Food and Nutrition Sciences
Vol.4 No.8A(2013), Article ID:35258,9 pages DOI:10.4236/fns.2013.48A008
Indigenous Purple Vegetable Extracts Protect against Hydrogen Peroxide-Induced DNA Damage in Human Lymphocytes
![]()
1Department of Food, Health and Nutrition Science, Chinese Culture University, Taipei, Taiwan; 2Institute of Applied Life Science, Chinese Culture University, Taipei, Taiwan; 3School of Nutrition and Health Sciences, Taipei Medical University, Taipei, Taiwan; 4Department of Horticulture and Landscape Architecture, National Taiwan University, Taipei, Taiwan; 5Graduate Institute of Biotechnology, Chinese Culture University, Taipei, Taiwan; 6Research Center for Biodiversity, Academia Sinica, Taipei, Taiwan; 7Taoyuan District Agricultural Research and Extension Station Council of Agriculture, Executive Yuan, Taoyuan, Taiwan.
Email: *pychao@faculty.pccu.edu.tw
Copyright © 2013 Pi-Yu Chao et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 4th, 2013; revised May 4th, 2013; accepted May 11th, 2013
Keywords: Antioxidant; Indigenous Purple-Leaved Vegetables; Comet Assay; Flavonols; Cynidin
ABSTRACT
The objectives of this study were to identify the antioxidants in acid hydrolysates of six of Taiwan’s indigenous purple-leaved vegetables (IPLV), including purple-leaved sweet potato (Ipomoea batatas L. Lamark), purple-leaved gynura line 1 (Gynura bicolor D.C.), purple-leaved gynura line 2 (Gynura bicolor D.C.), purple-leaved perilla line 1 (Perilla frutescents L. Britton), bicolored-leaved perilla line 2 (Perilla frutescens L. Britton), and heartleaf houttuynia (Houttuynia cordata Thumb.). Their antioxidant activity and ability to protect DNA from oxidative damage was also evaluated. The antioxidants identified included flavonoids, anthocyanidins and flavonols. Both purple-leaved perilla line 1 and bicolored-leaved perilla line 2 were found to be abundant in cynidin, malvidin, and myricetin. Moreover, purple-leaved sweet potato and heartleaf houttuynia were found to be abundant in quercetin. Purple-leaved sweet potato and purple-leaved perilla line 1 contained significantly higher inhibition percentages of conjugated diene formation than other vegetables. Purple-leaved sweet potato, heartleaf houttuynia, purple-leaved perilla line 1, and bicolored-leaved perilla line 2 had better inhibition percentages of tail DNA% and tail moment in the study. In conclusion, the abovementioned four indigenous purple-leaved vegetables abounded in antioxidants had strong antioxidant activity for protecting DNA in lymphocytes from oxidative damages.
1. Introduction
Many epidemiological studies suggest that increased consumption of vegetables, fruits, and other foods that contain antioxidants can protect against DNA damage and carcinogenesis [1-3]. Flavonoids such as flavonols, flavanols, and anthocyanins have strong antioxidative efficiencies and are common in vegetables and fruits. The antioxidant a-tocopherol decreases TPA-induced DNA fragmentation and the occurrence of carcinogenesis [4-6]. Hence, a number of phytochemicals commonly used in research have antioxidant activity that can protect cells from ROS-mediated DNA damage, resulting in mutation and subsequent carcinogenesis [7-11]. Cao [12] indicated that increased consumption of vegetables and fruits increases the plasma antioxidant capacity in human beings. Several vegetables, such as garlic, kale, spinach, Brussels sprouts, broccoli, red bell pepper, beets, corn, and eggplant, have antioxidant activity against hydroxyl radicals [13]. Some common vegetables, such as sweet potato (purpleand green-leaved) and onion outer layers, abound in quercetin and myricetin, scavenge DPPH, superoxide, and hydroxyl radicals, and inhibit lipid peroxidation [14].
Some of Taiwan’s indigenous vegetables, such as leafy sweet potato, perilla, Chinese knotweed, Gracilaria tenuistipitata, lettuce, pea shoot, and gynura are favored by Taiwanese as functional vegetables. Some health effects of vegetables have been reported including antioxidation [15-17], cholesterol lowering [18], inhibition of NO formation [19], and blood pressure reduction [20,21]. Previously, we also demonstrated that purple-leaved sweet potato exhibits free radical scavenging and has high polyphenolic content [22]. However, these studies focused on vitamins and glutathione contents, preventing microsomal lipid peroxidation, lowing plasma and liver lipid peroxidation, and preventing DNA from oxidative damage. Limited information is available regarding composition and antioxidant activity, and its relation to efficacy or ability to prevent oxidative damage to DNA of these indigenous purple vegetables, except for the purple-leaved sweet potato [22]. The objective of this study was to evaluate the antioxidant components, antioxidant activity, and the extent to which acid hydrolysates of six Taiwan indigenous vegetables with purple leaves could protect DNA in human lymphocytes from oxidative damage induced by H2O2. Our study explores the relationship between the composition and content of flavonols and anthocyanidin with antioxidative efficiency, and prevention of DNA oxidative damage afforded by indigenous purple-leaved vegetables.
2. Materials and Methods
2.1. Chemicals
Methanol, ethanol, acetone, hydrochloric acid, di-sodium hydrogen phosphate, potassium dihydrogen phosphate, formic acid, NaCl, KCl, Tris-HCl, and dimethyl sulfoxide (DMSO) were purchased from Merck Co. (Darmstadt, Germany). Linoleic acid, MgCl, d-glucose, calcium choloride dihydrate, EDTA, Tris (hydroxymethyl) aminomethane (Tris base), sodium lauryl sarcosinate, and ethidium bromide were purchased from Sigma Chemical Co (St Louis, MO, USA). Myricetin, morin, quercetin, kaempferol, cynidin, and malvidin were purchased from ROTH (Rheinzabern, Denmark). Ficoll-Paque was purchased from Amersham Biosciences (Uppsala, Sweden). Low melting gel agrose and triton X-100 were purchased from BDH (Poole, England). Normal melting gel agarose was purchased from Pantech Instruments (Darmstadt, Germany).
2.2. Vegetables
The vegetables we tested were purple-leaved sweet potato (Ipomoea batatas L. Lamark), purple-leaved gynura line 1 (Gynura bicolor DC.), purple-leaved gynura line 2 (Gynura bicolor DC.), purple-leaved perilla line 1 (Perilla frutescents L. Britton), bicolored-leaved perilla line 2 (Perilla frutescens L. Britton), and heartleaf houttuynia (Houttuynia cordata Thumb.). Green-leaved sweet potato (Ipomoea batatas L. Lamarck) was used as an indigenous green-leaved vegetable control. These purple indigenous vegetables were generously provided by Dr. T. R. Chang, Taoyuan District Agricultural Research and Extension Station Council of Agriculture, Executive Yuan, Taiwan.
2.3. Preparation of Vegetables Extract
The edible portion of a vegetable was weighed, lyophilized, and ground to powder. Each lyophilized vegetable powder was prepared according to Justesen et al. [23] with modifications as follows: 10 ml of 62.5% aqueous methanol containing BHT (2 g/l) was added to 1.25 g lyophilized samples, followed by adding 5 ml of 6 M HCl to bring up total volume to 12.5 ml. The final mixture consisted of 1.2 M HCl in 50% aqueous methanol. The extraction mixture was thereafter heated to 90˚C in a steam bath and refluxed for 2 h, allowed to cool in the refrigerator, sonicated for 5 min, and diluted to 50 ml with methanol to form the final extract. The acid hydrolysates methanolic extract was ready for HPLC and inhibition of conjugated diene formation in the linoleic acid assay.
2.4. Anthocyanidin Assay
The acid hydrolysates methanolic extract was used for anthocyanidin assays. Anthocyanidin was quantified according to the method of Mancinelli [24], and absorbances at 657 nm and 530 nm were measured.
2.5. Flavonoids Assay
The acid hydrolysates methanolic extract was used for flavonoids assays. Flavonoids were determined according to the method of Geissman [25], and absorbance at 540 nm was measured in supernatants.
2.6. Flanonols and Anthocyanidins Analysis
One ml of acid hydrolysates methanolic extract was filtered through a 0.45 μm filter prior to 20 μl injection into the HPLC. Samples were analyzed with a Spectra SYSTEM UV6000LP Photodiode Array Detection System (Thermo Separation Products, San Jose, USA) and an ODS column (250 × 4.6 mm, 5 μm; YMC, ODS-A, YMC Co., Kyoto, Japan). The mobile phase consisted of methanol-water (30:70, v/v) with 1% formic acid and 100% methanol. The gradient was 25% - 74% methanol in 40 min at a flow rate of 0.75 ml/min. Spectra were recorded at 365 and 528 nm for flavonol and anthocyanidin determination, respectively [23].
2.7. Inhibition of Conjugated Diene Formation in Linoleic Acid Emulsion Autoxidation System
The inhibition of conjugated diene formation was determined according to Mitsuda et al. [26]. Briefly, an aliquot of 0.1 ml of each diluted vegetable methanolic extract or blank was added to 2 ml of 10 mM linoleic acid emulsion (pH 6.6), mixed well, and incubated at 37˚C for 15 h. A sample of 0.2 ml for a 0 and 15 h incubation period was mixed with 7 ml of 80% methanol, followed by measuring the absorbance at 234 nm.
2.8. Isolated Human Peripheral Blood Lymphocytes
Blood samples were obtained from six donors, including four male and two female healthy non-smokers, 24 to 48 years old. Fresh whole blood (20 - 30 ml) from volunteers was taken with informed consent, and lymphocytes were isolated using a separation solution kit supplemented with Ficoll-Paque Plus lymphocyte isolation sterile solution (Pharmacia Biotech, Sweden) [27]. Cells were harvested within 1 day of blood samples taken, and cultured with AIM V medium containing serum-free lymphocyte medium (Gibco Invitrogen, USA) in a humidified atmosphere of 5% CO2 in air at 37˚C for 24 h.
2.9. Cell-Viability Testing
After culturing, lymphocytes were exposed to each of seven different vegetable extracts, including six purple-leaved and one green-leaved. Each lymphocyte was used at three concentrations (25, 50, and 100 μg/ml) for 30 min at 37˚C. DNA damage was induced by exposing lymphocytes to H2O2 (10 μM) for 5 min on ice. Treatment on ice minimized the possibility of cellular DNA repair after H2O2 injury. Cells were centrifuged (100 × g for 10 min), washed, and resuspended in the same medium as the Comet assay. All experiments were carried out in triplicate. Cell viability was tested using the tetrazolium/formazan (MTT) assay [28] both prior to and after either vegetable extract or H2O2 treatment.
2.10. DNA Single-Strand-Break Damage Estimation Using the Comet Assay
The standard Comet assay was performed as described in Szeto et al. [10]. Acid hydrolysates from six indigenous purple-leaved vegetables were used for the Comet assay, plus green-leaved sweet potato as a control. Briefly, 0.1 ml diluted extract aliquots with 1 ml lymphocyte suspension containing 20,000/ml were incubated for 30 min at 37˚C in a dark incubator together with untreated samples. After pre-incubation, samples were incubated with 10
μM H2O2 for 5 min together with untreated samples. Samples were centrifuged at 80 g for 10 min at 18˚C, and lymphocyte samples were resuspended in 0.25 ml of PBS and mixed well with 0.5 ml of low melting point agarose (0.5%). A 0.1 ml sample of the mixture was embedded on the top layer of a frosted slide with the normal agarose (0.65%) on the bottom layer, and covered with a cover glass in duplicate. After solidifying in a refrigerator for 30 min, only the cover glass was removed. Slides were dipped in the final lysing solution, which was freshly prepared with 1% Triton X-100 and 10% DMSO in 0.01 M Tris buffer with 1% sodium lauroyl sarcosinate, 2.5 M NaCl, and 0.1 M EDTA at pH 10 and 4˚C for 1 h in the dark. Slides were removed from the lysing solution and their surfaces washed slightly with Milli-Q water. They were then put on the anode side of the electrophoresis tank.
Electrophoresis buffer (0.3 M NaOH and 1 mM Na2EDTA, pH 13) was loaded up to 0.25 cm beyond the surface of the slides and left for about 20 min in the dark to unwind the DNA. Electrophoresis was started at a constant current of 300 mA and 25 V for 15 min. The surfaces of the slides were washed slightly with Milli-Q water, and after blotting the slides dry, drip neutralization buffer (0.4 M Tris-HCl, pH 7.5) was applied to the whole surfaces of the slides and left for 15 min to dry the buffer. These neutralization steps were repeated 3 times. Buffers on the slides were removed by edge-blotting with absorbent paper. A 50 μl staining solution (20 μg/ml ethidium bromide) was dripped onto it and the slide was banked to spread it over the whole surface, and then covered with a cover glass. The cover glass was stable after 30 min. Fluorescence-stained slides were then examined under a Zeiss-Axiovert 100 fluorescence microscope (Zwiss, Germany) at 400x VisCOMET (version 1.6, Impuls GmbH, Germany) was used to analyze the DNA damage by tail DNA% ((total brightness of tail area/total brightness of total area) × 100%) and moment (tail length × tail DNA%). Twenty spots from each slide were read to get the value for each treatment. Inhibition percentage of tail DNA% and moment were calculated relative to the 10 μM H2O2 treated group.
2.11. Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA), and the significance between means by the least significant difference (LSD) test. Pearson’s linear correlation was carried for correlation analysis.
3. Results and Discussion
3.1. Antioxidant Composition and Antioxidant Activity
The results in Table 1 show that anthocyanidins were abundant in both Perilla and purple-leaved sweet potato. Flavonoids were abundant in purple-leaved sweet potato, purple-leaved perilla line 1, bicolored-leaved perilla line 2, and purple-leaved gynura lines 1 and 2. Myricetin was abundant in purple-leaved perilla line 1 and bicolored-
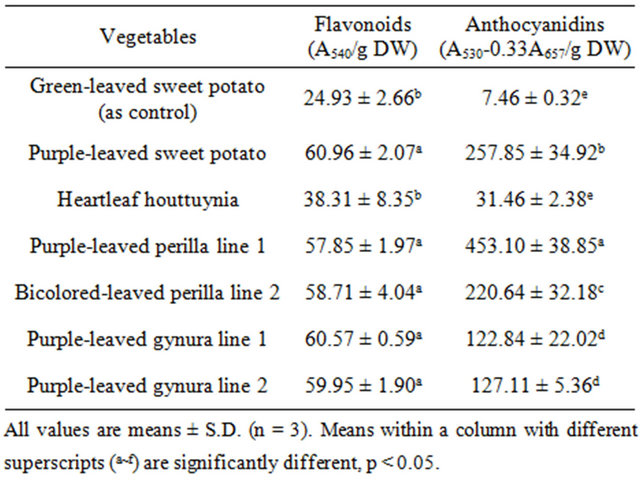
Table 1. The content of various antioxidative substances in acid hydrolysates of Taiwan-indigenous purple-leaved vegetables.
leaved perilla line 2 at levels of 1253.33 ± 83.27 and 1213.33 ± 61.10 μg/g DW, respectively (Table 2). In addition, quercetin was abundant in heartleaf houttuynia at a level of 2640.00 ± 105.83 μg/g DW, while purpleleaved sweet potato followed at a level of 2440.00 ± 320.00 μg/g DW. Morin was abundant in bicolored-leaved perilla line 2. Kaempferol was only present in heartleaf houttuynia and bicolored-leaved perilla line 2. Table 3 documents cynidins being abundant in purple-leaved perilla line 1 at a level of 893.33 ± 197.32 μg/g DW, and purple-leaved sweet potato followed at a level of 506.67 ± 100.66 μg/g DW. Malvidin was also abundant in purple-leaved perilla line 1 at a level of 813.33 ± 100.66 μg/g DW, while purple-leaved sweet potato and bicolored-leaved perilla line 2 followed behind that.
Flavonoid and anthocyanidin contents found in the extracted vegetables (Tables 1-3) were much lower than those in our previous study [29]. A possible reason is the use of a different extraction method. Likewise, inhibition of linoleic acid peroxidation activity was also lower compared to the previous study [29]. However, the relative order of antioxidant activity remained similar, where purple-leaved sweet potato appeared to have the highest activity level. The tested vegetables showed >73% inhibition of linoleic acid peroxidation at 625 μg/ml of extracts (Table 4). Therefore, purple-leaved sweet potato, heartleaf houttuynia, and purple-leaved gynura line 1 were particularly effective inhibitors. Purple-leaved sweet potato, heartleaf houttuynia, purple-leaved perilla line 1, bicolored-leaved perilla line 2, and green-leaved sweet potato exhibited better inhibition efficacy at lower concentrations. In general, purple-leaved sweet potato exhibited the highest inhibition of linoleic acid peroxidation, up to 91% (Table 4). The IC50 of inhibition of linoleic acid peroxidation in purple-leaved sweet potato, purple-leaved gynura line 1, purple-leaved gynura line 2, purpleleaved perilla line 1, bicolored-leaved perilla line 2, and heartleaf houttuynia were 120.15 ± 49.86, 322.51 ± 14.43, 239.81 ± 23.80, 135.74 ± 51.58, 277.98 ± 6.68, and 178.75 ± 21.12 μg/ml, respectively [30]. Compared to the IC50 of purple-leaved sweet potato, purple-leaved perilla line 1, and heartleaf houttuynia exhibited significantly higher effectiveness than the green-leaved sweet potato control (303.91 ± 49.86 μg/ml). The overall antioxidant activity in these purple-leaved vegetables was relatively high. Furuta et al. [31] indicated that, at 10 mg/ml of extracts, red sweet pepper, red cabbage, and red onion decreased lipid peroxidation by 65.8%, 41.5%, and 30.7%, respectively. However, the purple-leaved vegetables in our study showed greater than 73% inhibition of linoleic acid peroxidation at the much lower concentration of 625 μg/ml. Our previous study [29] also demonstrated that these purple-leaved vegetable extracts had much higher activity against LDL oxidation, while purple-leaved sweet potato exhibited stronger antioxidant activity compared to other vegetables.
The antioxidant activity of the tested vegetables was highly correlated with the content of cyanidin, malvidin (anthocyanidins), and quercetin (flavonol) [30]. Lee et al. [32] indicated that red peppers have high antioxidant activity due to high quercetin content. In the present study, purple-leaved sweet potato and heartleaf houttuynia contained abundant quercetin, while purple-leaved sweet potato and bicolored-leaved perilla line 2 were rich in malvidin and cynidin. Purple-leaved perilla line 1 and bicolored-leaved perilla line 2 had significant levels of the flavenoid myricetin (Tables 1-4). These different pigments may exhibit effective antioxidant activity alone or synergistically, and are a likely cause of the cultivar differences.
3.2. DNA Single-Strand-Break Damage Estimation
The effects of purple-leaved vegetable extracts on cell cytotoxicity were determined by an MTT assay. Lymphocytes were exposed to each of seven different vegetable extracts at three concentrations (25, 50, and 100 μg/ml) for 30 min at 37˚C. DNA damage was induced by exposing lymphocytes to H2O2 (10 μM) for 5 min on ice. No vegetable extracts were cytotoxic at the concentrations used, with >98% of cells remaining viable [30]. Therefore, concentrations of 25, 50, and 100 μg/ml were chosen to study the Comet assay.
The effects of pretreatment with purple-leaved vegetable extracts on 10 μM H2O2-induced DNA damage in human lymphocytes is presented in Figures 1 and 2. For each concentration of purple-leaved vegetable extractpretreated cells, a significantly reduced level of DNA sin-
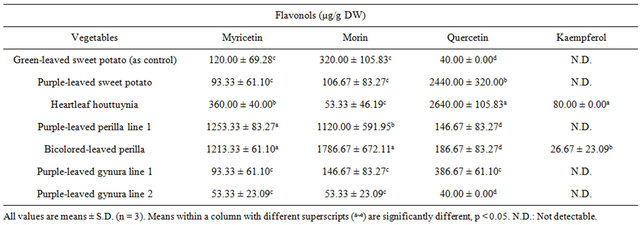
Table 2. The content of flavonols in acid hydrolysates of Taiwan-indigenous purple-leaved vegetables.
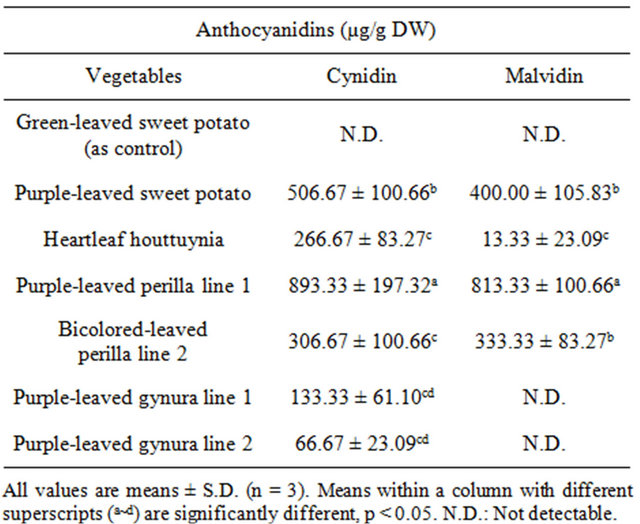
Table 3. The content of anthocyanidins in acid hydrolysates of Taiwan-indigenous purple-leaved vegetables.
gle-strand breaks following H2O2 exposure was observed (p < 0.05). These results indicate a protective effect of pretreatment on lymphocytes with each of the purpleleaved vegetable extracts at the lower dose (25 μg/ml). Those lymphocytes pretreated with 25 - 100 μg/ml extracts of purple-leaved sweet potato, purple-leaved perilla line 1, and bicolored-leaved perilla line 2 experienced a greater level of protection against H2O2 exposure than did lymphocytes exposed to other tested compounds, and in a dose-dependent manner per se (Figure 1). The maximum protective effect appeared in the pretreatment with 100 μg/ml purple-leaved sweet potato, purple-leaved perilla Line 1, and bicolored-leaved perilla line 2 at 83.35%, 89.14%, and 88.88% inhibition, respectively, compared to the H2O2 treated only (Table 5). The results of tail DNA% showed slight differences in heartleaf houttuynia, and had a greater level of protection against H2O2 exposure than did lymphocytes that were exposed to other
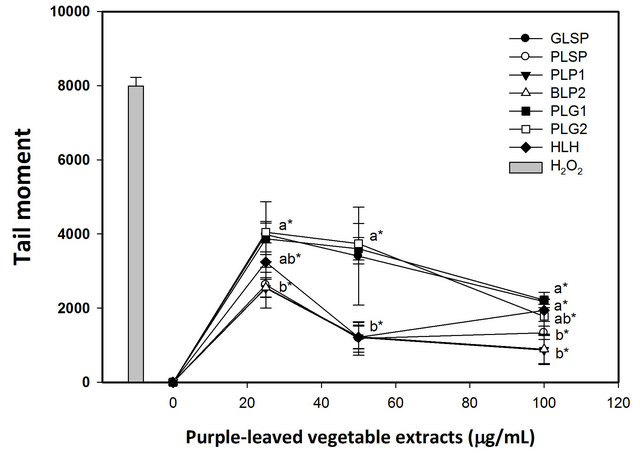
Figure 1. Effects of pretreatment with various acid hydrolysates from indigenous purple-leaved vegetables on 10 μM hydrogen peroxide-induced DNA damage to isolated human lymphocytes. DNA oxidative damage was measured using the Comet assay. Results are the mean ± SD. [●, Greenleaved sweet potato (GLSP); ○, Purple-leaved sweet potato (PLSP); ▼, Purple-leaved perilla line 1 (PLP 1); △, Bicolored-leaved perilla line 2 (BLP 2); ■, Purple-leaved gynura line 1 (PLG 1); □, Purple-leaved gynura line 1 (PLG 2); ◆, Heartleaf houttuynia (HLH)]. Values with different letters differ significantly with regard to oxidative damage when comparing different purple-leaved vegetable extracts (ANOVA, p < 0.05); *p < 0.05 refers to differences in oxidative damage as compared with the H2O2-alone treatment.
tested compounds, and in a dose (25 - 50 μg/ml)-dependent manner (Figure 2). The maximum protective effect of lymphocyte pretreatment was observed for pretreatment with 100 μg/ml purple-leaved perilla line 1 at 85.37% inhibition, compared to the H2O2 treated only (Table 6).
The tested vegetables showed at least a 73% inhibition of tail moment at the 100 μg/ml level, while purple-leaved perilla line 1, bicolored-leaved perilla line 2, and purpleleaved sweet potato had better inhibition efficacy compared to the rest of the tested sample extracts (Table 6).
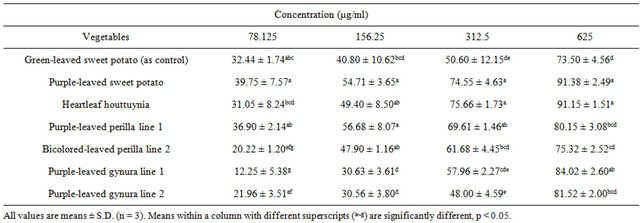
Table 4. Inhibition percentages of conjugated diene formation in the linoleic acid emulsion autoxidation system treated with various concentrations of acid hydrolysates of Taiwan-indigenous purple-leaved vegetables.
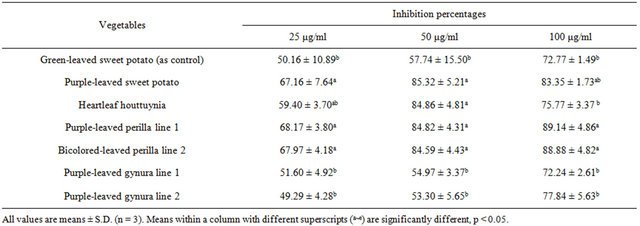
Table 5. Inhibition percentages of tail moment by treatment with various acid hydrolysates of Taiwan-indigenous purpleleaved vegetables in the H2O2-induced lymphocyte system.
Furthermore, at lower concentrations, purple-leaved perilla line 1, bicolored-leaved perilla line 2, heartleaf houttuynia, and purple-leaved sweet potato had better inhibition efficacy. The tested vegetables showed at least a 57% inhibition of tail DNA% at 100 μg/ml, with purpleleaved perilla line 1 having the best inhibition efficacy. At lower concentrations, purple-leaved perilla line 1, heartleaf houttuynia, and purple-leaved sweet potato had better inhibition efficacies compared to the rest of the tested extracts. In general, purple-leaved perilla line 1 had the highest inhibition percentage of both tail DNA% and tail moment up to 85% and 89%, respectively. Both 7990.55 ± 236.36 tail moment and 87.52 ± 3.43 tail DNA% were exhibited when the percentage of inhibition was compared to the DNA damage caused by treatment with 10 μM of H2O2 (Figures 1 and 2). The inhibition percentage of tail DNA% and tail moment were positively correlated with the content of cynidin (r = 0.59 ~ 0.83) and malvidin (r = 0.53 ~ 0.82) among the tested vegetables [30].
The inhibition percentage of tail DNA% and tail moment were also positively correlated with the content of myricetin, with the exception of the inhibition percentage of tail DNA% at 50 μg/ml of extract vegetables (r = 0.47 ~ 0.74) [30]. The inhibition percentage of tail DNA% (r = 0.66, p = 0.0010) and tail moment (r = 0.51, p = 0.0182) were positively correlated with the content of quercetin at 50 μg/ml of extract vegetables [30]. There was no correlation between kaempferol and the inhibition percentage of tail DNA% or tail moment, while morin showed only a partial positive correlation with inhibition efficacy at 25 and 100 μg/ml of vegetable extracts [30].
Quercetin was found to protect against H2O2-induced DNA damage in human lymphocytes at 10 μΜ [7] and at 3.1 to 25 μΜ [9]. Quercetin was also reported to induce DNA strand breaks in various cell types, but only at higher doses at 100 μM or above [7]. We noted there was less than 5% damage from quercetin when up to 50 μM was incubated with normal lymphocytes [30]. Similarly,
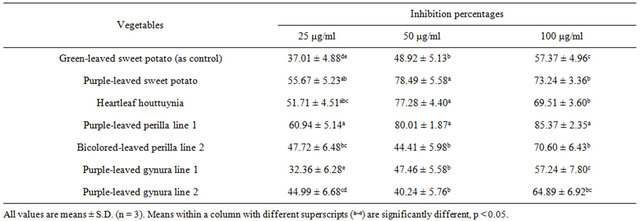
Table 6. Inhibition percentages of tail DNA% treatment with various acid hydrolysates of Taiwan-indigenous purple-leaved vegetables in the H2O2-induced lymphocyte system.
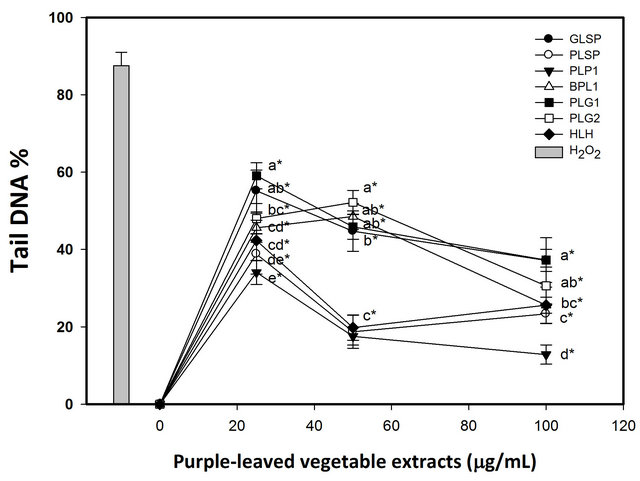
Figure 2. Effects of pretreatment with various acid hydrolysates of indigenous purple-leaved vegetables on 10 μM hydrogen peroxide-induced DNA damage on isolated human lymphocytes. DNA oxidative damage was measured using the Comet assay. Results are the mean ± SD. [●, Greenleaved sweet potato (GLSP); ○, Purple-leaved sweet potato (PLSP); ▼, Purple-leaved perilla line 1 (PLP 1); △, Bicolored-leaved perilla line 2 (BLP 2); ■, Purple-leaved gynura line 1 (PLG 1); □, Purple-leaved gynura line 1 (PLG 2); ◆, Heartleaf houttuynia (HLH)]. Values with different letters differ significantly with regard to oxidative damage when comparing different purple-leaved vegetable extracts (ANOVA, p < 0.05); *p < 0.05 refers to differences in oxidative damage as compared with the H2O2-alone treatment.
myricetin was also found to decrease oxidant-induced DNA damage at 100 μΜ, although α-tocopherol and β- carotene did not behave similarly [7]. Duthie et al. [7] proposed that the dihyroxy structure of quercetin and myricetin might be essential in protecting DNA against hydrogen peroxide. No such hydroxyl groups are present in the tocopherol molecule. This may reflect structure /activity relationships or the localization of the antioxidant relative to free radical generation within the cells. Noroozi et al. [33] demonstrated that in addition to quercetin, kaempferol could also inhibit H2O2-induced DNA strand breaks in human lymphocytes. However, in our study, no correlation was found between kaempferol and inhibition percentage of tail DNA% or tail moment in Comet assays, perhaps due to kaempferol variations in the tested genotypes. Zhu and Loft [34] reported that aqueous extracts of cooked and autolysed Brussels sprouts decreased DNA strand breaks in human lymphocytes, with the maximum inhibition being 38% and 39% at cooked and autolysed extract levels of 10 μg/ml and 5 μg/ml, respectively, and the inhibition effect decreasing at increasing concentrations up to 100 μg/ml. In our study, the inhibition percentages of tested purple vegetables ranged from 32% to 61% with acidic hydrolysate extract concentrations at 25 μg/ml, and the inhibitory effect increasing at increasing concentrations up to 50 μg/ml (Tables 1-4). The inhibition percentage of tail moment continued climbing up to 100 μg/ml, except for purple-leaved sweet potato and heartleaf houttuynia. The maximum inhibition percentage was 89% under 100 μg/ml extracts. Both purple-leaved perilla line 1 and bicolored-leaved perilla line 2 are rich in myricetin, cynidin, and malvidin. The inhibition percentage of tail DNA% and tail moment were positively correlated with the content of cynidin and malvidin among the tested vegetables [30]. Cynidin is effective against cytotoxicity, DNA single strand breaks, and lipid peroxidation induced by tert-butyl-hydroperoxide in rat smooth and hepatoma cell lines [11]. In the study, purple-leaved sweet potato, heartleaf houttuynia, purple-leaved perilla line 1, and bicolored-leaved perilla line 2 showed 85% inhibition percentage of tail moment at 50 μg/ml extracts. The results in inhibition percentage of tail DNA% were similar to the results in inhibition percentage of tail moment, except that bicolored-leaved perilla line 2 extracts had less inhibitory effect. Purple-leaved perilla line 1 extract was most effective against DNA single strand breaks and lipid peroxidation induced by hydroperoxide and air in human lyphocytes and the linoleic acid emulsion autoxidation system, indicating that it contains the hightest content of cynidin, malvidin, and myricetin. Purple-leaved sweet potato has more quercetin, cynidin, and malvidin, and inhibits lipid peroxidation and DNA damage better than green-leaved sweet potato. These results demonstrate that quercetin and anthocyanidin are important for preventing peroxdatition and oxidative damage to DNA.
4. Conclusion
In conclusion, purple-leaved perilla line 1 and bicoloredleaved perilla line 2 were particularly rich in cynidin, malvidin and myricetin, and purple-leaved sweet potato and heartleaf houttuynia were rich in quercetin. Purpleleaved sweet potato, heartleaf houttuynia, purple-leaved perilla line 1 and bicolored-leaved perilla line 2 had better inhibition percentage of tail DNA% and tail moment. The purple indigenous vegetables in the study have abundant antioxidants with strong antioxidant activity, and consequently can protect DNA in lymphocytes from oxidative damage.
5. Acknowledgements
This research was supported in part by grant NSC89- 2312-B-034-001 from the National Science Council, Taiwan.
REFERENCES
- W. Ren, Z. Qiao, H. Wang, L. Zhu and L. Zhang, “Flavonoids: Promising Anticancer Agents,” Medicinal Research Reviews, Vol. 23, No. 4, 2003, pp. 519-534. doi:10.1002/med.10033
- A. King and G. Young, “Characteristics and Occurrence of Phenolic Phytochemicals,” Journal of the American Dietetic Association, Vol. 99, No. 2, 1999, pp. 213-218. doi:10.1016/S0002-8223(99)00051-6
- M. P. Kähkönen and M. Heinonen, “Antioxidant Activity of Anthoxyanins and Their Aglycons,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 3, 2003, pp. 628-633. doi:10.1021/jf025551i
- G. Shklar, “Oral Mucosal Carcinogenesis in Hamsters: Inhibition by Vitamin E,” Journal of the National Cancer Institute, Vol. 68, No. 5, 1982, pp. 791-797.
- N. Ito and M. Hirose, “The Role of Antioxidants in Chemical Carcinogenesis,” Japanese Journal of Cancer Research, Vol. 78, No. 10, 1987, pp. 1011-1026.
- D. E. McClain, J. F. Kalinich and N. Ramakrishnan, “Trolox Inhibits Apoptosis in Irradiated MOLT-4 Lymphocytes,” FASEB Journal, Vol. 9, No. 13, 1995, pp. 1345- 1354.
- S. J. Duthie, A. R. Collins, G. G. Duthie and V. L. Dobson, “Quercetin and Myricetin Protect against Hydrogen Peroxide-Induced DNA Damage (Strand Breaks and Oxidised Pyrimidines) in Human Lymphocytes,” Mutation Research, Vol. 393, No. 3, 1997, pp. 223-231. doi:10.1016/S1383-5718(97)00107-1
- W. Lopaczynski and S. H. Zeisel, “Antioxidants, Programmed Cell Death, and Cancer,” Nutrition Research, Vol. 21, No. 1, 2001, pp. 295-307. doi:10.1016/S0271-5317(00)00288-8
- G. A. Liu and R. L. Zheng, “Protection against Damaged DNA in the Single Cell by Polyphenols,” Die Pharmazie, Vol. 57, No. 12, 2002, pp. 852-854.
- Y. T. Szeto, A. R. Collins and I. F. F. Benzie, “Effects of Dietary Antioxidants on DNA Damage in Lysed Cells Using a Modified Comet Assay Procedure,” Mutation Research, Vol. 500, No. 1-2, 2002, pp. 31-38. doi:10.1016/S0027-5107(01)00298-6
- M. C. Lazzé, R. Pizzala, M. Savio, L. A. Stivala, E. Prosperi and L. Bianchi, “Anthocyanins Protect against DNA Damage Induced by Tert-Butylhydroperoxide in Rat Smooth Muscle and Hepatoma Cells,” Mutation Research, Vol. 535, No. 1, 2003, pp. 103-115. doi:10.1016/S1383-5718(02)00285-1
- G. Cao, S. L. Brooth, J. A. Sadowski, R. L. Peior and A. J. Clin, “Increases in Human Plasma Antioxidant Capacity after Consumption of Controlled Diets High in Fruit and Vegetables,” Vol. 68, No. 5, 1998, pp. 1081-1087.
- G. Cao, E. Sofic and R. L. Prior, “Antioxidant Capacity of Tea and Common Vegetables,” Journal of Agricultural and Food Chemistry, Vol. 44, No. 11, 1996, pp. 3426- 3431. doi:10.1021/jf9602535
- Y. H. Chu, C. L. Chang and H. F. Hsu, “Flavonoid Content of Several Vegetables and Their Antioxidant Activity,” Journal of the Science of Food and Agriculture, Vol. 80, No. 5, 2000, pp. 561-566. doi:10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#
- F. Hayase and H. Kato, “Antioxidative Components of Sweet Potatoes,” Journal of Nutritional Science and Vitaminology, Vol. 30, No. 1, 1984, pp. 37-46. doi:10.3177/jnsv.30.37
- A. I. Mohamed and A. S. Hussein, “Chemical Composition of Purslane (Portulaca oleracea),” Plant Foods for Human Nutrition, Vol. 45, No. 1, 1994, pp. 1-9. doi:10.1007/BF01091224
- T. T. Jong and S. W. Chau, “Antioxidative Activities of Constituents Isolated from Pandanus Odoratissimus,” Phytochemistry, Vol. 49, No. 7, 1998, pp. 2145-2148. doi:10.1016/S0031-9422(98)00390-2
- E. D. Lund, “Cholesterol Binding Ccapacity of Fiber from Tropical Fruits and Vegetables,” Lipids, Vol. 19, No. 2, 1984, pp. 85-90. doi:10.1007/BF02534496
- M. Yoshikawa, T. Murakami, H. Shimada, S. Yoshizumi, M. Saka, J. Yamahara and H. Matsuda, “Medicinal FoodStuffs. XIV. On the Bioactive Constituents of Moroheiya (2): New Fatty Acids, Corchorifatty Acids A, B, C, D, E, and F, from the Leaves of Corchorus olitorius L. (Tiliaceae): Structures and Inhibitory Effect on NO Production in Mouse Peritoneal Macrophages,” Chemical & Pharmaceutical Bulletin, Vol. 46, No. 6, 1998, pp. 1008- 1014. doi:10.1248/cpb.46.1008
- K. Kar, V. N. Puri, G. K. Patnaik, R. N. Sur, B. N. Dhawan, D. K. Kulshrestea and R. P. Rastogi, “Pasmolytic Constituents of Cedrus deodara (Roxb.) Loud: Pharmacological Evaluation of Himachalol,” Journal of Pharmaceutical Sciences, Vol. 64, No. 2, 1975, pp. 258-266. doi:10.1002/jps.2600640213
- V. Molina Cuevas, M. L. Arruzazabala, D. Carbajal Quintana, R. Mas Ferreirom and S. Valdes Garcia, “Effect of Policosanol on Arterial Blood Pressure in Rats. Study of the Pharmacological Interaction with Nifedipine and Propranolol,” Archives of Medical Research, Vol. 29, No. 1, 1998, pp. 21-24.
- K. H. Lin, P. Y. Chao, C. M. Yang, W. C. Cheng, H. F. Lo and T. R. Chang, “The Effects of Flooding and Drought Stresses on the Antioxidant Constituents in Sweet Potato Leaves,” Botanical Studies, Vol. 47, No. 4, 2006, pp. 417-426.
- U. Justesen, P. Knuthsen and T. Leth, “Quantitative Analysis of Flavonols, Flavones, and Flavanones in Fruits, Vegetables and Beverages by High-performance Liquid Chromatography with Photo-diode Array and Mass Spectrometric,” Journal of Chromatography A, Vol. 799, No. 1-2, 1998, pp. 101-110. doi:10.1016/S0021-9673(97)01061-3
- A. L. Mancinelli, C. P. Huang-Yang, P. Lindquist, O. R. Anderson and I. Rabino, “Photocontrol of Anthocyanin Synthesis III the Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin,” Plant Physiology, Vol. 55, No. 2, 1975, pp. 251-257. doi:10.1104/pp.55.2.251
- T. A. Geissman, “Anthocyanins, Chalcones, Aurones, Flavones and Related Water-Soluble Plant Pigments,” In: K. Peach and M. V. Tracey, Eds., Modern Methods of Plant Analysis, Springer-Verlag, Berlin, 1955, pp. 420-433.
- H. Mitsuda, K. Yasumodo and K. Iwami, “Antioxidative Action of Indole Compounds during the Autoxidation of Linoleic Acid,” Eiyo to Shokuryo, Vol. 19, 1966, pp. 210- 214. doi:10.4327/jsnfs1949.19.210
- J. Cole, M. H. L. Green, S. E. James, L. Henderson and H. Cole, “A Further Assessment of Factors Influencing Measurements of Thioguanine-Resistant Mutant Frequency in Circulating T-Lymphocytes,” Vol. 204, No. 3, 1988, pp. 493-507.
- A. H. Cory, T. C. Owen, J. A. Barltrop and J. G. Cory, “Use of an Aqueous Soluble Tetrazolium/Formazan Assay for Cell Growth Assays in Culture,” Cancer Communications, Vol. 3, No. 7, 1991, pp. 207-212.
- S. C. Tang, H. F. Lo, K. H. Lin, T. J. Cheng, C. M. Yang and P. Y. Chao, “The Antioxidant Capacity of Extracts from Taiwan Indigenous Purple-leaved Vegetables,” Journal Taiwan Society for Horticultural Science, Vol. 59, No. 1, 2013, pp. 43-57.
- W. Y. Huang, “The Antioxidant Capacity of Flavonoids in Red Indigenous Vegetables and Their Protection Role in DNA Oxidative Damage of Lymphocytes,” M.S. Thesis, Chinese Culture University, Taipei, 2003.
- S. Furuta, Y. Nishiba and I. Suda, “Fluorometric Assey for Screening Antioxidative Activity of Vegetables,” Journal of Food Science, Vol. 62, No. 3, 1997, pp. 526-528. doi:10.1111/j.1365-2621.1997.tb04422.x
- Y. Lee, L. R. Howard and B. Villalόn, “Flavonoids and Antioxidant Activity of Fresh Pepper (Capsicum annuum) Cultivars,” Journal of Food Science, Vol. 60, No. 3, 1995, pp. 473-476. doi:10.1111/j.1365-2621.1995.tb09806.x
- M. Noroozi, W. J. Angerson and M. E. Lean, “Effects of Flavonoids and Vitamin C on Oxidative DNA Damage to Human Lymphocytes,” The American Society for Clinical Nutrition, Vol. 67, No. 6, 1998, pp. 1210-1218.
- C. Y. Zhu and S. Loft, “Effects of Brussels Sprouts Extracts on Hydrogen Peroxide-Induced DNA Strand Breaks in Human Lymphocytes,” Food and Chemical Toxicology, Vol. 39, No. 12, 2001, pp. 1191-1197. doi:10.1016/S0278-6915(01)00061-8
NOTES
*Corresponding author.

