily Duration of Vitamin D Synthesis in Human Skin with Relation to Latitude, Total Ozone, Altitude, Ground Cover, Aerosols and Cloud Thickness. Photochemistry and Photobiology, 81, 1287-1290.
http://dx.doi.org/10.1562/2004-11-19-RN-375 - 7. Czarnecki, D., Meehan, C.J. and Bruce, F. (2009) The Vitamin D Status of Australian Dermatologists. Clinical and Experimental Dermatology, 34, 624-625.
http://dx.doi.org/10.1111/j.1365-2230.2008.03002.x - 8. Holick, M.F. (2008) Sunlight, UV-Radiation, Vitamin D and Skin Cancer: How Much Sunlight Do We Need? Advances in Experimental Medicine and Biology, 624, 1-15.
http://dx.doi.org/10.1007/978-0-387-77574-6_1 - 9. Holick, M.F., Matsuoka, L.Y. and Wortsman, J. (1989) Age, Vitamin D, and Solar Ultraviolet. Lancet, 2, 1104-1105.
http://dx.doi.org/10.1016/S0140-6736(89)91124-0 - 10. Maggio, D., Cherubini, A., Lauretani, F., Russo, R.C., Bartali, B., Pierandrei, M., Ruggiero, C., Macchiarulo, M.C., Giorgino, R., Minisola, S. and Ferrucci, L. (2005) 25(OH)D Serum Levels Decline with Age Earlier in Women than in Men and Less Efficiently Prevent Compensatory Hyperparathyroidism in Older Adults. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 60, 1414-1419.
http://dx.doi.org/10.1093/gerona/60.11.1414 - 11. Misra, M., Pacaud, D., Petryk, A., Collett-Solberg, P.F. and Kappy, M. (2008) Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D Deficiency in Children and Its Management: Review of Current Knowledge and Recommendations. Pediatrics, 122, 398-417.
http://dx.doi.org/10.1542/peds.2007-1894 - 12. Holick, M.F., Biancuzzo, R.M., Chen, T.C., Klein, E.K., Young, A., Bibuld, D., Reitz, R., Salameh, W., Ameri, A. and Tannenbaum, A.D. (2008) Vitamin D2 Is as Effective as Vitamin D3 in Maintaining Circulating Concentrations of 25-Hydroxyvitamin D. Journal of Clinical Endocrinology & Metabolism, 93, 677-681.
http://dx.doi.org/10.1210/jc.2007-2308 - 13. Haddad, J.G., Matsuoka, L.Y., Hollis, B.W., Hu, Y.Z. and Wortsman, J. (1993) Human Plasma Transport of Vitamin D after Its Endogenous Synthesis. Journal of Clinical Investigation, 91, 2552-2555.
http://dx.doi.org/10.1172/JCI116492 - 14. Hollander, D., Muralidhara, K.S. and Zimmerman, A. (1978) Vitamin D-3 Intestinal Absorption in Vivo: Influence of Fatty Acids, Bile Salts, and Perfusate pH on Absorption. Gut, 19, 267-272.
http://dx.doi.org/10.1136/gut.19.4.267 - 15. Hollis, B.W., Lowery, J.W., Pittard 3rd, W.B., Guy, D.G. and Hansen, J.W. (1996) Effect of Age on the Intestinal Absorption of Vitamin D3-Palmitate and Nonesterified Vitamin D2 in the Term Human Infant. Journal of Clinical Endocrinology & Metabolism, 81, 1385-1388.
- 16. Chapuy, M.C., Schott, A.M., Garnero, P., Hans, D., Delmas, P.D. and Meunier, P.J. (1996) Healthy Elderly French Women Living at Home Have Secondary Hyperparathyroidism and High Bone Turnover in Winter. EPIDOS Study Group. The Journal of Clinical Endocrinology & Metabolism, 81, 1129-1133.
- 17. LeFevre, M.L. (2015) Screening for Vitamin D Deficiency in Adults: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine, 162, 133-140.
http://dx.doi.org/10.7326/M14-2450 - 18. LeBlanc, E.S., Zakher, B., Daeges, M., Pappas, M. and Chou, R. (2015) Screening for Vitamin D Deficiency: A Systematic Review for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 162, 109-122.
http://dx.doi.org/10.7326/M14-1659 - 19. Holick, M.F. (2007) Vitamin D Deficiency. The New England Journal of Medicine, 357, 266-281.
http://dx.doi.org/10.1056/NEJMra070553 - 20. Ricceri, F., Pescitelli, L., Tripo, L. and Prignano, F. (2013) Deficiency of Serum Concentration of 25-Hydroxyvitamin D Correlates with Severity of Disease in Chronic Plaque Psoriasis. Journal of the American Academy of Dermatology Home, 68, 511-512.
http://dx.doi.org/10.1016/j.jaad.2012.10.051 - 21. Adams, J.S., Clemens, T.L., Parrish, J.A. and Holick, M.F. (1982) Vitamin-D Synthesis and Metabolism after Ultraviolet Irradiation of Normal and Vitamin-D-Deficient Subjects. The New England Journal of Medicine, 306, 722-725.
http://dx.doi.org/10.1056/NEJM198203253061206 - 22. Lo, C.W., Paris, P.W., Clemens, T.L., Nolan, J. and Holick, M.F. (1985) Vitamin D Absorption in Healthy Subjects and in Patients with Intestinal Malabsorption Syndromes. The American Journal of Clinical Nutrition, 42, 644-649.
- 23. Argao, E.A., Heubi, J.E., Hollis, B.W. and Tsang, R.C. (1992) d-α-Tocopheryl Polyethylene Glycol-1000 Succinate Enhances the Absorption of Vitamin D in Chronic Cholestatic Liver Disease of Infancy and Childhood. Pediatric Research, 31, 146-150.
http://dx.doi.org/10.1203/00006450-199202000-00011 - 24. Heaney, R.P., Vieth, R. and Hollis, B.W. (2011) Vitamin D Efficacy and Safety. Archives of Internal Medicine, 171, 266.
http://dx.doi.org/10.1001/archinternmed.2010.528 - 25. Haddad, J.G., Walgate, J., Min, C. and Hahn, T.J. (1976) Vitamin D Metabolite-Binding Proteins in Human Tissue. Biochimica et Biophysica Acta (BBA)-General Subjects, 444, 921-925.
http://dx.doi.org/10.1016/0304-4165(76)90339-1 - 26. Vieth, R., Kessler, M.J. and Pritzker, K.P. (1990) Species Differences in the Binding Kinetics of 25-Hydroxyvitamin D3 to Vitamin D Binding Protein. Canadian Journal of Physiology and Pharmacology, 68, 1368-1371.
http://dx.doi.org/10.1139/y90-207 - 27. Kissmeyer, A., Mathiasen, I.S., Latini, S. and Binderup L. (1990) Pharmacokinetic Studies of Vitamin D Analogues: Relationship to Vitamin D Binding Protein (DBP). Endocrine, 3, 263-266.
http://dx.doi.org/10.1007/BF03021403 - 28. Hollis, B.W. (1984) Comparison of Equilibrium and Disequilibrium Assay Conditions for Ergocalciferol, Cholecalciferol and Their Major Metabolites. Journal of Steroid Biochemistry, 21, 81-86.
http://dx.doi.org/10.1016/0022-4731(84)90063-3 - 29. Smith, J.E. and Goodman, D.S. (1971) The Turnover and Transport of Vitamin D and of a Polar Metabolite with the Properties of 25-Hydroxycholecalciferol in Human Plasma. Journal of Clinical Investigation, 50, 2159-2167.
http://dx.doi.org/10.1172/JCI106710 - 30. Keenan, M.J. and Holmes, R.P. (1991) The Uptake and Metabolism of 25-Hydroxyvitamin D3 and Vitamin D Binding Protein by Cultured Porcine Kidney Cells (LLC-PK1). International Journal of Biochemistry, 23, 1225-1230.
http://dx.doi.org/10.1016/0020-711x(91)90220-h - 31. Nykjaer, A., Dragun, D., Walther, D., Vorum, H., Jacobsen, C., Herz, J., Melsen, F., Christensen, E.I. and Willnow, T.E. (1999) An Endocytic Pathway Essential for Renal Uptake and Activation of the Steroid 25-(OH) Vitamin D3. Cell, 96, 507-515.
http://dx.doi.org/10.1016/S0092-8674(00)80655-8 - 32. Powe, C.E., Evans, M.K., Wenger, J., Zonderman, A.B., Berg, A.H., Nalls, M., Tamez, H., Zhang, D., Bhan, I., Karumanchi, S.A., Powe, N.R. and Thadhani, R. (2013) Vitamin D-Binding Protein and Vitamin D Status of Black Americans and White Americans. The New England Journal of Medicine, 369, 1991-2000.
http://dx.doi.org/10.1056/NEJMoa1306357 - 33. Dopico, X.C., Evangelou, M., Ferreira, R.C., Guo, H., Pekalski, M.L., Smyth, D.J., Cooper, N., Burren, O.S., Fulford, A.J., Hennig, B.J., Prentice, A.M., Ziegler, A.G., Bonifacio, E., Wallace, C. and Todd, J.A. (2015) Widespread Seasonal Gene Expression Reveals Annual Differences in Human Immunity and Physiology. Nature Communications, 6, Article No. 7000.
http://dx.doi.org/10.1038/ncomms8000 - 34. Holick, M.F. (1995) Environmental Factors That Influence the Cutaneous Production of Vitamin D. The American Journal of Clinical Nutrition, 61, 638S-645S.
- 35. Edvardsen, K., Brustad, M., Engelsen, O. and Aksnes, L. (2007) The Solar UV Radiation Level Needed for Cutaneous Production of Vitamin D3 in the Face. A Study Conducted among Subjects Living at a High Latitude (68° N). Photochemical & Photobiological Sciences, 6, 57-62.
http://dx.doi.org/10.1039/B613263D - 36. Dhaliwal, R., Mikhail, M., Feuerman, M. and Aloia, J.F. (2014) The Vitamin D Dose Response in Obesity. Endocrine Practice, 20, 1258-1264.
http://dx.doi.org/10.4158/ep13518.or - 37. Hilger, J., Friedel, A., Herr, R., Rausch, T., Roos, F., Wahl, D.A., Pierroz, D.D., Weber, P. and Hoffmann, K. (2014) A Systematic Review of Vitamin D Status in Populations Worldwide. British Journal of Nutrition, 111, 23-45.
http://dx.doi.org/10.1017/S0007114513001840 - 38. Holick, M.F. (2004) Sunlight and Vitamin D for Bone Health and Prevention of Autoimmune Diseases, Cancers, and Cardiovascular Disease. The American Journal of Clinical Nutrition, 80, 1678S-1688S.
- 39. Holick, M.F. (2013) Vitamin D, Sunlight and Cancer Connection. Anti-Cancer Agents in Medicinal Chemistry, 13, 70-82.
http://dx.doi.org/10.2174/187152013804487308 - 40. Hossein-nezhad, A. and Holick, M.F. (2013) Vitamin D for Health: A Global Perspective. Mayo Clinic Proceedings, 88, 720-755.
http://dx.doi.org/10.1016/j.mayocp.2013.05.011 - 41. Holick, M.F., Chen, T.C., Lu, Z. and Sauter, E. (2007) Vitamin D and Skin Physiology: A D-Lightful Story. Journal of Bone and Mineral Research, 22, V28-V33.
http://dx.doi.org/10.1359/jbmr.07s211 - 42. Holick, M.F. (2009) Vitamin D Status: Measurement, Interpretation, and Clinical Application. Annals of Epidemiology, 19, 73-78.
http://dx.doi.org/10.1016/j.annepidem.2007.12.001 - 43. Lisse, T.S., Chun, R.F., Rieger, S., Adams, J.S. and Hewison, M. (2013) Vitamin D Activation of Functionally Distinct Regulatory miRNAs in Primary Human Osteoblasts. Journal of Bone and Mineral Research, 28, 1478-1488.
http://dx.doi.org/10.1002/jbmr.1882 - 44. Bouillon, R., Van Cromphaut, S. and Carmeliet, G. (2003) Intestinal Calcium Absorption: Molecular Vitamin D Mediated Mechanisms. Journal of Cellular Biochemistry, 88, 332-339.
http://dx.doi.org/10.1002/jcb.10360 - 45. Bouillon, R., Carmeliet, G., Verlinden, L., van Etten, E., Verstuyf, A., Luderer, H.F., Lieben, L., Mathieu, C. and Demay, M. (2008) Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocrine Reviews, 29, 726-776.
http://dx.doi.org/10.1210/er.2008-0004 - 46. Bouillon, R., Bischoff-Ferrari, H. and Willett, W. (2008) Vitamin D and Health: Perspectives from Mice and Man. Journal of Bone and Mineral Research, 23, 974-979.
http://dx.doi.org/10.1359/jbmr.080420 - 47. Takahashi, N., Udagawa, N. and Suda, T. (2014) Vitamin D Endocrine System and Osteoclasts. BoneKEy Reports, 3, Article No. 495.
http://dx.doi.org/10.1038/bonekey.2014.17 - 48. Eisman, J.A. and Bouillon, R. (2014) Vitamin D: Direct Effects of Vitamin D Metabolites on Bone: Lessons from Genetically Modified Mice. BoneKEy Reports, 3, Article No. 499.
http://dx.doi.org/10.1038/bonekey.2013.233 - 49. Sai, A.J., Walters, R.W., Fang, X. and Gallagher, J.C. (2011) Relationship between Vitamin D, Parathyroid Hormone, and Bone Health. The Journal of Clinical Endocrinology & Metabolism, 96, E436-E446.
http://dx.doi.org/10.1210/jc.2010-1886 - 50. Rosen, C.J. (2012) Building Bones by Knocking down Genes. Nature Medicine, 18, 202-204.
http://dx.doi.org/10.1038/nm.2630 - 51. Priemel, M., von Domarus, C., Klatte, T.O., Kessler, S., Schlie, J., Meier, S., Proksch, N., Pastor, F., Netter, C., Streichert, T., Püschel, K. and Amling, M. (2010) Bone Mineralization Defects and Vitamin D Deficiency: Histomorphometric Analysis of Iliac Crest Bone Biopsies and Circulating 25-Hydroxyvitamin D in 675 Patients. Journal of Bone and Mineral Research, 25, 305-312.
http://dx.doi.org/10.1359/jbmr.090728 - 52. Sanders, K.M., Stuart, A.L., Williamson, E.J., Simpson, J.A., Kotowicz, M.A., Young, D. and Nicholson, G.C. (2010) Annual High-Dose Oral Vitamin D and Falls and Fractures in Older Women: A Randomized Controlled Trial. JAMA, 303, 1815-1822.
http://dx.doi.org/10.1001/jama.2010.594 - 53. Rossini, M., Gatti, D., Viapiana, O., Fracassi, E., Idolazzi, L., Zanoni, S. and Adami, S. (2012) Short-Term Effects on Bone Turnover Markers of a Single High Dose of Oral Vitamin D3. The Journal of Clinical Endocrinology & Metabolism, 97, E622-E626.
http://dx.doi.org/10.1210/jc.2011-2448 - 54. Rossini, M., Adami, S., Viapiana, O., Fracassi, E., Idolazzi, L., Povino, M.R. and Gatti, D. (2012) Dose-Dependent Short-Term Effects of Single High Doses of Oral Vitamin D3 on Bone Turnover Markers. Calcified Tissue International, 91, 365-369.
http://dx.doi.org/10.1007/s00223-012-9637-y - 55. van Driel, M. and van Leeuwen, J.P. (2014) Vitamin D Endocrine System and Osteoblasts. BoneKEy Reports, 3, Article No. 493.
- 56. Francis, F., Hennig, S., Korn B., Reinhardt, R., de Jong, P., Poustka, A., Lehrach, H., Rowe, P.S.N., Goulding, J.N., Summerfield, T., Mountford, R., Read, A.P., Popowska, E., Pronicka, E., Davies, J.L.H., O’Riordan, K.E., Econs, M.J., Nesbitt, T., Drezner, M.K., Oudet, C., Pannetier, S., Hanauer, A., Strom, T.M., Meindl, A., Lorenz, B., Cagnoli, B., Mohnike, K.L., Murken, J. and Meitinger, T. (1995) A Gene (PEX) with Homologies to Endopeptidases Is Mutated in Patients with X-Linked Hypophosphatemic Rickets. Nature Genetics, 11, 130-136.
http://dx.doi.org/10.1038/ng1095-130 - 57. Lanske, B., Densmore, M.J. and Erben, R.G. (2014) Vitamin D Endocrine System and Osteocytes. BoneKEy Reports, 3, Article No. 494.
http://dx.doi.org/10.1038/bonekey.2013.228 - 58. Toffanello, E.D., Perissinotto, E., Sergi, G., Zambon, S., Musacchio, E., Maggi, S., Coin, A., Sartori, L., Corti, M.C., Baggio, G., Crepaldi, G. and Manzato, E. (2012) Vitamin D and Physical Performance in Elderly Subjects: The Pro.V.A Study. PLoS ONE, 7, e34950.
http://dx.doi.org/10.1371/journal.pone.0034950 - 59. Polly, P. and Tan, T.C. (2014) The Role of Vitamin D in Skeletal and Cardiac Muscle Function. Frontiers in Physiology, 5, 145.
http://dx.doi.org/10.3389/fphys.2014.00145 - 60. Villareal, D.T., Civitelli, R., Chines, A. and Avioli, L.V. (1991) Subclinical Vitamin D Deficiency in Postmenopausal Women with Low Vertebral Bone Mass. The Journal of Clinical Endocrinology & Metabolism, 72, 628-634.
http://dx.doi.org/10.1210/jcem-72-3-628 - 61. Collins, D., Jasani, C., Fogelman, I. and Swaminathan, R. (1998) Vitamin D and Bone Mineral Density. Osteoporosis International, 8, 110-114.
http://dx.doi.org/10.1007/BF02672505 - 62. Lamberg-Allardt, C.J., Outila, T.A., Karkkainen, M.U., Rita, H.J. and Valsta, L.M. (2001) Vitamin D Deficiency and Bone Health in Healthy Adults in Finland: Could This Be a Concern in Other Parts of Europe? Journal of Bone and Mineral Research, 16, 2066-2073.
http://dx.doi.org/10.1359/jbmr.2001.16.11.2066 - 63. Mezquita-Raya, P., Munoz-Torres, M., Luna, J.D., Luna, V., Lopez-Rodriguez, F., Torres-Vela, E. and Escobar-Jiménez, F. (2001) Relation between Vitamin D Insufficiency, Bone Density, and Bone Metabolism in Healthy Postmenopausal Women. Journal of Bone and Mineral Research, 16, 1408-1415.
http://dx.doi.org/10.1359/jbmr.2001.16.8.1408 - 64. Fradinger, E.E. and Zanchetta, J.R. (2001) Vitamin D and Bone Mineral Density in Ambulatory Women Living in Buenos Aires, Argentina. Osteoporosis International, 12, 24-27.
http://dx.doi.org/10.1007/s001980170153 - 65. Bischoff-Ferrari, H.A., Dietrich, T., Orav, E.J. and Dawson-Hughes, B. (2004) Positive Association between 25-Hydroxy Vitamin D Levels and Bone Mineral Density: A Population-Based Study of Younger and Older Adults. The American Journal of Medicine, 116, 634-639.
http://dx.doi.org/10.1016/j.amjmed.2003.12.029 - 66. Cauley, J.A., Lacroix, A.Z., Wu, L., Horwitz, M., Danielson, M.E., Bauer, D.C., Lee, J.S., Jackson, R.D., Robbins, J.A., Wu, C., Stanczyk, F.Z., LeBoff, M.S., Wactawski-Wende, J., Sarto, G., Ockene, J. and Cummings, S.R. (2008) Serum 25-Hydroxyvitamin D Concentrations and Risk for Hip Fractures. Annals of Internal Medicine, 149, 242-250.
http://dx.doi.org/10.7326/0003-4819-149-4-200808190-00005 - 67. Adami, S., Bertoldo, F., Braga, V., Fracassi, E., Gatti, D., Gandolini, G., Minisola, S. and Battista Rini, G. (2009) 25-Hydroxy Vitamin D Levels in Healthy Premenopausal Women: Association with Bone Turnover Markers and Bone Mineral Density. Bone, 45, 423-426.
http://dx.doi.org/10.1016/j.bone.2009.05.012 - 68. Ross, A.C., Manson, J.E., Abrams, S.A., Aloia, J.F., Brannon, P.M., Clinton, S.K., Durazo-Arvizu, R.A., Gallagher, J.C., Gallo, R.L., Jones, G., Kovacs, C.S., Mayne, S.T., Rosen, C.J. and Shapses, S.A. (2011) The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. The Journal of Clinical Endocrinology & Metabolism, 96, 53-58.
http://dx.doi.org/10.1210/jc.2010-2704 - 69. Napoli, N., Strollo, R., Sprini, D., Maddaloni, E., Rini, G.B. and Carmina, E. (2014) Serum 25-OH Vitamin D in Relation to Bone Mineral Density and Bone Turnover. International Journal of Endocrinology, 2014, Article ID: 487463.
http://dx.doi.org/10.1155/2014/487463 - 70. Barrett-Connor, E., Laughlin, G.A., Li, H., Nielson, C.M., Wang, P.Y., Dam, T.T., Cauley, J.A., Ensrud, K.E., Stefanick, M.L., Lau, E., Hoffman, A.R. and Orwoll, E.S., for the Osteoporotic Fractures in Men (MrOS) Research Group (2012) The Association of Concurrent Vitamin D and Sex Hormone Deficiency with Bone Loss and Fracture Risk in Older Men: The Osteoporotic Fractures in Men (MrOS) Study. Journal of Bone and Mineral Research, 27, 2306-2313.
http://dx.doi.org/10.1002/jbmr.1697 - 71. Elders, P., Lips, P., Netelenbos, J., VanGrinkel, F., Khoe, E., Van der VijghWet, W.J. and van der Stelt, P.F. (1994) Long-Term Effect of Calcium Supplementation on Bone Loss in Perimenopausal Women. Journal of Bone and Mineral Research, 9, 963-970.
http://dx.doi.org/10.1002/jbmr.5650090702 - 72. Reid, I., Mason, B., Horne, A., Ames, R., Reid, H., Bava, U., Bolland, M.J. and Gamble, G.D. (2006) Randomized Controlled Trial of Calcium in Healthy Older Women. The American Journal of Medicine, 119, 777-785.
http://dx.doi.org/10.1016/j.amjmed.2006.02.038