Food and Nutrition Sciences
Vol. 3 No. 4 (2012) , Article ID: 18492 , 13 pages DOI:10.4236/fns.2012.34068
Applicability of in Vitro Models in Predicting the in Vivo Bioavailability of Lycopene and β-Carotene from Differently Processed Soups
![]()
1Department of Chemical and Biological Engineering, Food Science, Chalmers University of Technology, Göteborg, Sweden; 2Physiology Department, Faculty of Biology, University of Murcia, Murcia, Spain; 3Unilever Discover R&D, Unilever Food and Health Research Institute, Vlaardingen, The Netherlands.
Email: *marie.alminger@chalmers.se
Received December 22nd, 2011; revised February 22nd, 2012; accepted March 1st, 2012
Keywords: In vitro digestion; human; uptake; Caco-2 cells; β-carotene; lycopene; carotene bioavailability
ABSTRACT
Presently, there is no clear consensus on the best approach to estimate carotenoid bioavailability. The best alternative would be to use human studies, but they are labour-intensive and expensive and can only be used to investigate a limited number of samples. Hence, a number of in vitro models have been developed to study pre-absorptive processes and factors affecting bioavailability. The question is, however, how well the results obtained by the various methods correlate to each other and to the in vivo situation. In the present paper, we have compared in vivo data from two human studies on differently processed soups containing carrots, tomato and broccoli, with results obtained by in vitro characterisation of the same soups. In vitro bioaccessibility was estimated by a static in vitro digestion investigating matrix release and micellarization of carotenoids and by uptake studies in a human intestinal cell line (Caco-2). In vivo data was obtained from clinical studies measuring total plasma carotenoid concentrations in human subjects after 4 weeks daily consumption of the soups. Comparison of the in vitro and in vivo results indicate that the combination of a two-step in vitro digestion and Caco-2 cells seems to be a useful tool for estimation of β-carotene bioaccessibility and screening of factors governing the release of β-carotene from this type of food. For lycopene the in vitro and in vivo results were less consistent, suggesting that reliable prediction of lycopene bioavailability might be more problematic.
1. Introduction
Carotenoids are a group of naturally occurring fat-soluble pigments that appear to have several important biological activities, like protection against the development of some forms of cancer and cardiovascular disease [1]. However, in order to be biologically active, the carotenoids much reach their site of action, i.e. they have to be bioavailable. The bioavailability of carotenoids is extremely variable, being influenced by many dietary and physiological factors such as the food matrix, carotenoid type, nutritional status and age [2,3]. The first steps in uptake of dietary carotenoids are release from the food matrix, which is improved by mechanical and enzymatic disruption. This is followed by incorporation into lipid droplets and the carotenoids then have to be solubilised in mixed micelles before intestinal uptake. Absorbed carotenoids are incorporated into chylomicrons assembled by the mucosal cells and can finally reach the blood via the lymphatic system. The carotenoids may also undergo different conversions during their passage through the gastrointestinal tract; β-carotene may for example be cleaved to form vitamin A in the intestinal mucosa [4]. Although the major form of lycopene in the diet is all-trans, representing about 80% - 97% of total lycopene in tomatoes and related products [5,6], human blood and tissues contain mainly cis-isomers [7,8]. Several studies have addressed the hypothesis that the cisisomers are more bioavailable [9-11], and although little is still known about the sites and mechanisms involved in the formation of cis-isomers of lycopene, recent studies suggest that lycopene isomerisation takes place within enterocytes during absorption in human subjects [12]. All these different steps and the multiple factors that may affect the bioavailability make reliable prediction of carotenoid bioaccessibility/availability difficult.
To enable the investigation of factors involved in the different steps of carotenoid absorption and metabolism, detailed in vitro approaches can be used to characterize the main determinants of carotenoid bioavailability from different food products. In vitro carotenoid bioaccessibility can be estimated by measuring the fraction of carotenoid transferred from the food matrix after simulated gastrointestinal digestion, either to a supernatant obtained after centrifugation/decantation [13-15] or to a micellar phase obtained after centrifugation/microfiltration [16-18]. Both methods have been considered to be appropriate tools for estimation of bioavailability of carotenoids in different plant matrices [19,20], but recent studies suggest that the bioaccessibility values obtained from the supernatant and micellar phase can be significantly different, even when samples from the same plant food have been investigated [21,22]. Cell culture models have also been utilised as part of in vitro digestion models, in particular the Caco-2 cell culture model has been used to estimate the absorption of bioactive components [23,24]. Even if not all aspects of bioavailability can be simulated, in vitro models can be used as simple, inexpensive and reproducible methods to study digestive stability, micellarization, intestinal transport and metabolism of carotenoids, and may allow prediction of bioavailability of different food components. During the past 10 years, a number of methods have been proposed to determine aspects of carotenoid bioavailability. However, due to the lack of consensus concerning the in vitro methodology, there is often limited comparability between the different studies. The aim of the present study was to assess the predictive value of some specific in vitro models often used for studies of carotenoid bioaccessibility by comparison of in vitro and in vivo data for the same fruit and vegetables soups. Results from two human studies evaluating the serum responses of long term consumption of the soups were compared with results obtained using different in vitro approaches.
2. Materials and Methods
2.1. Materials
The soups, used for the in vitro studies and two separate human studies [25,26] were produced by Unilever, the Netherlands within the “Healthy Structuring” project, supported by the European Commission. The project aimed to formulate validated processing solutions to improve the nutritional, structural and sensorial quality of readyto-eat fruit and vegetable products.
2.1.1. Test Products for Study 1
Several different soup prototypes were evaluated and a soup with a high content and in vitro bioaccessibility of carotenoids was selected for an initial evaluation in a human pilot study (n = 14). The optimised soup for Study 1 was produced in eight batches of 25 - 80 kg. Fresh broccoli and carrots were blanched (>85˚C). The soups were produced in the spring (March/April) and processing tomatoes were not available, tomato paste (tomato concentration 28% - 30%) was therefore used instead of fresh tomatoes. To create a purée with a similar Brix (7˚Brix) as freshly made tomato purée, 1 part tomato paste was diluted with 3 parts of water. All ingredients were blended to a homogenous mixture and heated to 80˚C, followed by high pressure homogenization (HPH) at 100 bar. The soup contained 5% olive oil. This soup will be referred to as the pilot soup.
2.1.2. Test Products for Study 2
For the larger human study (n = 72), one reference and one optimised soup was produced on a pilot plant scale. The reference soup was made using traditional processing conditions; fresh broccoli, carrots and tomato (ratio 1:1:1) were heated together in water, cooked until al dente and blended with 2.5% olive oil. For the optimised soup, processing was designed to improve the retention and bioavailability of nutrients. The broccoli, tomato and carrot (ratio 1:1:1) were each pre-processed separately, using different heating and blending conditions for each ingredient. They were then mixed together with 5% olive oil and passed through a high pressure homogenizer (HPH) at 100 bar. Three different batches of 250 - 300 kg were produced for each soup. [Further details on processing are subject to patent application and thus, cannot be disclosed at this time].
2.2. Methods
2.2.1. Extraction of Carotenoids
Lycopene and β-carotene in test products were extracted according to the method by Sadler et al. [27], with some modifications. The extractions were performed under subdued light in amber E-flasks. Duplicate samples of 5.0 g (±0.5) fresh product were dissolved in 50 ml (±0.1) iso-hexane: acetone: ethanol (50:25:25, v/v) containing 0.1% (w/v) butylated hydroxytoluene (BHT) and placed on a magnetic stirrer (500 ± 10 turns/min) for 20 min. Thereafter, 15 ml NaCl solution (25 g NaCl·L−1) was added and the extraction was continued for an additional 10 min. To allow phase separation the samples were left standing for 3 min before an aliquot of the upper layer was analysed by HPLC according to the method described below. For the extraction of soups used in the pilot human study (Study 1), duplicate samples from 6 soups from different batches were extracted. The extraction of soups from Study 2 was made on duplicate samples from three different batches of the optimised and reference soups.
2.2.2. HPLC Analysis
A reverse phase HPLC system was used, consisting of an auto sampler injector (Waters 717plus), pump (Waters 600), controller (Waters 600) and an UV-visible photodiode array detector (Waters 996). Data processing was made using Empower 2 Software. Separations were performed on a C30 column (4.6 mm × 25 cm, particle size 5 µm, YMC, Japan). The mobile phase consisted of methanol and methyl tert-butyl ether (MTBE). To obtain suitable separation of lycopene and β-carotene, a gradient from 70% methanol and 30% MTBE to 30% methanol and 70% MTBE over 17 min was used, followed by isocratic elution at 30% methanol (18 min). The methanol concentration was then changed back over 1 min and kept at 70% for 5 min. Flow rate was 1 ml/min and the injection volume 20 µl.
Carotenoid identification and quantification Carotenoids were identified using retention times and UV/vis absorption spectra. Absorption spectra were recorded between 250 and 500 nm, quantification was made at 452 nm based on linear calibration curves with eight concentrations. The lycopene standard curve was prepared from a stock solution of all-trans-lycopene in chloroform (range of concentrations: 0.18 - 45.12 g/ml) with the correlation coefficient of 0.995. The β-carotene standard curve was prepared from a stock solution of all-trans-β-carotene in iso-hexane (range of concentrations: 0.12 - 30.18 µg/ml) with the correlation coefficient 0.999). The purity of the standards was verified by HPLC and concentrations verified spectrophotometrically.
2.2.3. In Vitro Digestion Procedure
To estimate the bioaccessibility of carotenoids from the soups a previously described [28] static in vitro model was used with some modifications. Analyses were performed in duplicate on fresh material in subdued light. Samples were blanketed with nitrogen prior to incubations to minimize oxygen exposure. To simulate the initial part of the gastric phase, 5.0 (±0.5) g soup was mixed with 5 ml salt solution (0.9% (w/v) NaCl, 1% (w/v) ascorbic acid) and 5 ml electrolyte (51 mM NaCl, 14 mM KCl, 10 mM CaCl2·2H2O, 3.5 mM KH2PO4, 3.6 mM MgCl2·6H2O). The pH was reduced to 4 by addition of 1 M HCl, 5 ml pepsin solution (1 g pepsin, 2190 U/mg solid, dissolved in 50 ml electrolyte) was added and the samples incubated 30 min at 37˚C on a shaking plate. The second part of the gastric phase was initiated by lowering the pH to 2 with 1 M HCl followed by further incubation for 30 min. To mimic the intestinal digestion, pH was raised to 6.9 by addition of 1 M NaHCO3 and 3 ml intestinal solution (0.4%) (w/v) pancreatin, (2.5%) (w/v), bile extract containing 0.5% (w/v) pyrogallol and 1% (w/v) DL-α-tocopherol. After incubation for 120 min at 37˚C, samples were centrifuged (15 min, 4˚C, 5000 × g) and the supernatants vacuum filtered into amber E-flasks. The isolated supernatant was used to measure the amount of nutrients released from the food matrix. To estimate the efficiency of micellarization/ isolate the micellar fractions, aliquots of the supernatants were microfiltered (0.22 µm). Both fractions were collected and subsequently analyzed for their content of carotenoids. The relative in vitro bioaccessibility was calculated as the carotene content of the supernatant and micelles obtained from digested samples divided by the average carotene content of the soups.
To extract the carotenoids 2 ml digesta was added to a test tube followed by addition of 5 mL hexane: acetone: ethanol (50:25:25) solution containing 1 g·L−1 BHT. The test tubes were vortexed for 15 s, 1 mL NaCl solution (25 g NaCl·L−1) added; the tubes vortexed for another 15 s and centrifuged (5 min, 4000 × g). An aliquot of the upper organic phase was analysed by HPLC according to the method described above without any further treatment.
2.2.4. Caco-2 Cell Experiments
Caco-2 cells (HTB 37) were obtained from American Type Culture Collection (Rockville, MD, USA) at passage 20. The cells were maintained at 37˚C in a humidified atmosphere of 95% air and 5% CO2 and grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 17% (v/v) fetal bovine serum (FBS), nonessential amino acids and antibiotics. Cultures were split at 70% - 80% confluence to create a new passage. For the cellular uptake experiments, cells were seeded at a density of 65,000 cells·cm−2 in 6-well plates at passage 25 - 35 and the uptake experiments were performed 15 and 16 days post seeding. All cell culture media and reagents were purchased from Fischer Scientific GTF (Västra Frölunda, Sweden).
Micellar fractions from in vitro digested reference and optimised soups were used for the uptake experiments. The three batches of the optimised and reference soup were mixed into one “average” batch of reference or optimised soups prior to in vitro digestion. The micellar fractions were blanketed with N2 and stored at −80˚C until the uptake experiments, which were performed within one week after the in vitro digestion. For the uptake experiments, the micellar fractions were diluted 1:4 with DMEM. The dilution of the micellar fraction was made to avoid possible toxicity of bile salts and enzymes present in the digests. Growth medium was removed and the cells washed twice with phosphate buffer solution (PBS) prior addition of 4 ml diluted digesta to each well. The mixed reference and optimised soups were subjected to three independent in vitro digestions and the diluted digests were each added to 3 wells on 3 different 6-well plates. The cells were incubated on a rotary shaking plate at 37˚C for 6 h. After incubation the digesta was removed and the cells washed once with cold PBS containing 1% (v/v) FBS and twice with only PBS. The cells were lysed by addition of 2.5 ml 0.5 M NaOH to each well. The lysed cells were collected and stored at −80˚C until analysis. The carotenoid content of the cell lysates was analyzed within two weeks.
To extract the carotenes, 2 ml of the cell lysate was transferred to a glass test tube, 1.6 ml hexane (containing 1 g·L−1 BHT) added and the tube vortexed. Following the addition of 1 ml methanol, the tubes were vortexed a second time. The phases were separated by centrifugation (4000 × g, 5 min) and the upper hexane layer transferred to a new glass tube. An additional 1.5 mL hexane was added to the cell lysate and the extraction procedure repeated. The collected organic phase was evaporated at 35˚C under a stream of nitrogen and resuspended in 100 µL hexane. Carotenoids were separated and quantified using HPLC according to the method described above, but with an injection volume of 40 µl. Protein content of the lysed cells was analyzed with the Pierce BCA Protein assay kit (Thermo Scientific, Rockford, USA).
2.2.5. In Vivo Studies Used to Validate the in Vitro Models
To evaluate the usefulness and limitations with the in vitro methods the model-derived bioaccessibility values were compared with the bioavailability values observed in two published human studies using the same soups. Both human studies were conducted at the University of Murcia, Spain and the participants fulfilled the following eligibility criteria: normal biochemical and haematological profile; no smoking; no vitamin, mineral or other supplement use ≥8 weeks before the study; consumption of fruit and vegetables ≤ 4 portions/day.
2.2.5.1. Study 1
In the pilot human study [25], 14 male subjects consumed an optimized soup (300 ml/day) for 4 weeks. One serving of the soup contained 3.9 mg β-carotene, 4 mg lycopene and 5% olive oil. Baseline blood and urine samples were collected twice (days −1 and 0), and the mean value of these measurements were taken as baseline value. The age of the participants (mean ± SEM) was 24.0 ± 0.7 years old, their body mass index (BMI) was 22.3 ± 1.1 kg/m2 and body fat percentage 14.7 ± 1.4% (means ± SEM).
2.2.5.2. Study 2
In the second human study [26], 72 healthy volunteers (36 men/36 women) were recruited. Three subjects dropped out and sixty-nine participants completed the study (35 men/34 women). The subjects consumed 300 ml/day of the reference or the optimized soup during 4 weeks. Both soups contained the same proportions of carrot, tomato and broccoli, but with 5% olive oil in the optimised soup and 2.5% in the reference soup. The β-carotene content of the optimised/reference soups was 4.10/2.90 mg, and the lycopene content was 3.90/2.71 mg. Blood samples were collected before and after the supplementation periods for analysis of serum carotenoid concentrations. The participants were 30 ± 10 years old (mean ± SD) and had a BMI of 18.5 - 24.9 kg/m2.
2.2.6. Calculation of Bioavailability and Bioaccessibility Ratios
The mean bioavailability ratio was calculated as the ratio between mean increases in plasma carotenoid levels after 4 weeks intake of the optimised soup to that measured after 4 weeks intake of the reference or pilot soups. The bioaccessibility ratio was calculated by comparing the amount of carotenoids released from the food matrix or incorporated into the micellar phase, or the uptake in Caco-2 cells, after in vitro digestion of the optimised soup compared to the amounts measured after in vitro digestion of the reference soup. The carotenoid content was similar in the optimised and pilot soups, but lower in the reference soup. Since this most likely affected both the carotenoid content of the digesta after in vitro digestion and the plasma carotene levels, the difference in carotenoid content of the different soups was not normalized for during calculation of the ratios.
2.3. Statistical Analysis
2.3.1. HPLC Analysis of Soups and Digesta
For the HPLC data of carotenoid content of extracted samples the results are given as the mean value ± standard deviation (SD) of six independent samples (n = 6), analysed in duplicate. For the in vitro digested samples, data are based on three separate in vitro digestions made on each soup and analysed in duplicate. Values are presented as mean ± standard deviation (SD). The MannWhitney test (Wilcoxon two-samples test) was performed to determine significant differences of the mean between reference and optimised soups.
2.3.2. Statistical Analyses of Human Study 1
The results from Study 1 are expressed as mean ± standard error mean (SEM) with 95% CI. A general linear model of repeated-measures was used to compare the basal values and those obtained at different sampling times, followed by a post-hoc Bonferroni test. Differences were considered statistically significant for a P-value < 0.05. SPSS version 15.0 (Statistical Package for Social Sciences, SPSS Inc. Chicago, IL, USA) was used for the statistical analyses.
2.3.3. Statistical Analyses of Human Study 2
The results from Study 2 are expressed as mean ± SEM. Student’s paired t test was used to compare data obtained from samples collected at baseline and after the final treatment for each group. Differences between baseline characteristics from the optimised and reference groups, and differences arising from the intake of optimised or reference soup were compared using Student’s unpaired t test. In the case of differences between the baseline values of subjects from the reference and optimized groups, a univariate general linear model was used with the baseline value of the selected parameter as covariable. A Two-Way ANOVA analysis was used to estimate possible gender effects. The relationship between variables was determined using the Pearson correlation coefficient. Differences were considered statistically significant when P < 0.05. SPSS version 15.0 (Statistical Package for Social Sciences, SPSS Inc. Chicago, IL, USA) was used for the statistical analyses.
3. Results and Discussion
3.1. Effects of Storage and Preparation on Content and in Vitro Release of Carotenoids
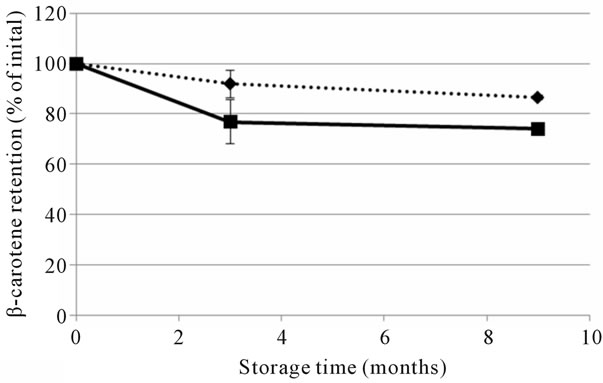
The optimised and reference soups were stored at ambient temperature and prepared by heating on a stove during the human studies. To evaluate the effect of storage on the nutritional quality of the soups, total content and in vitro accessibility of carotenoids was analyzed at the beginning (month 0) and at the end of the large human study (month 3), as well as after additional storage (at ambient temperature) for six months (month 9) (Figures 1 and 2). The β-carotene content of the two soups was reduced after the initial three months of storage, with the largest relative reduction in the optimised soup (Figure 1(a)). Lycopene was quite stable during the first three months of ambient storage, but a decrease was observed between month 3 and 9 (Figure 1(b)). The in vitro accessibility of β-carotene was lower in both the reference and optimised soup after 3 months storage, while the lycopene accessibility values appeared to be quite stable (Figure 2 and Table 1). The stored soups were also prepared with the same procedure as used by the participants of the human studies. The preparation was not found to have a significant effect on the total content (data not shown) or the in vitro release of carotenoids (Figure 2).
Comparison of the three different soups used for Study 1 and 2 showed that the total content of lycopene and
 (a)
(a)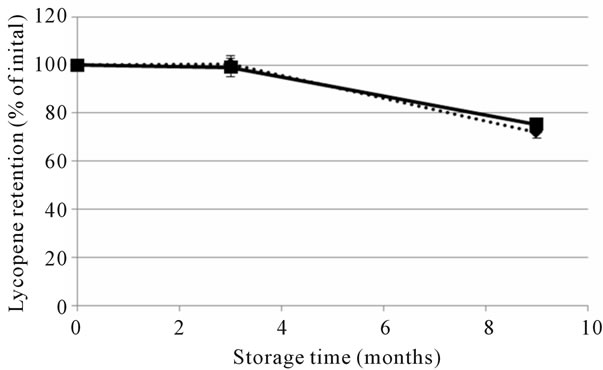 (b)
(b)
Figure 1. The retention of β-carotene (a) and lycopene (b) in optimised (solid line) and reference (dashed line) soups used in study 2, stored at ambient temperature up to 9 months. Values are shown as percentage of total initial amount and given as mean ± SD for the three batches of each soup.
β-carotene was significantly higher (P < 0.001) in optimised and pilot soups compared with the reference (Table 1). Although the content of both lycopene and olive oil was higher in the optimised soup compared with the reference soup used in Study 2, the total amount of in vitro accessible lycopene was not significantly different between the optimised and the reference soups (Table 1).
3.2. Release from the Matrix and Micellarization of Carotenoids
Estimations of carotenoid bioaccessibility are often given as either the fraction transferred from the food matrix to a supernatant or as the fraction transferred to a micellar phase. These two bioaccessibility estimates are compared for pooled batches (n = 3/soup) of the soups from Study 2 (Table 2). For the reference soup we found that the content of both β-carotene and lycopene transferred to the supernatant was significantly higher compared with the amount recovered in the micellar fraction (P < 0.1). The supernatant obtained from the optimised soup also

Table 1. Content and in vitro accessibility of β-carotene and lycopene in the soups produced for Study 1 and 2. In vitro digestions and analyses were made directly after the production of the soup for Study 1. For Study 2 soups were analysed both directly after production (Fresh = F) and after 3 months storage and heating (S + H). Values are given as means ± SD of the separate batches (n) produced for each soup.
 (a) (b)
(a) (b)
Figure 2. The in vitro accessibility of all-trans β-carotene (a) and lycopene (b) in the reference (dashed line) and optimised (solid line) soups used in Study 2. In vitro digestions were performed on freshly made soups (“Fresh”), on soups that had been stored at ambient temperature for the duration of the human trial, which was 3 months (“Stored”) and on heated soups (“Stored and heated”). Values are mean ± SD of the three batches of reference and optimised soup, respectively.
contained more carotenoids than the micellar phase, although those levels were not significantly higher. The supernatant fraction can be considered to include carotenoids incorporated into lipid droplets or mixed micelles as well as soluble (crystalline) aggregates of carotenoids not incorporated into lipid structures. Thus it seems reasonable that the supernatant fractions of the soups contained higher carotenoid concentrations. The microfiltration steps isolates the carotenoids incorporated to micelles. The degree of micellarization during the digestion process is often used as an indicator of carotenoid bioavailability since a higher degree of micellarization is suggested to facilitate the transfer of carotenoids to the brush border surface of enterocytes for uptake and subsequent absorption. The efficiency of this transfer has been found to vary depending on type of carotenoid, with very low efficiency for very polar compounds as lycopene and β-carotene [29]. Garrett et al. [30] found that less than 1% of lycopene in a meal with tomato paste was micellarised during in vitro digestion and that the efficiency of micellarization of lycopene was markedly less than that of lutein and β-carotene during digestion of a stir-fried meal containing spinach, carrot and tomato paste.
In Study 2, both the release from the food matrix and the amount of β-carotene obtained in the micellar phase after in vitro digestion of the optimised soup was significantly higher than the corresponding values obtained after in vitro digestion of the reference soup (Table 2). For lycopene, in vitro digested reference soup showed
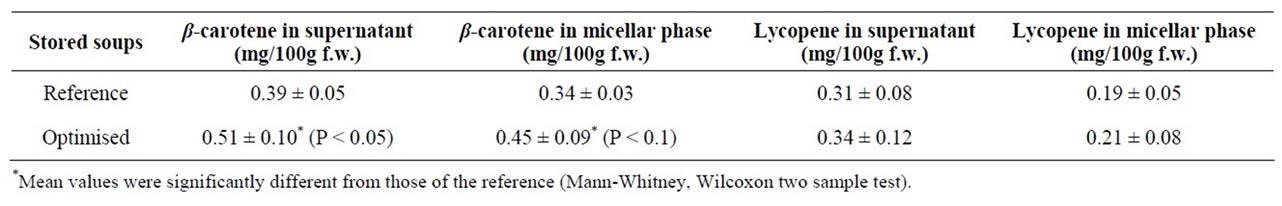
Table 2. In vitro release and micellar incorporation of all-trans β-carotene and total lycopene from the soups produced for Study 2. The soups were stored for 3 months and the three batches were mixed prior to in vitro digestion. Values are mean ± SD of six independent in vitro digestions performed on two subsequent days.
higher relative release and micellar efficiency, compared with the optimised soup, although the total amount of lycopene was slightly higher in the optimised soup (Table 2). Both extensive heating and mechanical processes have been found to enhance the bioavailability/accessibility of lycopene [31-33]. Thus, the higher lycopene accessibility observed in the optimised soup used in Study 1 (pilot soup) can be due to that it was prepared from a tomato paste (heat treated) whereas the soups for Study 2 was made using fresh tomatoes. Furthermore, unforeseen challenges with the provision of raw materials and the up scaling of the soups for Study 2, especially during the production of the optimised soup, created a soup with different characteristics and rheological properties, which may have adversely affected the bioaccessibility of lycopene in the optimised soup.
3.3. Caco-2 Cell Uptake
The cellular uptake of micellarised carotenoids from in vitro digested soups was investigated in the Caco-2 cell model. The reference and optimised soups used in Study 2 were investigated in two separate cell trials, one at the end of Study 2 (Cell study 1) and one in conjunction with the storage experiments performed six months later (Cell study 2). Cellular uptake from the pilot soup was investigated in conjunction with human Study 1, i.e. at a different time with different cells than the other soups. As shown in Table 3, this will largely influence the cellular uptake and the pilot soup is therefore not included.
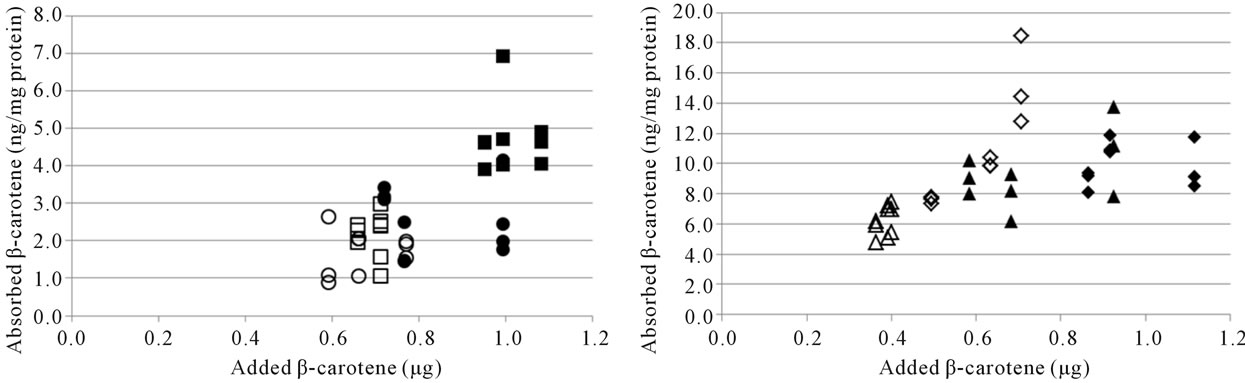
There was a trend of a higher cellular uptake of β- carotene from the optimised soup, but due to the relatively high SD no significant differences were observed (Table 3). Lycopene uptake was higher for the optimised soup in Cell study 1, but not in Cell study 2. Cellular uptake of carotenoids has previously been shown to be linearly dependent on the amount of carotenoids added to the cells [17,23,24,34]. The higher uptake of β-carotene from the optimised soup may therefore have been due to the higher carotene concentration in the micellar fraction (Table 2, Figures 3(a), (b)). Similarly, the inconsistencies in lycopene uptake could be due to the very similar lycopene content in the micellar fractions from the two soups (Table 2, Figures 3(c), (d)). However, even though micellarization is an important step for the uptake of carotenoids, high micellarization efficiency may not necessarily result in high cellular uptake since the localization of the carotenoids within the mixed micelles can have impact on the uptake. It is possible that the differences in uptake is related to structural changes during the storage of the soup, e.g. hydrolytic products of dietary lipids may modify physicochemical characteristics of the micelles [35]. The relative hydrophobicity of β-carotene and lycopene, their location and chemical form in the plant matrices, and their transfer between oil droplets and micelles are important, β-carotene in carrots are associated with proteins [36] surrounded by a thick membranous sheet and lycopene is present in the tomato as crystalloids that develop within or along thylakoid bodies [37]. In the study of Ryan et al. [38], comparing micellarization and cellular uptake of carotenoids after processing of courgette, red pepper and tomato samples using 4 different cooking procedures, it was also reported that an enhanced release of carotenoids did not necessary lead to a higher micellarization and/or absorption efficiency. Possible reasons for this may be a higher susceptibility of carotenoids for degradation and isomerization when released from the food matrix, and related consequences for the micellarization efficiency and uptake of carotenoids [39]. Serrano et al. [40] showed a significant inverse correlation between in vitro availability of carotenoids and content of Klason lignin, non-starch polysaccharides and protein. Thus, a more extensive release of different plant matrix components such as fiber and phytosterols [41], and formation of components during processing can also have an impact on the uptake of carotenoids.
3.4. Comparison of in Vitro Bioaccessibility with Bioavailability Values from the Human Studies
3.4.1. Study 1
Study 1 was performed as a pilot study to investigate changes in serum concentration of carotenoids during four weeks consumption of a fruit and vegetables soup [25]. Serum concentrations of both β-carotene and lycopene increased significantly compared to baseline values
 (a)
(a)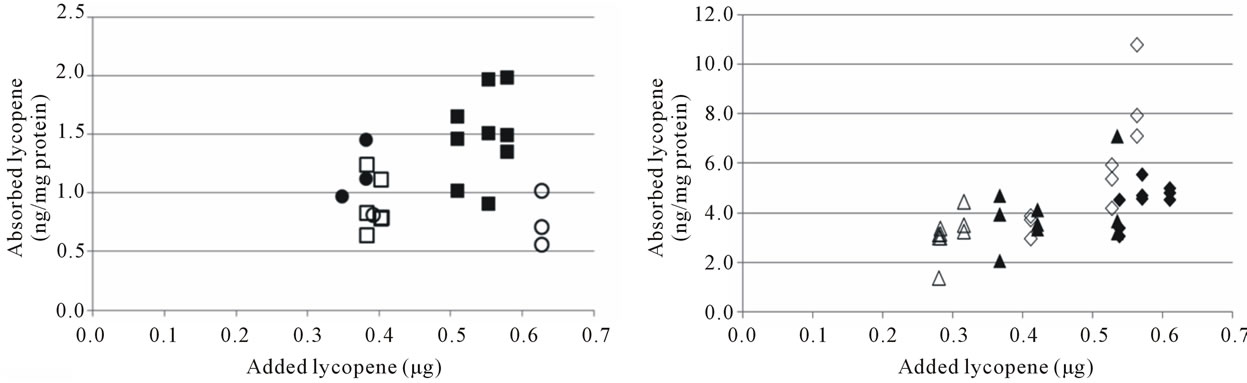 (b)
(b)
Figure 3. Cellular uptake of all-trans β-carotene (a), (b) and total lycopene (c), (d) in Cell study 1 (a), (c) and Cell study 2 (b), (d). Open markers denote reference soups and filled markers optimised soups. The uptake experiments were performed on two different days, marker shape indicate day of experiment. The lycopene content of the cells was below the detection limit for several wells in Cell study A, these have been excluded from the dataset.
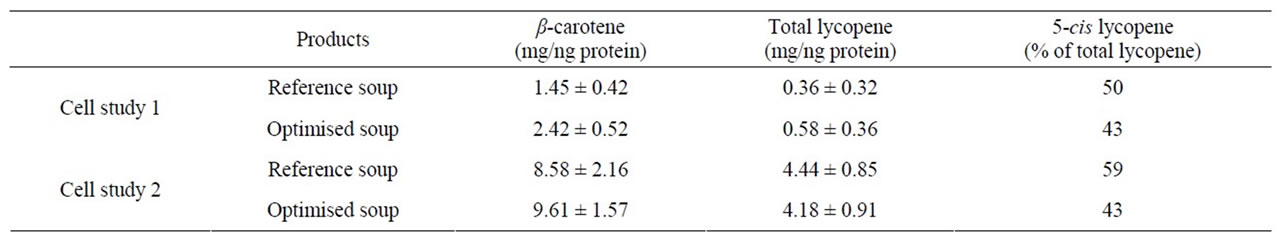
Table 3. Cellular uptake of carotenoids from the micellar fraction of in vitro digested soups from Study 2. Results are given as mean ± SD.
following 3 and 4 weeks intake of the pilot soup produced for Study 1 (Table 4). After 4 weeks, serum β- carotene was increased by 141% and lycopene by 132%. The higher relative increase for β-carotene is consistent with the higher relative and total bioaccessibility of β-carotene observed in vitro. The relative in vitro bioaccessibility of the pilot soup was 55% of the total content for β-carotene and 43% for lycopene.
3.4.2. Study 2
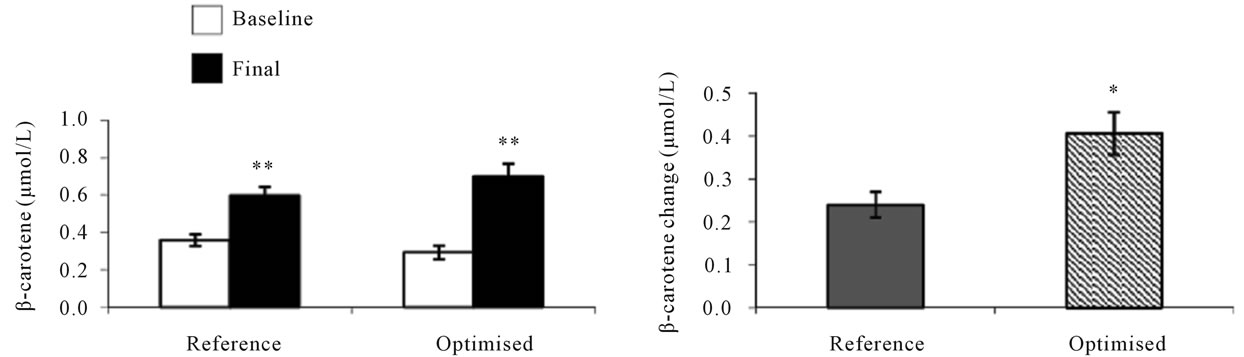
Study 2 was made to investigate the effects of daily intakes of differently processed soups on human serum carotene concentrations and oxidative stress markers [26]. The mean serum concentration of carotenoids increased significantly in response to 4 weeks of soup consumption (Figure 4). The serum β-carotene response was significantly (P < 0.001) higher in subjects consuming the optimised soup compared with those consuming the reference soup and was increased by 139% compared to baseline values compared with an increase of 67% in subjects consuming the reference soup. Whereas for lycopene, serum responses were higher in subjects consuming the
 (a)
(a) (b)
(b)
Figure 4. Serum carotenoid responses in human subjects [26] after 4 weeks consumption of reference or optimised soup produced for study 2. Baseline and final carotenoid concentrations ((a) and (c)). Serum carotenoid changes after 4 weeks consumption ((b) and (d)). Values are means ± SEM, n = 34 for group with reference soup, n = 35 for group with optimised soup. *Represents statistically significant differences, *P < 0.05; **P < 0.001. (Student’s paired t test, (a) and (c); Student’s unpaired t test, (b) and (d)).

Table 4. Human serum concentrations of β-carotene and lycopene of subjects at baseline and after 3 and 4 weeks consumption of the soups used in study 1 [25]1.
reference soup, resulting in an average increase of 54% compared with baseline values and with only an increase by 24% for the optimised soup.
For comparison of the bioaccessibility values obtained by in vitro methods with the in vivo results from the long-term human study, the bioavailability/accessibility ratios were calculated (as described in the materials and methods section). Table 5 shows the ratios calculated from results obtained from in vitro digestion and cell uptake studies (Cell study 1, which was performed with soups stored until the end of the human study (3 months storage)). Table 6 shows the results from in vitro digestion experiments with stored (optimised and reference) soups and with cell uptake experiments in Cell study 2 (9 months storage). In Table 7, the comparison between in vivo and in vitro values for the optimised and pilot soup from Study 1 is shown. Results from cell uptake studies made with the pilot soup are not included as these experiments were made on different occasions.
As shown in Tables 5-7, for β-carotene the bioavailability ratio measured in humans was in the same range as the bioaccessibility values measured with in vitro methods, but for lycopene the results were less consistent. The largest discrepancy for lycopene was obtained when comparing in vitro release and micellarization followed by cellular uptake from optimised and reference soups in Cell study 1 with in vivo data for the same soups. Here, the in vitro data indicated a higher degree of micerallization and cellular uptake for the optimised soup whereas the human data showed that the lycopene bioavailability was higher from the reference than the optimised soup. Reboul et al. [19] have compared data derived from in vitro digestion and sampling of small intestinal contents from human subjects fed carotenoid rich vegetables with data from published human studies. They concluded that measurements of the carotenoid micellarization during in vitro digestion are a reliable estimate of the in vivo bio-
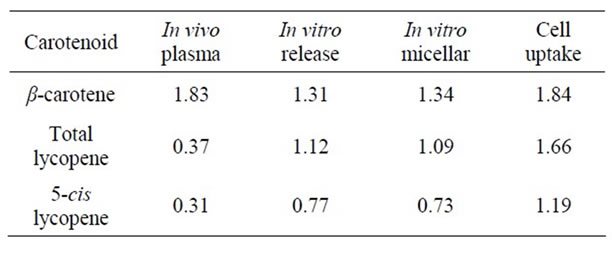
Table 5. Bioavailability/accessibility ratios of optimized vs. reference soups Cell study 1.
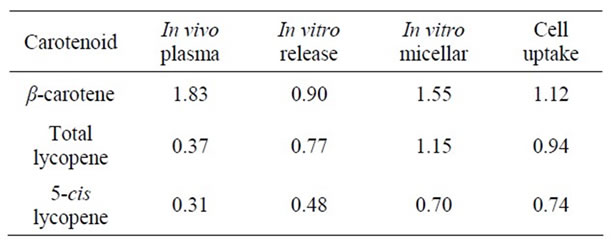
Table 6. Bioavailability/accessibility ratios of optimized vs. reference soups Cell study 2.

Table 7. Bioavailability/accessibility ratios of optimized vs. pilot soups.
accessibility, especially for β-carotene. In their study, Reboul and co-workers [19] found that the percentage β-carotene recovered in micelles in vivo from a carrot puree were in the same range as those measured with the in vitro model (5.01 ± 0.72 vs 4.39% ± 0.18%, mean ± SEM) whereas the percentage of lycopene recovered in micelles in vivo and in vitro, was 2.23 ± 0.64 and 1.11% ± 017% (mean ± SEM), respectivley.
Tomato, broccoli and carrot also contain other carotenoids such as e.g. lutein, β-carotene and pre-cursors of lycopene, which were not determined in the present study. The transfer efficiency of five carotenoids (astaxanthin, lutein, β-cryptoxanthin, β-carotene and lycopene from emulsion lipid droplets to micelles was investigated by Tyssandier et al. [42] and found to be very different: from 0.5% for lycopene to 19.8% for astaxanthin. These authors also studied interactions between carotenoids and found a marked effect of adding either lycopene or lutein on the transfer of β-carotene. However, different human studies have reported varying results, where simultaneous intake of different carotenoids increased, decreased or did not affect the bioavailability depending on speciation and ratios [43-45], thus it is possible that although there might be interactions between different carotenoids this does not always result in adverse effects on medium/long-term plasma status of carotenoids in humans.
The content of olive oil was lower in the reference soup, 2.5% compared to 5% in the optimised soup. In a static in vitro system, the lycopene/oil ratio may have a large impact on lycopene solubilisation and micellarization. In the dynamic environment of the gastrointestinal tract other factors, such as the higher relative release of lycopene from the food matrix illustrated in Figure 2(d) and the higher proportion of cis-isomers in the reference soup may have a larger impact on the in vivo bioavailability of lycopene. The fraction of total and in vitro accessible cis-lycopene was higher in both the reference soup and the pilot soup compared with the optimised soup (Table 1). This could have contributed to the higher in vivo bioaccessibility of lycopene observed from these soups compared with the optimised soup (Tables 5-7). A higher relative uptake of 5-cis lycopene was also observed in the Caco-2 model (Table 3). In addition, there seems to be huge variability in lycopene uptake in intestinal cell lines, and data from human studies in general report low bioavailability of lycopene, a large inter-individual variation of lycopene bioavailability in humans has been reported by several authors [46,47]. Thus, means calculated from human studies with a relatively small number of subjects may not always be representative.
The primary finding of this study was a consistently lower bioaccessibility and bioavailability of lycopene compared to β-carotene both in vitro and in vivo. Similarly, previous investigations of the efficiency of transfer of individual carotenoids from a food matrix to the aqueous or micellar fraction in response to in vitro digestion have shown that β-carotene is more readily micellarised than lycopene [29,30,35]. These findings therefore support the validity of in vitro models for comparisons of the relative bioavailability of different carotenes.
4. Conclusion
The comparison of the in vitro and in vivo results suggests that the combination of a two-step in vitro digestion and Caco-2 cells can be useful for estimation of β-carotene bioaccessibility. The results from the in vitro digestion experiments of β-carotene appeared to be relatively consistent in terms of general bioaccessibility and can be used to improve our understanding of the factors governing release and micellarisation of β- carotene from a fruit and vegetable matrix. For lycopene the in vitro and in vivo results were less consistent, suggesting that reliable prediction of lycopene bioavailability might be more problematic. Further evaluation of the applicability of in vitro/cell uptake methods to study lycopene bioaccessibility is needed.
5. Acknowledgements
This research was financially supported by the Commission of the European Communities, Framework 6, Priority 5 “Food Quality and Safety”, STREP Project Healthy Structuring (2006-023115).
REFERENCES
- A. V. Rao and L. G. Rao, “Carotenoids and Human Health,” Pharmacological Research, Vol. 55, No. 3, 2007, pp. 207-216. doi:10.1016/j.phrs.2007.01.012
- K. J. Yeum and R. M. Russell, “Carotenoid Bioavailability and Bioconversion,” Annual Review of Nutrition, Vol. 22, 2002, pp. 483-504. doi:10.1146/annurev.nutr.22.010402.102834
- L. Yonekura and A. Nagao, “Intestinal Absorption of Dietary Carotenoids,” Molecular Nutrition & Food Research, Vol. 51, No. 1, 2007, pp. 107-115. doi:10.1002/mnfr.200600145
- R. S. Parker, “Absorption, Metabolism, and Transport of Carotenoids,” FASEB Journal, Vol. 10, No. 5, 1996, pp. 542-551.
- J. Shi and J. Le Maguer, “Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing,” Critical Reviews in Biotechnology, Vol. 20, 2000, pp. 293-334. doi:10.1080/07388550091144212
- M. L. Nguen and S. J. Schwarz, “Lycopene Stability during Food Processing,” Proceedings of the Society for Experimental Biology and Medicine, Vol. 218, 1998, pp. 101-105.
- S. K. Clinton, C. Emenhiser, S. J. Schwarz, D. G. Bostwick, A. W. Williams, B. J. Moore and J.-W. Erdman, “Cis-Trans Lycopene Isomers, Carotenoids, and Retinol in the Human Prostate,” Cancer Epidemiology, Biomarkers & Prevention, Vol. 5, 1996, pp. 823-833.
- J. Schierle, W. Bretzel, I. Buhler, N. Faccin, D. Hess, K. Steiner and W. Schuep, “Content and Isomeric Ratios of Lycopene in Food and Human Blood Plasma,” Food Chemistry, Vol. 59, No. 3, 1997, pp. 459-465. doi:10.1016/S0308-8146(96)00177-X
- C. Gärtner, W. Stahl and H. Sies, “Lycopene Is More Bioavailabile from Tomato Paste than from Fresh Tomatoes,” American Journal of Clinical Nutrition, Vol. 66, No. 1, 1997, pp. 116-122.
- A. C. Boileau, N. R. Merchen, K. Wasson, C. A. Atkinson and J. W. Erdman, “Cis-lycopene Is More Bioavailable than Trans-Lycopene in Vitro and in Vivo in Lymph-Cannulated Ferrets,” Journal of Nutrition, Vol. 129, No. 6, 1999, pp. 1176-1181.
- N. Z. Unlu, T. Bohn, D. Francis, H. N. Nagaraja, S. K. Clinton and S. J. Schwartz, “Lycopene from HeatInduced Cis-Isomer-Rich Tomato Sauce Is More Bioavailable than from All-Trans-Rich Tomato Sauce in Human Subjects,” British Journal of Nutrition, Vol. 98, No. 1, 2007, pp. 140-146. doi:10.1017/S0007114507685201
- M. Richelle, B. Sanchez, I. Tavazzi, P. Lambelet, K. Bortlik and G. Williamsson, “Lycopene Isomerization Takes Place within Enterocytes during Absorption in Human Subjects,” British Journal of Nutrition, Vol. 103, No. 12, 2010, pp. 1800-1807. doi:10.1017/S0007114510000103
- E. Hedrén, V. Diaz and U. Svanber, “Estimation of Carotenoid Accessibility from Carrots Determined by an in Vitro Digestion Method,” European Journal of Clinical Nutrition, Vol. 56, No. 5, 2002, pp. 425-430. doi:10.1038/sj.ejcn.1601329
- F. Granado-Lorencio, B. Olmedilla-Alonso, C. HerreroBarbudo, B. Perez-Sacristan, I. Blanco-Navarro and S. Blazquez-Garcia, “Comparative in Vitro Bioaccessibility of Carotenoids from Relevant Contributors to Carotenoid Intake,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 15, 2007, pp. 6387-6394. doi:10.1021/jf070301t
- F. Granado-Lorencio, B. Olmedilla-Alonso, C. HerreroBarbudo, I. Blanco-Navarro, B. Perez-Sacristan and S. Blazquez-Garcia, “In Vitro Bioaccessibility of Carotenpids and Tocopherols from Furits and Vegetables,” Food Chemistry, Vol. 102, No. 3, 2007, pp. 641-648. doi:10.1016/j.foodchem.2006.05.043
- D. A. Garrett, M. L. Failla and R. J. Sarama, “Development of an in Vitro Digestion Method to Assess Carotenoid Bioavalability from Meals,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 10, 1999, pp. 4301-4309. doi:10.1021/jf9903298
- S. K. Thakkar, B. Maziya-Dixon, A. G. O. Dixon and M. L. Failla, “β-Carotene Micellarization during in Vitro Digestion and Uptake by Caco-2 Cells Is Directly Proportional to β-Carotene Content in Different Genotypes of Cassava,” Journal of Nutrition, Vol. 137, No. 10, 2007, pp. 2229-2233.
- S. Veda, A. Kamath, K. Platel, K. Begum and K. Srinivasan, “Determination of Bioaccessibility of Beta-Carotene in Vegetables by in Vitro Methods,” Molecular Nutrition & Food Research, Vol. 50, No. 11, 2006, pp. 1047-1052. doi:10.1002/mnfr.200600076
- E. Reboul, M. Richelle, E. Perrot, C. DesmoulinsMalezet, V. Pirisi and P. Borel, “Bioaccessibility of Carotenoids and Vitamin E from Their Main Dietary Sources,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 23, 2006, pp. 7849-8755. doi:10.1021/jf061818s
- F. Granado, B. Olmedilla, C. Herrero and B. Perez-Sacristan, “Bioavailability of Carotenoids and Tocopherols from Broccoli: In Vivo and in Vitro Assessment,” Experimental Biology and Medicine, Vol. 231, 2006, pp. 1733-1738.
- A. Bengtsson, M. Larsson Alminger and U. Svanberg, “In Vitro Bioaccessibility of β Carotene from Heat-Processed Orange-Fleshed Sweet Potato,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 20, 2009, pp. 9693- 9698. doi:10.1021/jf901692r
- A. Bengtsson, C. Brackmann, A. Enejder, M. Larsson Alminger and U. Svanberg,” Effects of Thermal Processing on the in Vitro Bioaccessibility and Microstructure of β-Carotene in Orange-Fleshed Sweet Potato,” Journal of Agricultural and Food Chemistry, Vol. 58, No. 20, 2010, pp. 11090-11096. doi:10.1021/jf1024104
- D. A. Garrett, M. L. Failla, R. J. Sarama and N. Craft, “Accumulation and Retention of Micellar β-Carotene and Lutein by Caco-2 Human Intestinal Cells,” Journal of Nutritional Biochemistry, Vol. 10, No. 10, 1999, pp. 573- 581. doi:10.1016/S0955-2863(99)00044-3
- A. During, M. Mahmood Hussain, D. W. Morel and E. H. Harrisson, “Carotenoid Uptake and Secretion by Caco-2 Cells: β-Carotene Isomer Selectivity and Carotenoid Interactions,” Journal of Lipid Research, Vol. 43, No. 7, 2002, pp. 1086-1095. doi:10.1194/jlr.M200068-JLR200
- R. Martínez-Tomás, E. Larqué, D. González-Silvera, M. Sánchez-Campillo, M. I. Burgos, A. Wellner, S. Parra, L. Bialek, M. Alminger and F. Pérez-Llamas, “Effect of the Consumption of a Fruit and Vegetable Soup with High in Vitro Carotenoid Accessibility on Serum Carotenoid Concentrations and Markers of Oxidative Stress in Young Men,” European Journal of Nutrition, 2011, pp. 1-9.
- R. Martínez-Tomás, F. Pérez-Llamas, M. SánchezCampillo, D. González-Silvera, A. I. Cascales, M. García-Fernández, J. Ángel López-Jiménez, S. Zamora, M. I. Burgos, F. López-Azorín A. Wellner, S. Parra, L. Bialek, M. Alminger and E. Larqué, “Daily Intakes of Fruit and Vegetable Soups Processed in Different Ways Increase Human Serum Carotenoid Responses and Reduced Levels of Several Oxidative Stress Markers,” Food Chemistry, 2012, D-11-02661R.
- G. Sadler and J. Davies, “Rapid Extraction of Lycopene and β-Carotene from Reconstituted Tomato Paste and Pink Grapefruit,” Journal of Food Science, Vol. 55, No. 5, 1990, pp. 1460-1461. doi:10.1111/j.1365-2621.1990.tb03958.x
- C. Svelander, E. Tibäck, L. Ahrné M. Langton U. Svanberg and M. Alminger, “Processing of Tomato: Impact on in Vitro Bioaccessibility of Lycopene and Textural Properties,” Journal of the Science of Food and Agriculture, Vol. 90, No. 10, 2010, pp. 1665-1672. doi:10.1002/jsfa.4000
- V. Tyssandier, B. Lyan and P. Borel, “Main factors Governing the Transfer of Carotenoids from Emulsion Lipid Droplest to Micells,” Biochimica et Biophysica Acta, Vol. 1533, 2001, pp. 285-292.
- D. A. Garrett, M. L. Failla and R. J. Sarama, “Estimation of Carotenoid Bioavailability from Fresh Stir-Fried Vegetables Using an in Vitro Digestion/Caco-2 Cell Culture Model,” Journal of Nutritional Biochemistry, Vol. 11, No. 11, 2000, pp. 574-580. doi:10.1016/S0955-2863(00)00122-4
- W. Stahl, H. van den Berg, J. Arthur, A. Bast, J. Dainty, R. M. Faulks, C. Gärtner, G. Haenen, P. Hollman, B. Holst, F. J. Kelly, M. C. Polidori, C. Rice-Evans, S. Southon, T. van Vliet, J. Vina-Ribes, G. Williamsson and S. B. Astely, “Bioavailability and Metabolism,” Molecular Aspects of Medicine, Vol. 23, No. 1-3, 2002, pp. 39-100. doi:10.1016/S0098-2997(02)00016-X
- E. A. Tibäck, C. A. Svelander, I. J. P. Colle, A. I. Altskär, M. A. G. Alminger, M. E. G. Hendrickx, L. M. Ahrné and M. I. B. C. Langton, “Mechanical and Thermal Pretreatments of Crushed Tomatoes: Effects on Consistency and in Vitro Accessibility of Lycopene,” Journal of Food Science, Vol. 74, No. 7, 2009, pp. 386-395. doi:10.1111/j.1750-3841.2009.01255.x
- I. Colle, S. van Buggenhout, A. van Loey and M. Hendrickx, “High Pressure Homogenization Followed by Thermal Processing of Tomatopulp: Influence on Microstructure and Lycopene in Vitro Bioaccessibility,” Food Research International, Vol. 43, No. 8, 2010, pp. 2193- 2200. doi:10.1016/j.foodres.2010.07.029
- A. Bengtsson, N. Scheers, T. Andlid, M. Alminger, A. S. Sandberg and U. Svanberg, “Impaired Uptake of BetaCarotene by Caco-2 Human Intestinal Cells in the Presence of Iron,” International Journal of Food Sciences and Nutrition, Vol. 60, No. S5, 2009, pp. 125-135. doi:10.1080/09637480802641270
- T. Huo, M. G. Ferruzzi, S. J. Schwartz and M. L. Failla, “Impact of Fatty Acyl Composition and Quantity of Triglycerides on Bioaccessibility of Dietary Carotenoids,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 22, 2007, pp. 8950-8957. doi:10.1021/jf071687a
- J. D. Bryant, J. D. McCord, L. K. Unlu and J. W. Erdman, “Isolation and Partial Characterization of α- and β- Carotene-Containing Carotenoprotein from Carrot (Daucus carota L.) Root Chromoplast,” Journal of Agricultural and Food Chemistry, Vol. 40, No. 4, 1992, pp. 545-549. doi:10.1021/jf00016a003
- W. P. Mohr, “Pigment Bodies Found in Fruits of Crimson and High Pigment Lines of Tomatoes,” Annals of Botany, Vol. 44, 1979, pp. 427-434.
- L. Ryan, O. O’Connell, L. O’Sullivan, S. A. Aherne and N. M. O’Brien, “Micellarisation of Carotenoids from Raw and Cooked Vegetables,” Plant Foods for Human Nutrition, Vol. 63, No. 3, 2008, pp. 127-133. doi:10.1007/s11130-008-0081-0
- M. L. Failla, C. Chitchumroonchokchai and B. Ishida, “In Vitro Micellarization and Intestinal Uptake of Cis-Isomers of Lycopene Exceed Those of All-Trans Lycopene,” Journal of Nutrition, Vol. 138, No. 3, 2008, pp. 482-486.
- J. Serrano, I. Goni and F. Saura-Calixto, “Determination of β Carotene and Lutein Available from Green Leafy Vegetables by an in Vitro Digestion and Colonic Fermentation Method,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 8, 2005, pp. 2936-2940. doi:10.1021/jf0480142
- P. Borel, “Factors Affecting Intestinal Absorption of Highly Lipohilic Food Microconstituents (Fat-Soluble Vitamins, Carotenoids and Phytosterols,” Clinical Chemistry and Laboratory Medicine, Vol. 41, No. 8, 2003, pp. 979-994.
- V. Tyssandier, E. Reboul, J. F. Dumas, C. BouteloupDemange, M. Armand, J. Marcand, M. Sallas and P. Borel, “Processing of Vegetable-Born Carotenoids in the Human Stomach and Duodenum,” American Journal of Physiology—Gastrointestinal and Liver Physiology, Vol. 284, 2003, pp. 913-923.
- H. Van den Berg, “Carotenoid Interactions,” Nutrition Reviews, Vol. 57, No. 1, 1999, pp. 1-10. doi:10.1111/j.1753-4887.1999.tb01769.x
- V. Tyssandier, N. Cardinault, C. Caris-Veyrat, M.J. Amiot, P. Grolier, C. Bouteloup, V. Azai-Braesco and P. Borel, “Vegetable-Borne Lutein, Lycopene, and β-Carotene Compete for Incorporation into Chylomicrons with No Adverse Effect on the Medium-Term (3-wk) Plasma Status of Carotenoids in Humans,” American Journal of Clinical Nutrition, Vol. 75, 2002, pp. 526-534.
- K. J. Yeum and R. M. Russell, “Carotenoid Bioavailability and Bioconversion,” Annual Review of Nutrition, Vol. 22, 2002, pp. 483-504. doi:10.1146/annurev.nutr.22.010402.102834
- P. Borel, P. Grolier, N. Mekki, Y. Boirie, Y. Rochette, B. Le Roy, M. C. Alexandre-Gouabou, D. Lairon and V. Azais-Braesco, “Low and High Responders to Pharmacological Doses of Beta-Carotene: Proportion in the Population, Mechanisms Involved and Consequences on Beta-Carotene Metabolism,” Journal of Lipid Research, Vol. 39, No. 11, 1998, pp. 2250-2260.
- M. E. O’Neill and D. I. Thurnham, “Intestinal Absorption of β-Carotene, Lycopene and Lutein in Men and Women Following a Standard Meal: Response Curves in Triacylglycerol-Rich Lipoprotein Fraction,” British Journal of Nutrition, Vol. 79, No. 2, 1998, pp. 149-159. doi:10.1079/BJN19980026
NOTES
*Corresponding author.

