Food and Nutrition Sciences
Vol. 2 No. 7 (2011) , Article ID: 7235 , 8 pages DOI:10.4236/fns.2011.27101
Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”
![]()
1Dipartimento di Scienze degli Alimenti e dell’Ambiente, Università di Messina, Messina, Italy; 2Malta Centre for Fisheries Sciences (MCFS/MRRA), Fort San Lucjan, Malta; 3Hellenic Centre of Marine Research, (HCMR), Institute of Aquaculture, Agios Kosmas, Helliniko, Greece; 4Dipartimento di Matematica, Università di Messina, Messina, Italy.
Email: *dgiuffrida@unime.it
Received April 15th, 2011; revised July 20th, 2011; accepted July 27th, 2011.
Keywords: Thunnus thynnus, Raw Roe, Bottarga, Fatty Acids, Health Lipid Indices, GC
ABSTRACT
The fatty acids composition and the related health lipid indices (IA, atherogenic and IT thrombogenic) of Blue Fin Tuna’s (Thunnus thynnus L.) raw roe and their cured product bottarga, both considered a delicacy, were studied. The fatty acid (FA) composition of tuna’s roe and bottarga showed a relevant proportion (40.87% and 36.62% respectively) of poly-unsaturated fatty acids (PUFAs) with a prevalence of the n – 3 series, that showed values almost ten folds higher than those of n – 6 fatty acids, in both classes of analyzed samples. The IA and IT indices resulted comparable in tuna’s roe and in the bottarga samples respectively. To the best of our knowledge, this is the first detailed report on the fatty acids composition and the related lipid health indices in tuna’s raw roe and in their cured product “bottarga”.
1. Introduction
Recently great interest has been devoted to the study of the lipid composition of fish and fish products which are recognized as important sources of n – 3 fatty acids. In fact a wide class of health beneficial fatty acids characterizes their nutritional value and their therapeutic effects are raising the commercial interest of this food mainly for its high content of healthy fatty acids [1]. It is well known that PUFA’s in fish have a beneficial effect on health by, for example, decreasing the risk of stroke, reducing serum triacylglycerol levels, reducing blood pressure, and insulin resistance and modulating the glucose metabolism [2]. Among the PUFA’s are known components with anti-atheratogenic action like LA (18:2n – 6) belonging to the n – 6 PUFA’s class, and in the more important n – 3 PUFA’s class, components such as LNA (18:3n – 3), EPA (20:5n – 3) and DHA (22:6n – 3) which are appreciated for their anti-thrombogenetic effect [3]. On the contrary, amongs the saturated fatty acids (SFAs), lauric acid (C12:0), myristic acid (C14:0) and palmitic acid (C16:0), are recognized as health risk factors [3]. Diets reach in monounsaturatred fatty acids (MUFAs) resulted very efficient in reducing the coronaric deseases risk. Indeed MUFA have been recognized as beneficial as the PUFA’s n – 3 class for human health because of their effect in lowering blood cholesterol, in particular the DHA. In this study we focused our interest on tuna’s (Thunnus thynnus) raw roe and their cured product tuna botargo (bottarga di tonno), which are both considered a food delicacy. Bottarga is an ancient autochthonous delicacy produced nowadays mainly in Sicily and Sardinia (Italy), Greece and Japan. Bottarga’s production seems to be connected to ancient techniques introduced by the Phoenicians into the Mediterranean area since the 17th century. It is a cured product, a relish made of roes of mullet (in Sardinia) or tuna (in Sicily), lightly salted, pressed and sundried, which can be used as a fresh preparation or can also be stored up to 3 years. Briefly, the industrial production of bottarga starts from crude eggs extracted from mullet or tuna that are cleaned salted, dried, and then selected to be marketed whole or grounded. Mullets (in Sardinia) or tunas (in Sicily) are eviscerated and their ovarian sacs are cut off. Veins and blood are removed. Ovarian sacs are then maintained under marine salts for several hours, according to their weight, then washed with salt water and dried for some days, resting on a wooden table. After this first drying, lasting about 5 days, ovarian sacs are then pressed and kept hanging for further drying. Salting, spice-salting, and marinating are typical ways to process raw roe. The primary purpose of salting is preservation, but, at the same time, the ripening continues, giving the product its typical taste, flavour and texture. From a regional food status (Sicily and Sardinia), bottarga has been gaining a wider reputation during last time [4]. The aim of this study was to characterize the fatty acids component and the related health lipid indices of tuna’s raw roe and bottarga in order to add information on their nutritional quality. This data will provide useful information on health and nutritional values of these delicacies, considering the importance of these micronutrients in our diet and of a precise knowledge of the food composition and quality.
2. Materials and Methods
2.1. Samples.
Samples of captive raw roe of Blue Fin Tuna (T. thynnus) were kindly supplied by the Departement of Animal Health and Well Being of the University of Bari during a sampling campaign in summer 2009. A total of nine batchs of raw roe of tuna’s samples were obtained from sexually mature females from a capture-based tuna aquaculture, farming and fattening sited in the Tyrrhenian Sea. Samples were stored at –20˚C until the analysis. The samples of tuna’s botargo (T. thynnus) was purchased in Marsala (Sicily, Italy) in a specialized fish food factory and contained, as stated in the label, tuna roes and salt.
2.2. Chemicals and Solvents
All solvents used were of the highest availability purity. Fatty acids methyl esters standard compounds and reagents were purchased from Sigma-Aldrich (Milan, Italy). All the other chemicals used in this study were of analytical grade.
2.3. Extraction of Lipids
Lipid extraction was preceded on the samples of raw roe of tuna and on the samples of bottarga. Total lipids were extracted after homogenization of various samples aliquots (1 g), in a mixture of isopropanol and exane (3:2) containing 0.05% butylated hydroxytoluene (BHT) as antioxidant, basically according to Hara and Radin [5]. All the analyses were carry out in triplicates.
2.4. Fatty Acids Analysis
Fatty acid methyl esters were prepared by using a basecatalyzed transesterification according to Christie [6]. Fatty acids methyl esters were determined on a gas chromatograph Shimadzu GC 2010 (Milan, Italy), equipped with a capillary column Supelco Omegawax 250 (length 30 m, 0.25 mm inner diameters, 0.25 µm film thickness) and a flame ionization detector (FID). Hydrogen was used as carrier gas at a column flow rate of 1.46 ml/min. The injector was split/splitless and the split rate was 1:100. The oven temperature was set from 50˚C (hold time 2 min) to 240˚C (hold time 15 min) with a rate of 4˚C/min, injector was maintained at 180˚C and detector at 260˚C. The fatty acid methyl esters were identified by comparing the retention times with those of standard compounds. The percentage of composition of individual fatty acids was calculated by using the method of internal normalization.
2.5. Indexes of Lipid Quality
From the data on the fatty-acid composition, the following were calculated:
1) Index of atherogenicity (IA): indicating the relationship between the sum of the main saturated fatty acids and that of the main classes of unsaturated, the former being considered pro-atherogenic (favouring the adhesion of lipids to cells of the immunological and circulatory system), and the latter anti-atherogenic (inhibiting the aggregation of plaque and diminishing the levels of esterified fatty acid, cholesterol, and phospholipids, thereby preventing the appearance of microand macrocoronary diseases) [3,7].
The following equation was applied:

2) Index of thrombogenicity (IT): showing the tendency to form clots in the blood vessels. This is defined as the relationship between the pro-thrombogenetic (saturated) and the anti-thrombogenetic fatty acids (MUFAs, PUFAs – n6 and PUFAs – n3), [3,7].
The following equation was applied:
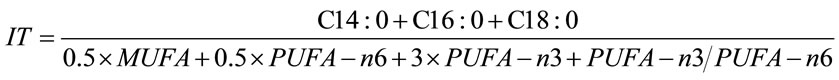
2.6. Statistical Analysis
Statistical analysis was performed using STATISTICA package for Windows (version 6.0, 2000). A non-parametric multivariate analysis was applied on data through the test of Mann-Whitney and successively by applying a Principal Component Analysis (PCA). The test of differences between groups (independent samples) of Mann Whitney was applied to investigate distinction between samples of raw roes and bottarga according to their fatty acids concentration. Moreover a PCA was also carried out to visualize similarities between samples and correlations between variables. Under this multivariate technique new variables summarizing the original variables are created, they are called principal components, the first principal components (PC1) is calculated as to explain the largest variation, followed by the second component (PC2) and so forth. Every principal component are composed by the weighted original variables and the loadings express the correlation between each component and variables.
The factor-scores plot, explaining the two components score (PC1 and PC2) for each samples, displays grouping similarities between samples according to their fatty acids concentration.
3. Results and Discussion
The results presented in this study provide a nutritional evaluation of the fatty acids components and their healthrelated lipid indices revealed in tuna’s whole roe and their cured product bottarga. By gas chromatographic analyses several FA components were detected and their concentrations were expressed as area percentages (%) as reported in Table 1. The lipid content in raw roe and bottarga were previously determined to be respectively 5.56% ± 0.41% and 4.32% ± 0.82%, in their proximate composition. We restricted our statistical analysis especially to PUFA’s and the two health-related lipid indices, the atherogenic index (IA) and thrombogenic index (IT).
The results showed that, in all examined samples, the most abundant fatty acids were poly n – 3 (C22:6n – 3, C20:5n – 3) and MUFA’s (C16:1n – 7, C18:1n – 9, C22:1n – 9). By applying Mann-Whitney test to both classes samples, a significant difference between raw roe and bottarga data in relation to their concentration of C18: 1n – 9, C20:5n – 3, C22:6n – 3, C18:2n – 6, and C20:4n – 6 were found, whereas a weakly difference for the IA and IT variables were shown. In fact the resulting p-level suggests rejecting the hypothesis that data are coming from the same population (Table 2).
By applying PCA, two principal components were extracted. That explains almost 85% of the total variance.
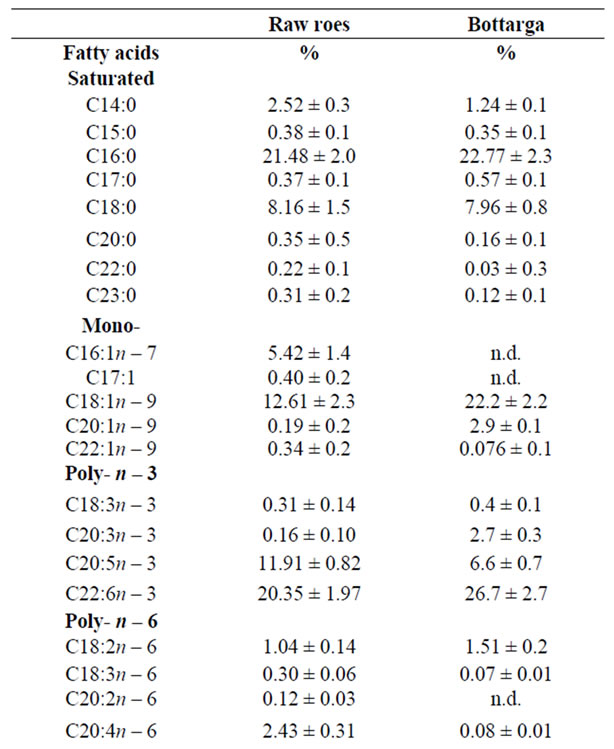
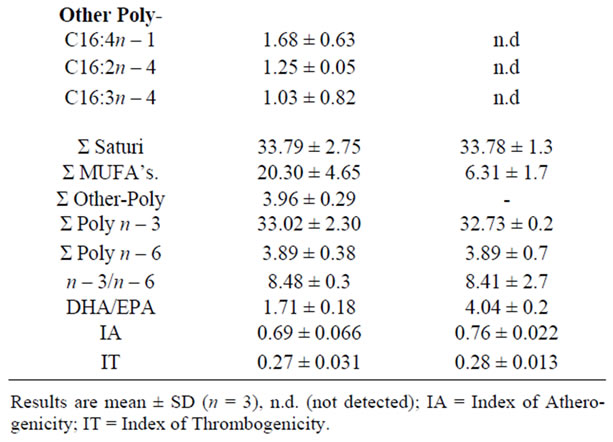
Table 1. Fatty acids composition (%), IA and IT of tuna’s raw roes and bottarga.
In Table 3 the PCA component are listed together with the variable correlations (factor loadings) with the component scores. Principal component one (PC1), explaining about the 55% of the total variance is mainly associated positively with variable C18:1n – 9, C22:6n – 3 and C18:2n – 6 while it is negatively correlated with variables C20:4n – 6, C20:5n – 3, IA and IT. PC2 explains about 29% of the total variance and it is positively associated with fatty acid C18:3n – 3, C16:0 and C18:0.
By displaying the component scores for data belonging
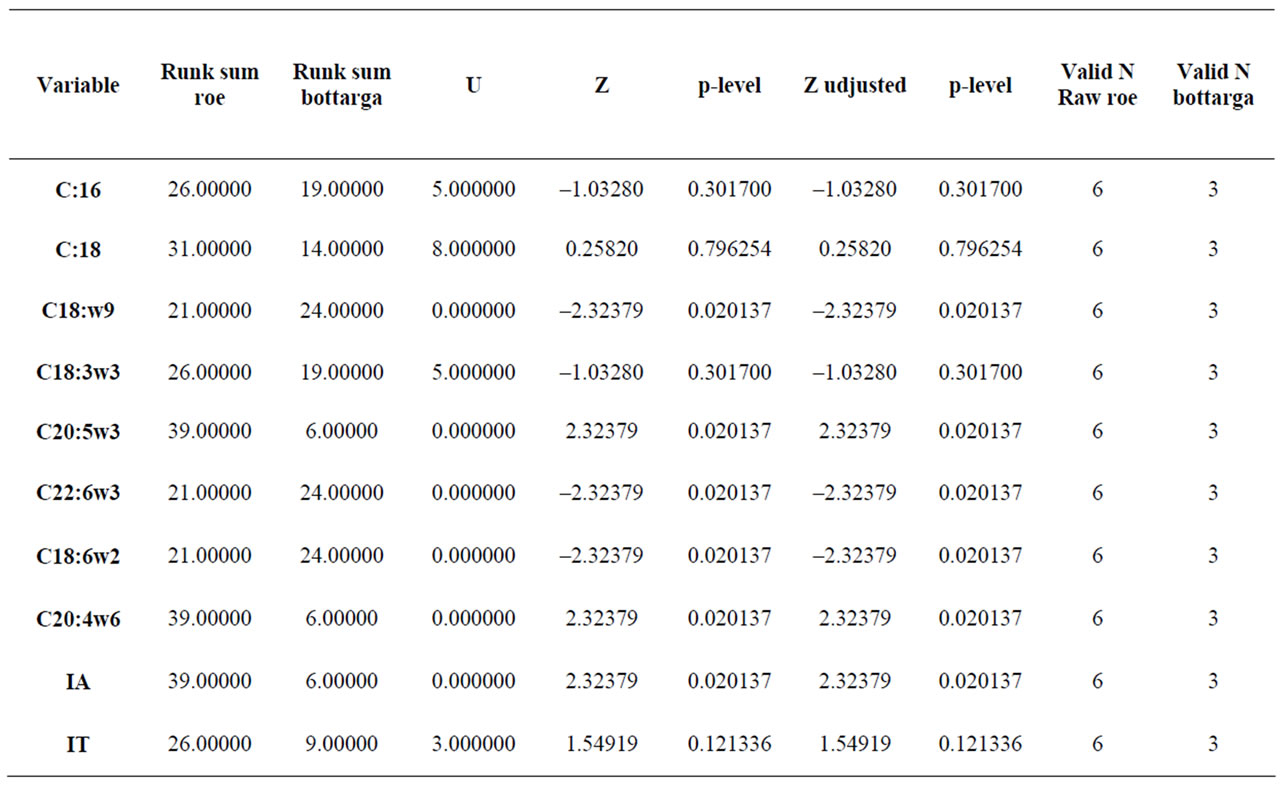
Table 2. Mann-Whitney test differences among tuna’s raw roes and bottarga.
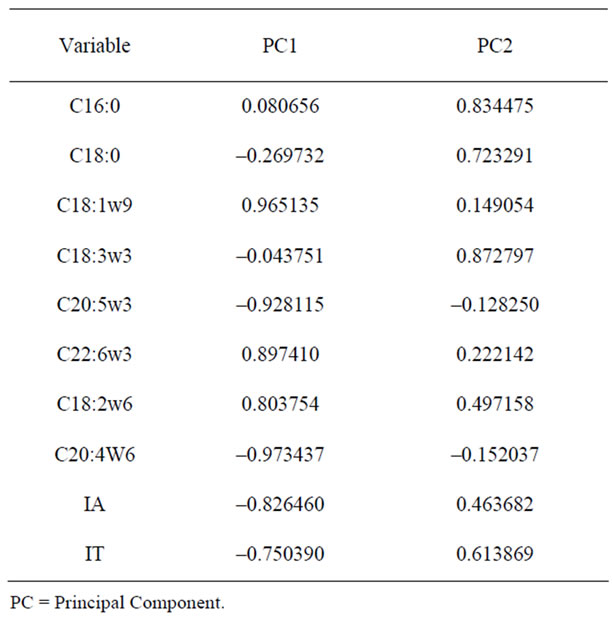
Table 3. Component-variable correlations (factor loadings), based on correlations.
to each classes samples (Figure 1) it was shown that in tuna’s roe samples the main fatty acids revealed were arachidonic acid (AA; 20:4n – 6) and eicosapentaenoic acid (EPA; 20:5n – 3); whereas oleic acid (18:1n – 9)docosahexaenoic acid (DHA; 22:6n – 3) and linoleic acid (LA; 18:2n – 6) resulted to characterized the bottarga samples. In particular, by comparing the tuna’s roes vs bottarga samples, the PCA analysis showed a significative differences for the following variables: oleic acid (18:1n – 9), linoleic acid (LA; 18:2n – 6), arachidonic acid (AA; 20:4n – 6), eicosapentaenoic acid (EPA; 20:5n – 3), docosahexaenoic acid (DHA; 22:6n – 3). Similar value were found for palmitic acid (16:00), stearic acid (18:00) and α-linolenic acid (LNA; 18:3n – 3). These results highlighted that lipid composition of tuna (T. thynnus) raw roes and their derivate product bottarga, are a source of fatty acids with functional properties to human health and therefore can be conveniently inserted in a correct diet.
Fish and fish derivates consumption are recommended by health authorities, not only for their high-quality protein content, but also for being a source of fatty acids considered highly beneficial for human health (n – 3 and n – 6) [7]. Recently special attention has been paid to the ratio of n – 3/n – 6 fatty acids because a very high intake of n – 6 acids has been recognized to be less desirable [8, 9]. Nutritionists believe that the desirable ratio n – 3/n – 6 should be 5 and that the addition of n – 3 polyunsaturated fatty acids (n – 3 PUFA) could improve nutritional value and prevent diseases [10]. Polyunsaturated fatty

Figure 1. The factor-scores plot, explaining the two components score (PC1 and PC2) for each samples of tuna’s raw roes and bottarga, displays grouping similarities between samples according to their fatty acids concen-tration.
acids from fish food, decrease the blood cholesterol level maintaining the proper blood fluidity especially when fish food is well stored and preserved from oxidation. It was shown by Scano et al. [11], that in bottarga samples (from raw mullet roe) the levels of EPA and DHA were not affected by the salting and drying procedures and the different storage conditions did not induce a marked oxidative degradation of n – 3 PUFA. Interestingly, in this study, we reported similar values for the sum of PUFAs – n3 fatty acids in tuna’s raw roe and bottarga and the same occurred also for the PUFAs n – 6 class (Table 1), although the samples were coming from different population. Table 1 also shows similar values for LA (18:2n – 6) in both classes of samples analyzed. LA is the precursor of the AA (20:4n – 6), which is reported to be advantageous to consumer’s cardiovascular health, only when it is present in low levels, due to its antagonistic effect to the health benefits of the n – 3 fatty acids [12-14]. We found a low content of AA in tuna’s roes and just traces in bottarga.
The human body can synthesize all the PUFAs from the two precursors the LNA (18:3n – 3) and the LA (18:2n – 6) by two different enzymes. The elongasi that acts lengthening the carbon chain and the desaturasi that acts increasing the number of double bonds getting so two series of compounds, the PUFAs n – 3 and PUFAs n – 6.
It was reported that DHA decreases the concentration of low density lipoprotein cholesterol in plasma [15], and EPA is recognized as the most important essential fatty acid of the n – 3 series in the human diet because it is the precursor to the 3-series eicosanoids [16]. On the contrary the saturated fatty acids cause a raise in the blood cholesterol level because are easily deposited on the walls of the arteries. Fish roes products are proposed as rich sources of n – 3 PUFA, containing high amounts of EPA and DHA [4,17,18], and this was also confirmed by our research. In both whole tuna roes and bottarga the DHA (22:6n – 3) and EPA (20:5n – 3) resulted as those fatty acids occurring in the highest proportion among the n – 3 PUFA series. Among the health benefits of dietary n – 3 fatty acids are included protection against heart disease, reduced susceptibility to mental illness, improved brain and eye function in infants, and alleviation of rheumatoid arthritis symptoms [19-22]. In our study, differences were observed in n – 3 and n – 6 PUFAs composition, with prevalence of n-3 series that shows values almost ten folds higher than those of n – 6 fatty acids in both classes of analyzed samples. In fact, as reported in Table 1, the n – 3/n – 6 ratio was about 8 in both samples due to the low level of n – 6 acids and high levels of EPA and DHA. The results presented in this investigation are in agreement with similar study on salted herring (Clupea harengus) products as reported by Aro et al. [9], and also with previous studies on the general lipid composition in the ovary of Bluefin Tuna (BFT) T.thynnus by Mourente et al. [23] during sex maturation. It is worth to highlight the role of both DHA and EPA in the ovary of this T. thynnus during sex maturation and spawning, in order to better understand the results obtained in our research. The high DHA levels may be considered as an essential character of the tuna family and may result from the unceasing accumulation of DHA originated from their prey fish, as the tuna is a top predatory fish in the marine food chain. It is proved that T. thynnus as well as other tunids accumulate DHA in almost all their tissues from very early stages of sex maturation until spawning [24-27]. The eggs spawned by BFT females are of the high lipid content type, so much so that EPA and mainly DHA, are unceasing accumulated in blue fin tuna ovary at spawning, as egg-specific lipoproteins component [23,28]. Besides it is well documented, that in this specimen there is a strong selection against monoenes such as 20:1n – 9 and 22:1n – 11 during sex maturation and which might be a preferred substrate for oxidation in the muscles tissues to be used as fuel in reproductive migration and spawning activity [28]; this features could be considered in accordance to the values of these monoenes here reported.
Among unsaturated fatty acids, oleic (18:1n – 9) and linoleic (18:2n – 6) are the most important contributors to the enrichment the aromatic components [29-31] and are considered as high nutritional because of their protective role against cardiovascular diseases [32]. Regarding the SFAs series, even if saturated fatty acid sensu latu are involved in atherogenic and thrombogenic processes, not all of them express the same behaviour as regards the increase of serum cholesterol. Lauric (C12:0), myristic (C14:0) and palmitic (C16:0) SFAs show a tendency to increase the haematic cholesterol concentration (myristic is more atherogenic), while there is a very high correlation between the sum of three acids (myristic, palmitic and stearic) and the thrombus formation [3]. Myristic acid has an iper-cholesterolemic effect four folds higher than palmitic one, while stearic acid is considered neutral [33].
In our study two distinct indexes were investigated: 1) Atherogenic index (IA); and 2) Thrombogenic index (IT). These indexes take into account the different effects that single fatty acid might have on human health and in particular on the probability of increasing the incidence of pathogenic phenomena, such as atheroma and/or thrombus formation.
Examining the values reported in Table 1 and can be evidenced that both atherogenic and thrombogenic indexes are similar in bottarga and in whole raw roe; those values can be considered as low, in agreement with literature reports [3,7,33].
The samples of tuna bottarga manufactured in Sicily and the whole roes, resulted both strongly characterized by palmitic acid in the SFAs series, oleic acid in the MUFAs series and DHA and EPA in the poly n – 3 series. The results here reported are in agreement with those reported in a similar study of mullet bottarga [34,11].
4. Conclusions
This study showed that tuna bottarga is a good and stable source of long-chain n – 3 fatty acids due to the low level of n – 6 and high level of DHA, EPA and the noticeable n – 3/n – 6 ratio when compared to recommended daily intake. Besides, the present study, through the determination of the FA composition and its related health lipid indexes in tuna’s roes product, could contribute to the valorization of the salted and semidried tuna ovary product, know as “bottarga” which has been regarded as a delicacy since the 17th century in several Mediterranean countries but still maintains an exceptional reputation as a traditional food in particularly in the NW area of Sicily and Sardinia and also in other countries such as Greece and Japan where is named respectively “Mesolongi botarga” and “Masagi” or “Karasumi”. To the best of our knowledge, this is the first detailed report on the fatty acids composition and the related lipid health indices in the whole tuna roes and its cured product “bottarga.”
REFERENCES
- W. E. Conner, “Importance of n-3 in Health and Disease,” The American Journal of Clinical Nutrition, Vol. 17, No. 1, 2000, pp. 171S-175S.
- D. Li, O. Bode, H. Drummond and A. J. Sinclair, “Lipid for Functional Food and Nutriaceuticals, Omega-3 (n-3) Fatty Acid,” The Oily Press, Bridgewater, U.K., 2003.
- T. L. V. Ulbritch and D. A. T. Southgate, “Coronary Heart Disease: Seven Dietary Factors,” Lancet, Vol. 338, 1991, pp. 985-992. doi:10.1016/0140-6736(91)91846-M
- R. Bernasconi, E. Bolzacchini, G. Galliani, F. Gugliersi, B. Rindone, M. Rindone, M. T. Tacconi and A. Terraneo, “Determination of Content of Wax Esters in Some Sea Foods and Their Molecular Composition. A Comparison with ω-3 Enriched Wax Esters,” LWT―Food Science and Technology, Vol. 40, No. 4, 2007, pp. 569-573.
- A. Hara and N. S. Radin, “Lipid Extraction of Tissue with Low Toxicity Solvent,” Analytical Biochemistry, Vol. 90, No. 1, 1978, pp. 420-426. doi:10.1016/0003-2697(78)90046-5
- W. A. Christie, “Simple Procedure for Rapid Transmethylation of Glycerolipids and Cholesteryl Esters,” The Journal of Lipid Research, Vol. 23, 1982, pp. 1072-1075.
- L. Senso, M. D. Suarez, T. Ruiz-Cara and M. GarciaGallego, “On the Possible Effects of Harvesting Season and Chilled Storage on the Fatty Acid Profile of the Fillet of Farmed Gilthead Sea Bream (Sparus aurata),” Food Chemistry, Vol. 101, No. 1, 2007, pp. 298-307. doi:10.1016/j.foodchem.2006.01.036
- L. Horrocks and Y. Yeo, “Health Benefits of Docosahexaenoic Acid (DHA),” Pharmacological Research, Vol. 40, No. 3, 1999, pp. 211-225. doi:10.1006/phrs.1999.0495
- T. L. Aro, P. S. Larmo, C. Backman, H. Kallio and R. Tahvonen, “Fatty Acids and Fat-Soluble Vitamins in Salted Herring (Clupea harengus) Products,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 5, 2005, pp. 1482-1488. doi:10.1021/jf0401221
- A. B. Moreira, J. V. Visentainer, N. E. de Souza and M. Matsushita, “Fatty Acids Profile and Cholesterol Contents of Three Brazilian Brycon Freshwater Fishes,” Journal of Food Composition and Analysis, Vol. 14, No. 6, 2001, pp. 565-574. doi:10.1006/jfca.2001.1025
- P. Scano, A. Rosa, S. Mereu, C. Piras, A. Atzeri and M. A. Dessi, “Multivariate Fatty Acid and Fatty Alcohol Profile of Mullet Bottarga,” European Journal of Lipid Science and Technology, Vol. 112, No. 12, 2010, pp. 1369-1374. doi:10.1002/ejlt.201000321
- J. E. Kinsella, “Food Components with Potential Therapeutic Benefits: The n-3 Polyunsaturated Fatty Acids in Fish Oils,” Food Technology, Vol. 40, No. 2, 1986, pp. 89-97.
- M. Boscarini and R. Corio, “Gli Acidi Grassi Della Serie Omega e le Loro Funzioni,” Natural, Vol. 1, 2005, pp. 66-75.
- Y. Ozogul, F. Ozogul, “Fatty Acid Profiles of Commercially Important Fish Species from the Mediterranean, Aegen and Black Seas,” Food Chemistry, Vol. 100, No. 4, 2007, pp. 1634-1638. doi:10.1016/j.foodchem.2005.11.047
- M. T. Childs, I. B. King and R. H. Knopp, “Divergent Lipoprotein Responses to Fish Oils with Various Ratios of Icosapentaenoic and Docosahexaenoic Acids,” The American Journal of Clinical Nutrition, Vol. 52, No. 4, 1990, pp. 632-639.
- I.-C. Chen, F. A. Chapman, C.-I. Wei, K. M. Porteir and S. F. O’Keefe, “Differentiation of Cultured and Wild Sturgeon (Acipencer oxyrinchus desotoi) Based on Fatty Acid Composition,” Journal of Food Science, Vol. 60, No. 3, 1995, pp. 631-635.
- G. E. Bledsoe, C. D. Bledsoe and B. Rasco, “Caviars and Fish Roe Products,” Critical Reviews in Food Science and Nutrition, Vol. 43, No. 3, 2003. pp. 317-356. doi:10.1080/10408690390826545
- N. Shirai, T. Higuchi and H. Suzuki, “Analysis of Lipid Classes and the Fatty Acid Composition of the Salted Fish Roe Food Products, Ikura, Tarako, Tobiko and Kazunoko,” Food Chemistry, Vol. 94, No. 1, 2006, pp. 61-67. doi:10.1016/j.foodchem.2004.10.050
- G. Boran, H. Karaçam and M. Boran, “Changes in the Quality of Fish Oils Due to Storage Temperature and Time,” Food Chemistry, Vol. 98, No. 4, 2006, pp. 693- 698. doi:10.1016/j.foodchem.2005.06.041
- C. Cahu, P. Salen and M. de Lorgeril, “Farmed and Wild Fish in the Prevention of Cardiovascular Diseases: Assessing Possible Differences in Lipid Nutritional Values,” Nutrition, Metabolism & Cardiovascular Diseases, Vol. 14, No. 1, 2004, pp. 34-41. doi:10.1016/S0939-4753(04)80045-0
- K. S. Sidhu, “Health Benefits and Potential Risks Related to Consumption of Fish or Fish Oil,” Regulatory Toxicology and Pharmacology, Vol. 38, No. 3, 2003, pp. 336- 344.
- A. P. Simopoulos, “Omega-3 Fatty Acids in Health and Disease and in Growth and Development,” The American Journal of Clinical Nutrition, Vol. 54, No. 3, 1991, pp. 438-463.
- G. Mourente, C. Megina and E. Diaz-Salvago, “Lipids in Female Northern Bluefin Tuna (Thunnus thynnus thynnus L.) during Sexual Maturation,” Fish Physiology and Biochemistry, Vol. 24, No. 4, 2002, pp. 351-363. doi:10.1023/A:1015011609017
- T. Murase and H. Saito, “The Docosahexaenoic Acid Content in the Lipid of Albacore Thunnus Alalunga Caught in Two Separate Localities,” Fisheries Science, Vol. 62, No. 4, 1996, pp. 634-638.
- H. Saito and K. Ishihara, “Docosahexaenoic Acid Content of Fatty Acids in the Lipids of Two Species of Frigate Mackerel, Auxis Rocheri and Auxis Thazard,” Bioscience Biotechnology & Biochemistry, Vol. 60, No. 6, 1996, pp. 1014-1016. doi:10.1271/bbb.60.1014
- H. Saito, K. Ishihara and T. Murase, “The Fatty Acid Composition Characteristic of a Highly Migratory Fish, with Seasonal Variation of Docosahexahenoic Acid Content in Lipid of Bonito (Euthynnus pelamis),” Bioscience Biotechnology & Biochemistry, Vol. 59, No. 11, 1995, pp. 2186-2188. doi:10.1271/bbb.59.2186
- H. Saito, K. Ishihara and T. Murase, “The Fatty Acid Composition in Tuna (Bonito, Euthynnus pelamis) Caught at Three Different Localities from Tropics to Temperate,” Journal of the Science of Food and Agriculture, Vol. 73, No. 1, 1997, pp. 53-59. doi:10.1002/(SICI)1097-0010(199701)73:1<53::AID-JSFA707>3.0.CO;2-5
- M. D. Wiegand, “Composition, Accumulation and Utilization of Yolk Lipids in Teleost Fish,” Reviews in Fish Biology and Fisheries, Vol. 6, 1996, pp. 259-286. doi:10.1007/BF00122583
- R. Shródter, J. Schliemann and G. Woim, “Lipid Oxidation and Their Importance in the Formation of Meat Aroma,” Nahrung, Vol. 30, No. 8, 1986, pp. 799-808.
- R. Ramarathnan, L. Rubin and L. L. Diosady, “Studies of Meat Flavor. Qualitative and Quantitative Differences in Uncured Pork,” Journal of Agricultural and Food Chemistry, Vol. 39, No. 2, 1991, pp. 344-350. doi:10.1021/jf00002a024
- J. S. Elmore, D. S. Mottram, M. Enser and J. D. Wood, “Effect of the Polyunsaturated Fatty Acid Composition of Beef Muscle on the Profile of Aroma Volatiles,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 4, 1999, pp. 1619-1625. doi:10.1021/jf980718m
- G. Hornstra, “Lipids in Functional Foods in Relation to Cardiovascular Disease,” Lipids, Vol. 12, 1999, pp. 456- 466.
- J. D. Higgs, “The Changing Nature of Red Meat: 20 Years of Improving Nutritional Quality,” Trends in Food Science & Technology, Vol. 11, 2000, pp. 11-95. doi:10.1016/S0924-2244(00)00055-8
- A. Barra, V. L. Garau, A. Dessi, G. Sarais, E. Ceretti, M. Alorio, J. D. Coisson and P. Cabras, “Chemical Characterization and DNA Tracking of Sardinian Botargo by Mugil Cephalus from Different Geographical Origins,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 22, 2008, pp. 10847-10852. doi:10.1021/jf802363k

