Food and Nutrition Sciences
Vol. 2 No. 5 (2011) , Article ID: 5757 , 6 pages DOI:10.4236/fns.2011.25061
Evaluation of Sensory Properties of Probiotic Yogurt Containing Food Products with Prebiotic Fibres in Mwanza, Tanzania
![]()
1National Institute for Medical Research, Mwanza, Tanzania; 2Division of Food and Nutritional Sciences, Brescia University College, The University of Western Ontario, London, Canada.
Email: hekmat@uwo.ca
Received April 1st, 2011; revised May 5th, 2011; accepted May 12th, 2011.
Keywords: Probiotics, Prebiotics, Yogurt, Functional Food
ABSTRACT
Yogurt becomes a functional food upon incorporating probiotics-live microorganisms which when adequately administered confer health benefits. Prebiotics are fermentable fibres that nourish beneficial gastrointestinal microflora enhancing the functionality of probiotics. This research aimed to improve the acceptability and functionality of probiotic yogurt produced in Mwanza, Tanzania by incorporating prebiotic food ingredients. The probiotic culture Lactobacillus rhamnosus GR-1 and standard yogurt cultures Lactobacillus delbrueckii bulgaricus and Steptococcus thermophilus were used to manufacture yogurt, then locally available prebiotic food ingredients containing fructooligosaccharide/inulin were incorporated. A nine-point facial hedonic scale was used to evaluate five yogurt samples. A mean score between one and three indicated that the sample product was well accepted. Probiotic yogurt containing onions, garlic and sweet potato received a score of 1.6 ± 0.84 (p < 0.01); banana and honey was 2.5 ± 1.72 (p = 0.02); and leafy greens, onions and garlic was 2.6 ± 1.54 (p = 0.04). Samples containing beans, 4.4 ± 1.99 (p > 0.90), and plantains, 5.3 ± 2.56 (p > 0.90) were not well accepted. Sweet, mildly flavored prebiotic ingredients were most successfully incorporated into probiotic yogurt in Mwanza.
1. Introduction
Microbial fermentation of dairy products has long been recognized for its beneficial effects on human health, wellness and longevity [1]. The addition of active bacteria cultures into the diet improves digestion and nutrient absorption and also promotes food safety [2,3]. Certain microbes are capable of colonizing the lower intestine, improving gastrointestinal health and consequently enhancing immune function [2]. As a low cost technique available at the household level, fermentation can be particularly useful in areas where food security, malnutrition, food borne illness and disease resulting in immunosuppression such as HIV/AIDS, are highly prevalent [4].
The addition of specific lactic acid bacteria to milk used in the production of yogurt enhances the digestibility and nutrient value of milk. First, the active cultures used in the fermentation process metabolize the milk sugar lactose, improving tolerance to this disaccharide, notoriously difficult to digest. Secondly, yogurt manufacturing induces the denaturation of proteins, improving overall bioavailability. Thirdly, lactic acid bacteria assist in the manufacturing of several B-complex vitamins and vitamin K in vivo, and also improve the absorption of minerals such as calcium, phosphorous and magnesium [5,6].
Food safety can also be enhanced via fermentation. Lactic acid is generated by bacteria as a result of the breakdown of carbohydrates. This process effectively lowers the pH of the food product to the point where the proliferation of pathogenic microorganisms such as Staphylococcus aureus, Escherichia coli, Campylobacter jejuni, Vibrio cholera and coliform bacteria becomes inhibited [2,5]. Milk fermentation to produce yogurt also extends the shelf life of the product and allows for the maintenance of food safety outside of cold storage for a significantly longer duration of time when compared to fluid milk.
The acidic properties of yogurt further pose an optimal environment to support the viability of probiotics, “live microoganisisms which when administered in adequate amounts confer a health benefit on the host” [7]. Probiotics enhance immune function by increasing the production of immunoglobulin A and by improving the integrity of the mucosa, and strengthening intestinal tight junctions. Overall, this reduces the surface area available for the binding of pathogenic organisms, competitively inhibiting microbial colonization and translocation within the intestinal tract. Probiotics are thus able to reduce the incidence of acute gastroenteritis and chronic diarrhea, as well as hasten recovery from these debilitating ailments [8]. Probiotic supplementation has been indicated for use as both a prophylaxis and treatment for diarrhea. Probiotic supplements have been shown to be particularly useful for children as well as the immune-compromised, such as people living with HIV/AIDS [9,10].
The efficacy of probiotics can be enhanced by the addition of prebiotics, “selectively fermentable fibres which promote specific changes in the composition and/or activity of the gastrointestinal microflora, conferring benefits upon host well being and health” [11]. Onions, garlic, bananas, plantains, eggplant, dark leafy green vegetables, beans and honey each contain inulin and/or fructooligosacchardide-functional prebiotic fibres which stimulate the growth of beneficial species of lactobacilli and bifidobacteria residing in the gut [11-14]. This task is accomplished upon fermentation of prebiotic fibres in the colon, by which short chain fatty acids (SCFA) are produced. SCFA provide nourishment for the colonic microbiota and enhance the colonization of beneficial organisms by improving their survival, growth and metabolism in vivo [6,12].
Enhancing the colonization of beneficial flora not only improves host resistance to enteric pathogens, but also hastens recovery from dysentery and diarrheal disease, specifically among children [15] and people living with HIV/AIDS [16]. Beyond the immunomodulatory and antimicrobial functional properties, several prebiotic food ingredients also exhibit antifungal, antioxidant, antihypertensive, antidiabetic, anticarcinogenic and antinflammatory effects [17].
The term synbiotics is used to describe the synergistic relationship between probiotics and prebiotics [6]. Synbiotic utilization may provide significant, measurable improvements in quality of life and nutritional status in regions were food safety and food security is a concern and the prevalence of diarrheal disease and HIV infection greatly influence national morbidity and mortality rates. The increase of synbiotics consumption be an exemplary method for improving nutritional status and therefore health outcomes in developing countries.
In 2005, the Western Heads East (WHE) project was initiated as the University of Western Ontario’s (UWO) response to the HIV/AIDS endemic in Africa. Established in Mwanza, Tanzania, WHE is a partnership between UWO, the National Institute for Medical Research (NIMR), Mwanza, and the Kivulini Women’s Rights Organization, Mwanza. WHE is a grass-roots initiative in which a group of ten local women manu-facture and sell probiotic yogurt within their village and also donate a portion to community members living with HIV/AIDS. Although plain probiotic yogurt has been accepted within Mabatini, the village where yogurt production takes place, verbal discussions with villagers identified two major factors currently discouraging widespread consumption. First, the strong, sour taste of plain yogurt [18] is unfamiliar and second, a pronounced social stigma exists regarding the consumption of dairy products after infancy, a stigma previously observed in Western Africa [19].
The objective of this study was to include prebiotic food ingredients in probiotic yogurt to enhance the organoleptic properties of plain yogurt to improve consumer acceptability and to identify methods for improving the nutrient profile and possible therapeutic effectiveness of plain probiotic yogurt.
2. Materials and Methods
2.1. Preparation of Probiotic Microorganisms and Inoculation of Fluid Milk
The probiotic mother culture containing Lactobacillus rhamnosus GR-1 [20] was prepared in the laboratory facilities at NIMR. The probiotic microorganisms were added to sterile MRS broth then anaerobically incubated using BBL gas packs at 37˚C for 16 hours. Fluid milk was then inoculated with a 1% by weight sample of the probiotic broth mixture and 0.4% yeast extract. The inoculated milk was then incubated at 37˚C for 18 hours then stored at 4˚C in an airtight container prior to pickup.
2.2. Manufacturing of Probiotic Yogurt
Standardized milk with 3.25% fat was heated to 85˚C for 30 minutes, cooled to 40˚C, then an 8% by weight sample of the standard yogurt cultures Lactobacillus delbrueckii subsp. bulgaricus and Steptococcus thermophilus was used to inoculate the milk. A 4% by weight sample of the prepared probiotic strain Lactobacillus rhamnosus GR-1 was then added and the total mixture incubated at 37˚C for six hours. Table 1 presents the nutrient profile of one 200 ml serving of probiotic yogurt manufactured in the yogurt production facilities in Mabatini village, Mwanza, Tanzania [21].
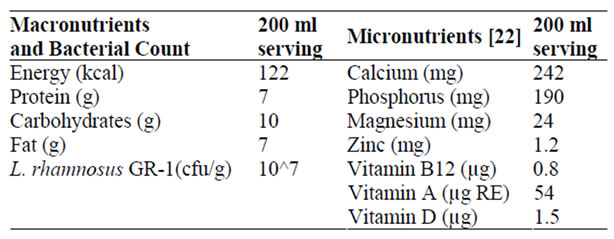
Table 1. Nutrient profile of probiotic yogurt produced in Mabatini Village, Mwanza, Tanzania.
2.3. Production of Probiotic Yogurt containing Food Ingredients with Prebiotic Fibres
Food ingredients containing prebiotic fibres were selected for incorporation into probiotic yogurt based on availability and evidence of therapeutic functionality [12, 14]. Recipe design considered local food preferences and were created to ensure ease of preparation within a household of limited resources. Ten recipes underwent preliminary testing by nine untrained panelists to determine which prebiotic food ingredients best improved the organoleptic profile of plain probiotic yogurt, of which five recipes were selected for appraisal by the sensory panelists.
2.4. Sample Preparation for Sensory Panel
Sample 1: Mashed Sweet Potato Sweet potatoes (1.5 kg) were boiled for approximately 30 minutes, then peeled and mashed. Approximately 330 g of finely chopped onions and 35 g of crushed and rubbed garlic were sautéed in 60 ml of oil on low heat for approximately 10 minutes. Once cooled, these ingredients were combined with 1000 ml of probiotic yogurt, and salt and pepper were added to taste.
Sample 2: Tropical Fruit Yogurt Four large ripe yellow bananas (450 g) and one whole papaya (350 g) were peeled and mashed then incorporated into 1000 ml of probiotic yogurt. 175 ml of locally produced, pasteurized honey was stirred into the mixture and 60 ml of honey drizzled on top of the final product prior to serving.
Sample 3: Creamy, Savory Greens A mixture of approximately 100 g each of freshly picked kale, spinach and onions were finely chopped and 30 g of garlic was crushed and rubbed, then added to 60 ml of oil in a hot pan. The ingredients were wilted and sautéed for approximately 15 minutes, salt and pepper were added to taste, then the mixture was cooled prior to adding 1000 ml of probiotic yogurt.
Sample 4: Bean Dip Dried kidney beans (360 g) and garbanzo beans (300 g) were soaked for 12 hours, boiled in salted water then drained, mashed and cooled. Roughly chopped onions (125 g) and garlic (35 g), the juice of half of one lemon (50 ml), oil (60 ml) and salt and pepper were added to taste. Finally, 1000 ml of probiotic yogurt were incorporated into the mixture.
Sample 5: Egg-Plantain Sauce Eggplant (150 g) and plantains (250 g) were cut into cubes, sautéed in hot oil (60 ml) and once soft, were mashed and cooled. Chopped raw onions (125 g) and garlic (50 g) were added, followed by 1000 ml of probiotic yogurt and salt and pepper to taste.
2.5. Panel Selection
To be eligible for participation, subjects had to be over the age of 18 years, capable of under-standing the intent of the study and able to provide informed consent. Fortyeight untrained panelists were selected to evaluate five variations of probiotic yogurt combined with prebiotic food ingredients. A total of 22 untrained male panelists and 26 untrained female panelists between the ages of 18 - 61 years old completed the sensory evaluation.
2.6. Sensory Evaluation
Sensory evaluations were privately conducted while participants were seated in a quite area behind a privacy divider in the yogurt manufacturing facilities in Mabatini village, Mwanza. Tastings occurred between the hours of 10:00 am - 2:00 pm and 4:00 pm - 8:00 pm to correspond with regular meal times. Prior to the tastings, all ingredients were disclosed to the participants to ensure cultural or religious restrictions would not be violated by participation. Each panelist was given a pen for recording on her/his evaluation sheet and a glass of water to cleanse the mouth between tastings.
A nine-point facial hedonic scale in which 1 = “liked extremely”, 5 = “neither liked nor disliked” and 9 = “disliked extremely” was used by each participant for sample evaluation. Approximately 50 ml of each sample were presented in a glass bowl with a plastic spoon. A control sample of plain probiotic yogurt was first tasted and then the remaining five samples were served in a random order. Plain probiotic yogurt was used as a control product to eliminate first order bias in which consumers may unintentionally rate a first sample more or less favorably than other samples, regardless of the product under evaluation [22]. All study procedures were in accord with the University of Western Ontario Research Ethics Board for Human Subjects and met the Brescia University College Ethical Review Committee standards.
2.7. Statistical Analysis
Results from the facial hedonic scale record sheets were collated and input into a Microsoft Excel 2007 database, then imported into the Statistical Package for Social Sciences (SPSS) version 15.0 for analysis. Mean, standard deviations and p-values were calculated for each sample. p-values less than 0.05 were considered statistically significant.
3. Results
The mean acceptability score and standard deviation for each sample are presented in Table 2. A score of 1-3 on a numerical rating scale indicated recipes that were well accepted. The control product of plain probiotic yogurt received a mean score of 2.9 ± 1.69, p = 0.04 and was generally well accepted among the group of selected panelists. Sample one containing sweet potatoes, sautéed onions and garlic received the best overall score (1.6 ± 0.84, p < 0.01) and had the least inter-subject variation. Sample two containing banana, honey and papaya and sample three containing sautéed spinach, kale, garlic and onions were also well received (2.5 ± 1.97, p = 0.03; 2.9 ± 1.75, p = 0.04, respectively). Sample four containing kidney beans, garbanzo beans, raw onions and garlic, and sample five containing plantains, raw onions and garlic were liked slightly (4.4 ± 1.99, p > 0.90) and neither liked nor disliked (5.3 ± 2.56, p > 0.90) respectively.
4. Discussions
Food ingredients containing prebiotic fibres can successfully be incorporated into plain probiotic yogurt and can increase consumer acceptability in Mwanza, Tanzania. Overall, participants preferred yogurt products which were sweet or subtly flavored and those which maintained a smooth, creamy, homogenous texture. For samples one and three, the prebiotic ingredients onions and garlic were caramelized prior to incorporation into yogurt, yielding a sweet taste which decreased the acidic, sour flavor produced during fermentation. The sweet attributes elicited a pleasant aroma, flavor and color and likely influenced the positive mean acceptability score. Samples four and five containing raw onions and garlic
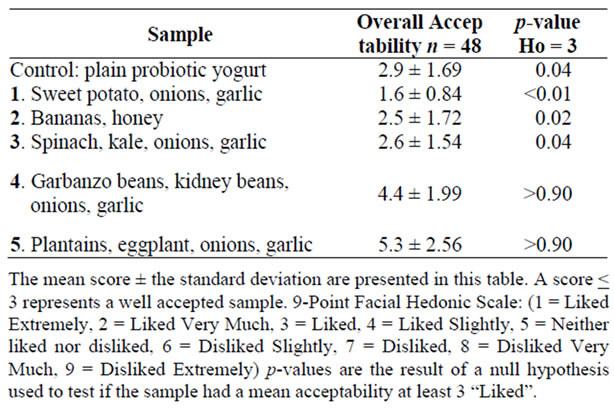
Table 2. Recipe scoring results for various samples.
were pungent flavored and were not as well received. In the future, these prebiotic food ingredients should be prepared as in samples one and three then re-tested to evaluate acceptability.
Several participants mentioned sample two containing tropical fruits would be very well accepted by children. This is a finding to be ruminated for several reasons, primarily because it is well known that diarrhea represents a high burden of disease amongst children in low-income countries. It is the third leading cause of death in the developing world overall and accounts for over half of all child deaths in sub-Saharan Africa annually [23,25]. Furthermore, vitamin and mineral deficiencies including calcium, vitamin D, iron, vitamin B12 and folate are substantial causes of rickets, poor bone mineralization, poor muscle and brain development in children in sub-Saharan Africa [24,25]. Yogurt, providing a substantial amount of protein and easily absorbable micronutrients is an optimal source of nutrition for children. Additional synbiotic components may also mitigate the risk of morbidity and mortality associated with diarrhea in childhood, making synbiotic foods particularily advantageous for this age cohort.
Previous health based research conducted within this community and affiliated with the WHE yogurt project demonstrated that probiotic yogurt consumption is associated with a reduced incidence of diarrhea and increased immune function measured by CD4+ cell count among people living with HIV/AIDS [26]. In the future, it will therefore be beneficial to evaluate the health impacts of synbiotic yogurt produced in Mwanza among people living with HIV/AIDS to determine the significance of the correlation between synbiotic yogurt consumption and selected health status indicators.
The findings from this study can be applied within Mwanza and in communities of similar economic status as a low-cost method to improve dietary quality, gastrointestinal health and to strengthen immune function. Fermentation is an indigenous practice to many African nations and can be used to enhance the cultural acceptability of dairy products. Introducing beneficial bacterial cultures to the diet in a fashion compatible with local food preferences may significantly impact quality of life in this community and beyond. Moreover, the establishment of a microenterprise based on nutritional interventions has the added benefit of capacity building, provides financial security for members within the community and promotes a resonating sense of empowerment for those manufacturing this nutritious carrier.
There are limitations to the scope of the future implications of this research. Due to the yogurt projects affiliations with the UWO and NIMR, probiotic cultures are supplied to the yogurt manufacturing facility in Mwanza free of charge. The synthesis of these probiotic cultures requires nutrient broth and yeast extract for proliferation, both of which are not readily available or affordable for members of the general public. Consuming regular yogurt, however, is still advantageous in terms of nutrient provision and food safety [1-4]. The further addition of prebiotic ingredients will favor the growth of beneficial intestinal microflora, impeding the growth of pathogenic organisms [13] regardless of prebiotic-probiotic synergism.
Devising affordable, sustainable methods to improve food safety, food security and nutritional status in developing countries is a priority in line with the World Health Organizations Millennium Development Goals, especially goals numbers one, four, six and eight which relate to combating malnutrition, child mortality and HIV/ AIDS and to the establishment of global partnerships for development, respectively [23]. Grassroots initiatives such as that WHE project is an excellent model for a successful and sustainable nutrition intervention that further provides financial security, community empowerment and facilitates knowledge transfer.
5. Conclusions
Food ingredients containing prebiotic fibres can successfully be incorporated into plain probiotic yogurt and can increase consumer acceptability in Mwanza, Tanzania. Prebiotic food ingredients which reduce the sour taste of plain yogurt and elicit sweet flavors appear to be the most promising means of increasing probiotic yogurt consumption within this community.
REFERENCES
- J. M. Conly and L. B. Johnston, “Coming Full Circle: From Antibiotics to Probiotics and Prebiotics,” The Canadian Journal of Infectious Disease and Medical Microbiology, Vol. 15, No. 3, 2004, pp. 161-163.
- C. Duggan, J. Gannon and W. A. Walker, “Protective Nutrients and Functional Foods for the Gastrointestinal Tract,” American Journal of Clinical Nutrition, Vol. 75, No. 5, 2002, pp. 789-808.
- P. Mensah, “Fermentation-The Key to Food Safety Assurance in Africa?” Food Control, Vol. 8, No. 5-6, 1997, pp. 271-278. doi:10.1016/S0956-7135(97)00020-0
- O. B. Oyewole, “Lactic Fermented Foods in Africa and their Benefits,” Food Control, Vol. 8, No. 5-6, 1997, pp. 289-297. doi:10.1016/S0956-7135(97)00075-3
- S. Hekmat and L. Koba, “Fermented Dairy Products: Knowledge and Consumption,” Canadian Journal of Dietetic Practice and Research, Vol. 67, No. 4, 2006, pp. 199-201. doi:10.3148/67.4.2006.199
- M. de Vrese and J. Schrezenmeir, “Probiotics, Prebiotics and Synbiotics,” Advances in Biochemical Engineering/Biotechnology, Vol. 111, 2008, pp. 1-66. doi:10.1007/10_2008_097
- Food and Agricultural Organization and the World Health Organization (FAO/WHO), “Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria,” 2009. http://www.who.int/entity/foodsafety/publications/fs_management/en/probiotics.pdf
- S. Guandalini, “Probiotics for Children with Diarrhea: An Update,” Journal of Clinical Gastroenterology, Vol. 42, 2008, pp. S53-S57. doi:10.1097/MCG.0b013e3181674087
- K. Anukam and G. Reid, “Providing Probiotics to SubSaharan Africa: Ethical Principles to Consider,” Journal of Complementary and Integrative Medicine, Vol. 2, No. 1, 2005, Article 10. doi:10.2202/1553-3840.1015
- G. Reid, S. Anand, M. O. Bingham, G. Mbugua, T. Wadstrom, R. Fuller, K. Anukam and M. Katsivo, “Probiotics for the Developing World,” Journal of Clinical Gastroenterology, Vol. 39, No. 6, 2005, pp. 485-488. doi:10.1097/01.mcg.0000165648.32371.38
- M. Roberfroid, “Prebiotics: The Concept Revisited,” The Journal of Nutrition, Vol. 137, 2007, pp. S830-S837.
- J. L. Buttriss and C. S. Stokes, “Dietary Fibre and Health: An Overview,” Nutrition Bulletin, Vol. 33, No. 3, 2008, pp. 186-200. doi:10.1111/j.1467-3010.2008.00705.x
- G. R. Gibson, “Prebiotics as Gut Microflora Management Tools,” Journal of Clinical Gastroenterology, Vol. 42, Suppl. 2, 2008, pp. S75-S79. doi:10.1097/MCG.0b013e31815ed097
- Z. Zdunczyk, “Physiological Effect of Low Digestible Oligosaccharides in Diets for Animals and Humans,” Polish Journal of Food and Nutrition Sciences, Vol. 13, No. 54, 2004, pp. 115-130.
- G. H. Rabbani, S. Ahmed, M. F. Hossain, R. Islam, F. Marni, M. Akhtar and N. Majid, “Green Banana Reduces Clinical Severity of Childhood Shigellosis: A DoubleBlind, Randomized, Controlled Clinical Trial,” Pediatric Infectious Disease Journal, Vol. 28, No. 5, 2009, pp. 420-425. doi:10.1097/INF.0b013e31819510b5
- G. Reid, M. E. Sanders, H. R. Gaskins, G. R. Gibson, A. Mercenier, R. Rastall, M. Roberfroid, I. Rowland, C. Cherbut and T. R. Klaenhammer, “New Scientific Paradigms for Probiotics and Prebiotics,” Journal of Clinical Gastroenterology, Vol. 37, No. 2, 2003, pp. 105-118. doi:10.1097/00004836-200308000-00004
- A. M. E. El-Arab, S. M. Girgis, E. M. Hegazy and A. B. A. El-Khalek, “Effect of Dietary Honey on Intestinal Microflora and Toxicity of Mycotoxins,” BMC Complementary and Alternative Medicine, Vol. 6, 2006, pp. 1-13. doi:10.1186/1472-6882-6-1
- D. L. Barnes, S. J. Harper, F. W. Bodyfelt and M. R. McDaniel, “Prediction of Consumer Acceptability by Sensory and Analytical Measures of Sweetness and Sourness,” Journal of Dairy Science, Vol. 74, No. 11, 1991, pp. 3746-3754. doi:10.3168/jds.S0022-0302(91)78566-4
- C. P. Nana, I. D. Brouwer, N. M. Zagre, F. J. Kok and A. S. Traore, “Community Assessment of Availability, Consumption, and Cultural Acceptability of Food Sources of (pro)vitamin A: Toward the Development of a Dietary Intervention Among Preschool Children in Rural Burkina Faso,” Food and Nutrition Bulletin, Vol. 26, No. 4, 2005, pp. 356-365.
- S. Hekmat and G. Reid, “Survival of Lactobacillus Reuteri RC-14 and Lactobacillus Rhamnosus GR-1 in Milk,” International Jouornal of Food Science & Technology, Vol. 42, No. 5, 2007, pp. 615-619.
- Z. Lukmanji, E. Hertzmark, N. Mlingi, V. Assey, G. Ndossi and W. Fawzi, “Tanzania Food Composition Tables,” Tanzania Food and Nutrition Centre and the Harvard School of Public Health, Dar es Salaam, Tanzania and Boston, 2008.
- C. C. Meilgaard, “Sensory Evaluation Techniques 3rd Edition,” CRC Press, Boca Raton, 1999. doi:10.1201/9781439832271
- World Health Organization, “WHO Health and Millennium Development Goals,” 2009. http://www.who.int/mdg/en/.
- G. T. Keusch, O. Fontaine, A. Bhargava, C. Boschi-Pinto, E. Bhutta, J. Rivera, J. Chow, S. Shahid-Salles and R. Laxminarayan, “Disease Control Priorities in Developing Countries 2nd Edition,” Oxford University Press, New York, 2006.
- J. Bryce, C. Boschi-Pinto, K. Shilbuya and R. E. Black, “WHO Estimates of the Causes of Death in Children,” Lancet, Vol. 365, No. 9465, 2005, pp. 1147-1152. doi:10.1016/S0140-6736(05)71877-8
- S. L. Irvine, R. Hummelen, S. Hekmat, C. W. N. Looman, J. D. F. Habbema and G. Reid, “Probiotic Yogurt Consumption is Associated with an Increase of CD4 Count Among People Living with HIV/AIDS,” Journal of Clinical Gastroenterology, Vol. 44, No. 9, 2010, pp. 201-205. doi:10.1097/MCG.0b013e3181d8fba8

