Modern Research in Inflammation
Vol.3 No.3(2014), Article ID:49190,11 pages
DOI:10.4236/mri.2014.33016
Enhancement of Gingival Wound Healing by Local Application of Silver Nanoparticles Periodontal Dressing Following Surgery: A Histological Assessment in Animal Model
Ghanbari Habiboallah1, Zakery Mahdi2, Zakeri Majid2*, Saghravanian Nasroallah3, Amir Moeen Taghavi1, Ali Forouzanfar1, Nooshin Arjmand2
1Department of Periodontics, School of Dentistry and Dental Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
2School of Dentistry and Dental Research Center, Mashhad University of Medical Science, Mashhad, Iran
3Department of Oral and Maxillofacial Pathology, School of Dentistry and Maxillofacial Diseases Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Email: *zakeri1987@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 June 2014; revised 20 July 2014; accepted 18 August 2014
Abstract
Background: The value of periodontal dressing and its effects on periodontal wound healing have been questioned. Besides, the early healing of the gingival and periodontal wounds in oral cavity should be emphasized to promote the treatment outcome. Several studies pointed out a potential positive effect of silver nanoparticles (NPs) on the healing process of skin wounds. The present work aimed to design silver NPs periodontal dressing and then, evaluated its effects on gingival wound healing following operation. Methods: Thirty healthy adolescent female rabbits were used in this study. They had intact teeth and the clinical and radiographic examination revealed no periodontal disease. Silver NPs were added to carbonate methyl cellulose as base with two different concentrations of 25% v/v (Group A) and 50% v/v (Group B). On the basis of simple randomized allocation, gingivectomy was performed at the buccal gingiva of the left and right mandibular incisors and subsequently, untreated wounds in the control area were covered with periodontal pack and periodontal dressings A and B were applied in the test regions. Histological changes were monitored in day 4 and 7 post operation to evaluate the inflammatory and repair stage of healing process. Results: We observed significant difference in the inflammatory and repair parameters of the healing process between surgical sites treated with this periodontal dressing and control group. Besides, wounds treated with silver NPs dressing in general terms had all the hallmarks of relatively complete healing one week post operatively. Conclusion: The results indicated positive potential therapeutic effects for this new formulation of periodontal dressing on acceleration of surgical wound healing that led to improvement of periodontal treatment consequences following operation.
Keywords:Silver Nanoparticles, Wound Healing, Dressing, Periodontal Pack, Wound Dressing, Gingivectomy

1. Introduction
As a new emerging technique in the nanotechnology industry, the silver nanoparticles (NPs) are regarded as the fastest growing product in the market such as wound dressings, contraceptive devices, surgical instruments and bone prostheses [1] -[3] . The results of previous studies suggested that silver NPs had a potent anti-inflammatory effect and could accelerate wound healing [4] [5] .
In addition, oral cavity environment is full of pathogenic and opportunistic bacteria that are favorable for acute and chronic infections in the presence of any cut or wound in the epithelium. Without controlling the microbial activity, the regenerative, respective surgeries and oral implants fail [6] . Due to the vital important effect of silver-coated dressing in burn wound regeneration and their antiseptic properties, such biological dressings play a significant role in the treatment of burnt skin [7] -[10] . Min et al. showed that the application of silk fibroin spongy wound dressing (SFSD) incorporated with silver NPs promoted the healing process of full-thickness skin defects of dorsal side of rabbits suggested that SFSD may provide a good component for the development of new wound dressings [11] .
Antimicrobial properties of silver NPs dressing have already been established because the silver ions inhibit bacterial growth through certain procedure [12] . Recently Singh et al. found that polyvinyl pyrrolidone alginate hydrogels containing silver NPs with efficient fluid handling capacity and antimicrobial activity are suitable to use as biological wound dressing [13] .
On the other hand, after the completion of surgical periodontal procedure, the area is covered with a surgical pack. The pack minimizes the likelihood of post-operative infection and hemorrhage and facilitates the healing process by preventing surface trauma during mastication [14] . In general, dressing has no curative properties; however, they assist healing by protecting the tissue rather than providing “healing factor” [15] . In some cases, the packs increase plaque formation [16] and irritate the healing tissue [17] and thus, cause little damage to the periodontium as result of inflammation. However, efforts should be directed to enhance the repair of mucosa wounds particularly in oral cavity and consequently reestablish function and esthetic in due course as well as less discomfort and pain for patients.
The overall results of previous studies indicated a relatively beneficial effect for biomaterials containing silver NPs particularly in skin wound healing. Thus, in the first step of the present work, we aimed to make silver NPscoated periodontal dressing to improve the healing process of mucosal wounds in oral cavity particularly traumatized soft and hard tissue of periodontium following surgery. Subsequently, in the second step, we established a preliminary assessment to evaluate the effects of this periodontal dressing on inflammatory and repair stages of gingival wound healing based on histological observation in animal model.
2. Methods and Materials
2.1. Animals
The procedures and protocol design described here were completely in accordance with the guidelines and regulations of the Animal Research Center of Mashhad Medical Science. Thirty healthy white adolescent female rabbits (2500 ± 300 g body weight) were used in this study. They had intact teeth and the clinical and radiographic survey showed no pervious periodontal disease. Pre-study preparation included scaling and daily plaque control to obtain gingival health.
2.2. Materials
High Shear Mixer (Ultra-high Shear Mixer, Charles Ross & Son Company, USA) was used to homogenize and uniform the base and complementary materials such as carbonate methyl cellulose (CMCCAS No. 9000-11-7, Zhengzhou Sinbis Industrial Products Ltd., China) as absorbent for silver NPs so as to create dressing particles of even dispersion in different parts. It is important to note that this reduction of “particle size” refers to the breakdown of agglomerates and not necessarily of individual particles. Typically, the primary particles retain their integrity and the measured size distribution narrows down as a result of the break-up of agglomerates. Silver was turned into the nanoparticles through chemical reduction method (Imen Daroo Company, Khorasan Science and Technology Park, Iran) and then, were added to the base. This mentioned process was performed by a small laboratory-scalereactor that guaranteed the isolated environment. Besides, the thermal resistance of dressing was measured in order to prevent silver particles oxidization. Silver NPs was added to the base with two different concentrations of 25% v/v (Periodontal Dressing A) and 50% v/v (Periodontal Dressing B) in order to evaluate the effect of different silver NPs dosages on the healing process and obtain the optimal concentration.
2.3. Surgical Protocol
All surgical procedures were performed under general an esthesia in sterile conditions. Medetomidine hydrochloride (0.05 mg/kg, Dormitor, Orion Corporation, Espoo, Finland) was administered intramuscularly as a premedication. General anesthesia was achieved using intravenous sodium thiopental injection (25 mg/kg, Ravonal, Tanabe. Osaka, Japan), and spontaneous breathing was monitored. Routine dental infiltration anesthesia (lidocaine hydrochloride 2%, 36 mg in each cartridge, with epinephrine 1:80,000, Astra Pharmaceuticals, Westborough, MA) was used at the surgical sites. Gingivectomy was performed at the buccal gingiva of the left and right mandibular incisors teeth On the basis of a simple randomized allocation, untreated wounds in the control areas were covered with periodontal pack (Coe-PACK, GC, Japan) and the nanosilver-coated periodontal dressing A and B were applied in test regions as well. This study was conducted in a double blind manner because both the researchers and the pathologist were not aware of silver nanoparticles dosage in periodontal dressing. In order to eliminate any effect of the materials on each others, a minimum space was considered between the above mentioned areas. An aluminum foil with 0.2 mm thickness (Toos Corporation, Mashhad, Iran) was placed on the pack as well as dressing and was sutured (Silk 0_3, 20 mm, Reverse cutting, Gore & Associated Inc., Flagstaff, AZ) so as to prevent the removal of pack from the region of surgery.
2.4. Wound Management
A soft diet (Pedigree, KalKan Foods Inc., Vernon, CA) was chosen to reduce potential mechanical interference with healing during food intake. Plaque control was maintained daily during healing phase by swabbing the teeth with chlorhexidine (chlorhexidine gluconate 20%, ICI Pharmaceutical Group, Wilmington, DE; 40 ml of a 2% solution).
2.5. Preparation of Histological Specimens
While the rabbits were under general anesthesia, biopsies were taken from healing buccal gingivae surrounding the teeth in the regions where gingivectomy was done of 15 rabbits at day 4 and 15 remaining ones at day 7 after surgery. Samples were fixed in 10% neutral formalin and embedded in paraffin (Paraffin tissue processor HMP 110, Microm International, Heidelberg, Germany). Sections of 4 µm thickness were stained with hematoxylin and eosin and evaluated under light microscopy (Eclipse, E800, Nikon, Japan).
2.6. Histologic Analysis
The parameters used for evaluation were inflammatory cells count, necrosis, acute hemorrhage, chronic hemorrhage and edema as the inflammatory parameters and neovascularization, fibroblast proliferation, collagen density and epithelialization as the repair parameters of the healing process [18] .
Each of the parameters was scored from 0 to 3, with 0 = normal, 1 = mild increase, 2 = moderate increase, 3 = marked increase. Sparsely scattered neutrophils, plasma cells, macrophages, eosinophils and mast cells arranged in a random fashion were considered normal. Localization of 3 - 10, 11 - 30, or 31 or more cells in the wound tissue per 400× magnification field was considered a mild, moderate, and marked increased, respectively.
Sparsely scattered necrotic cell debris, extravasated erythrocytes (indicative of acute hemorrhage), and haemosiderin-laden macrophages (indicative of chronic hemorrhage) were considered a mild increase for each of these parameters. Focal dense accumulations of these components in the wound tissue were considered a moderate increase. Extensive tissue necrosis, massive hemorrhage involving the surrounding tissue, and the presence of greater than 30 haemosiderin-laden macrophages per 400× magnification fields were considered a marked increase for each of these parameters, respectively.
Slight separation of cells and collagen from one another in the wound tissue by non-staining or poorly staining acellular material was considered a mild edema. Separation of 30 - 50 µm by this acellular material was considered a moderate edema. While separation of greater than 50 µm was considered a marked edema.
Fibroblasts and collagen organized in a random pattern, typical of the normal connective tissue of gingiva, were considered normal. Localization of fibroblasts and capillary buds in the wound tissue in number of 3 - 10, 11 - 30, and greater than 31 per 400× magnification field were considered a mild, moderate, and a marked increase, respectively, for fibroblast and neovascularization. Scant collagen bundles slightly separating fibroblast in the wound tissues were considered a mild increase in collagen density. Somewhat dense accumulation of collagens between fibroblasts in the wound tissues was considered a moderate increase in collagen density. Extensive separation of fibroblast by abundant collagen bundles was considered a marked increase in collagen density.
Regeneration and migration of epithelial cells from wound margin was considered a mild epithelialization. Approach of regenerated epithelial cells that migrated from wound margin, was considered a moderate epithelialization. Complete continuity regenerated epithelial cells that covered the wound surface and formed intercellular bridge, was considered a marked epithelialization.
2.7. Statistical Analysis
All samples were evaluated for one parameter before proceeding to the next which allowed for more accurate comparison of individual parameters. Mean scores were used for objective evaluation of histological changes. The data was statistically analyzed by Kruskal-Wallis test using commercial software (GraphPad Prism Version 4, GraphPad Software, San Diego, CA). Differences were considered to be staisticaly significant at P < 0.05.
3. Results
3.1. Statistical Finding
The result of this study showed that the local application of this novel periodontal dressing promote the healing process remarkably in comparison with control group. Scores of all inflammatory and repair parameters suggested a statistically meaningful differences at both days 4 and 7 between the control group and the regions in which periodontal dressings A and B were applied (Table 1 & Table 2). On comparing the healing process of surgical sites treated with periodontal dressings A with B in order to achieve the optimal concentration of silver NPs, we observed significant statistically differences between the edema (P = 0.034) and collagen density (P = 0.05) at day 4 so that more new collagens formed in the regions treated with periodontal dressing A than dressing B while the amount of edema was noticeable lower in group A (Table 1). However; there was a marginal difference of all parameters between dressings A and B one week following operation (Table 2).
3.2. Histological Observation (Day 4)
Histological analysis of untreated wounds of control group at day 4 revealed abundant infiltration of inflammatory cells in the connective tissue and particularly in gingival epithelium as exocytose, limited amount of vessels buds, extensive edema and poor repair parameters (Figure 1). However, we observed relatively moderate inflammatory response with diffused chronic inflammatory cells among the gingival epithelial rete processes and in connective tissue, relatively marked new collagen synthesis with irregular orientation for wounds treated with periodontal dressing A and B (Figure 2 & Figure 3). Neovascularization in the first post-operative days results in migration of inflammatory cells for cleaning necrotic remains and releasing some mediators to activate synthesis of extracellular matrix. Our study demonstrated that neovascularization was more prominent in regions treated with particularly periodontal dressing A as well as dressing B in comparison with control group.
Table 1. Histopathologic parameters for surgical wound at day 4.
Number in parentheses = standard error, Cell Coun = Cell Count, Edema = Edema, Nec = Necrosis, Fibro = Fibroblast, Neovas = Neovascularization, Coll Den = Collagen Density, Epithe = Epithelialization.
Table 2. Histopathologic parameters for surgical wound at day 7.
Number in parentheses = standard error, Cell Coun = Cell Count, Edema = Edema, Nec = Necrosis, Fibro = Fibroblast, Neovas = Neovascularization, Coll Den = Collagen Density, Epithe = Epithelialization.
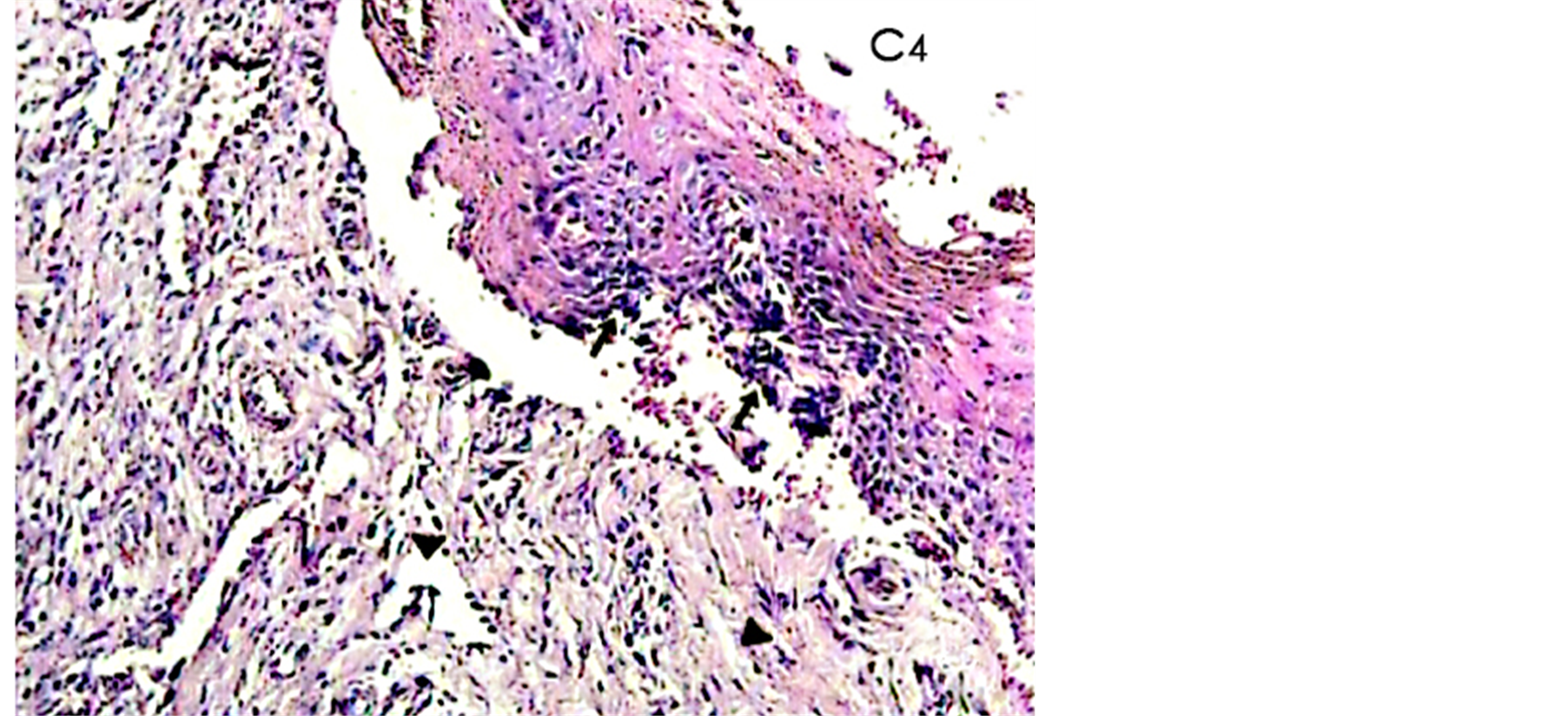
Figure 1. A histological photomicrograph of hematoxylin and eosin stained sections of surgical wound at day 4 after the operation. (Control, C4) Untreated wounds of control group suggested arelatively abundant infiltration of inflammatory cells in epithelial layer (arrow) with extensive edema (arrow heads) and poor repair parameters (original magnification 10×).
On the other hand, the amount of inflammatory parameters particularly edema was marginally higher in sites treated with dressing B compared with dressing A. abundant new vessels buds and collagen synthesis directly below the gingival epithelium was apparent in region where dressing A were applied which probably results from the higher percentage of silver NPs in comparison with dressing B. However, wounds covered by dressing B showed mild proliferated epithelial cells on the wound surface.
3.3. Histological Observation (One Week Post-Operatively)
In the histological survey at day 7, in the control group, we observed moderate to severe chronic inflammatory
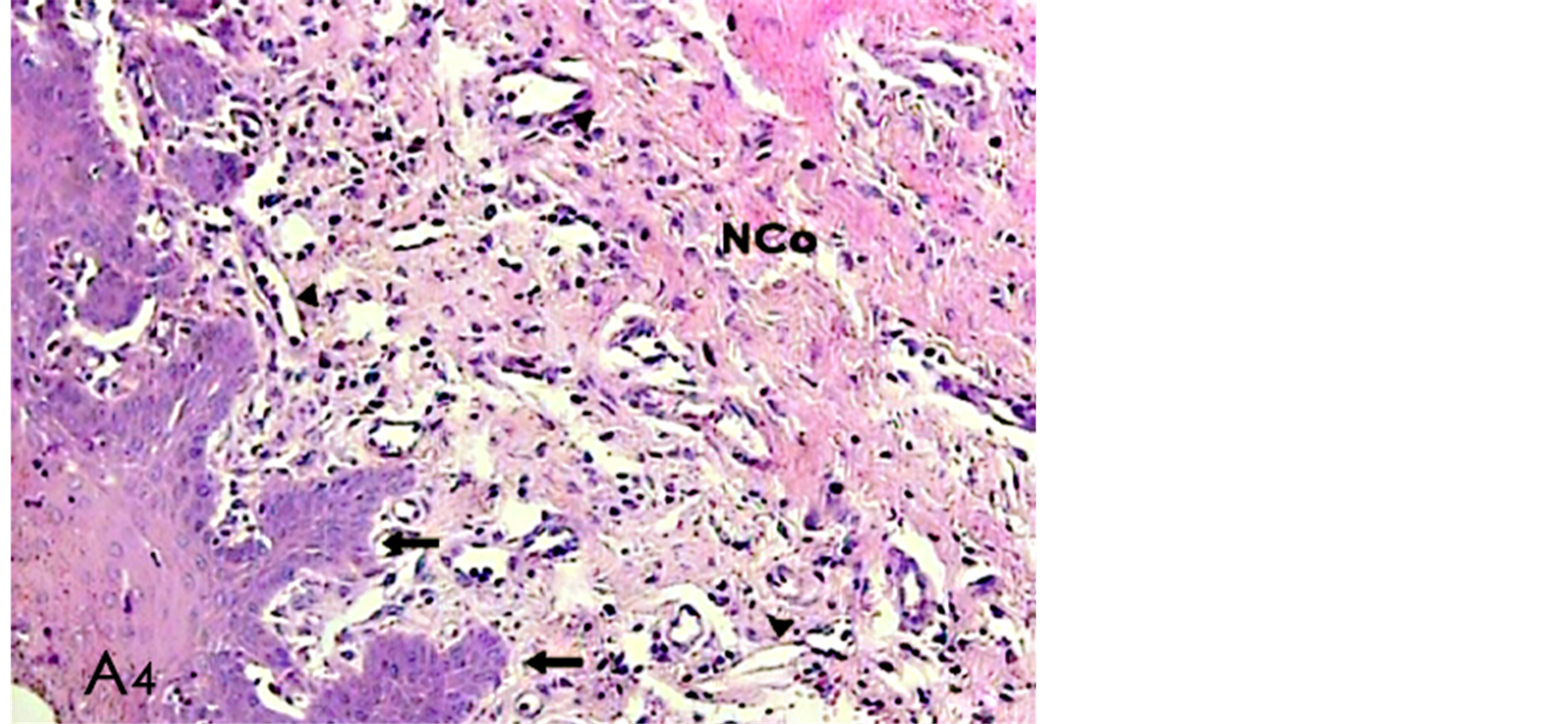
Figure 2. A histological photomicrograph of hematoxylin and eosin stained sections of surgical wound at day 4 after the operation. (A4) Surgical sites treated with periodontal dressing A reveals scant inflammatory cells with epithelial reaction to inflammatory response (arrows) and moderate new collagen synthesis (NCO) with irregular orientation; blood vessels (arrow heads) (original magnification 10×).
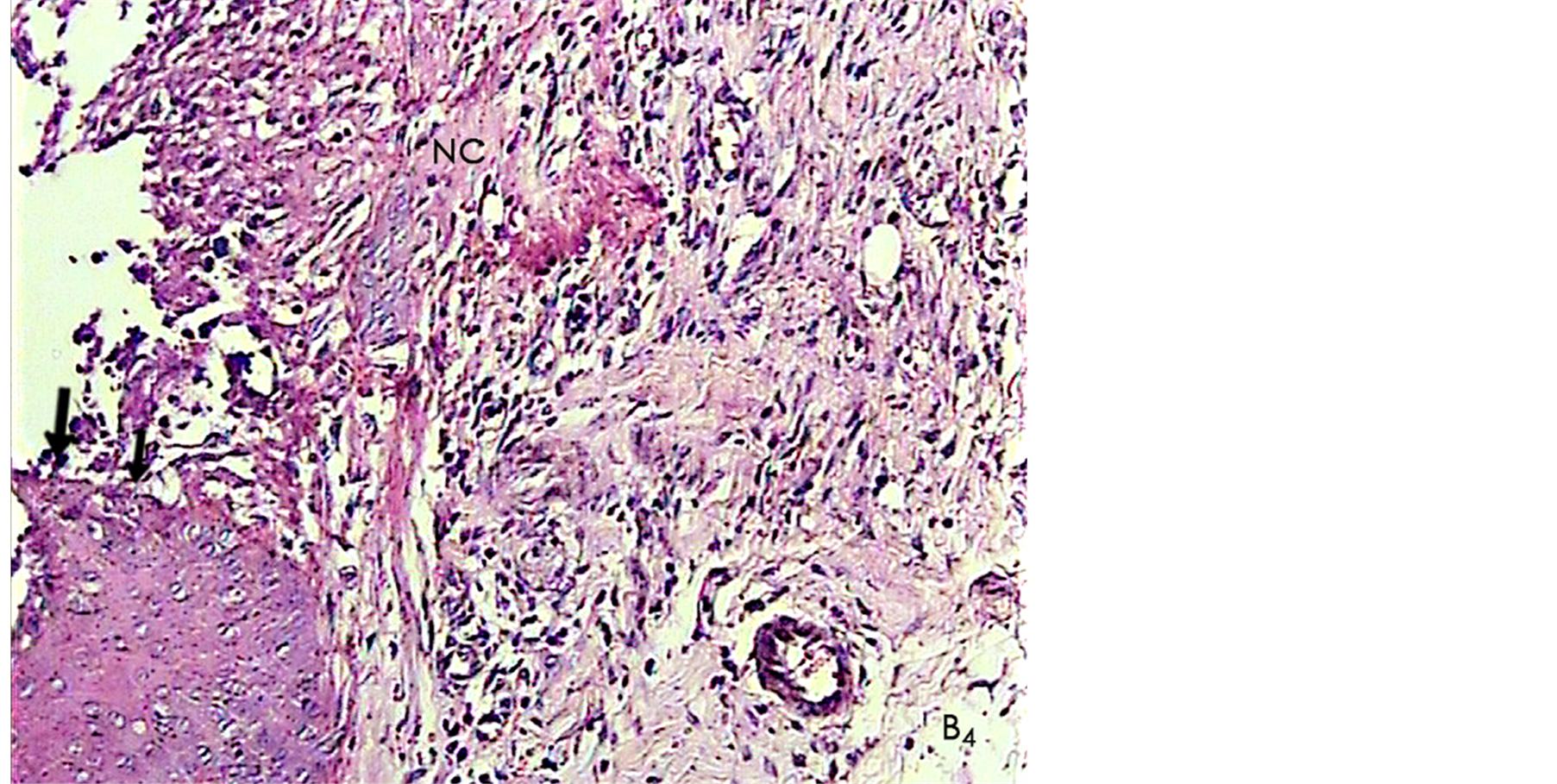
Figure 3. A histological photomicrograph of hematoxylin and eosin stained sections of surgical wound at day 4 after the operation. (B4) Wounds treated with periodontal dressing B reveals moderate inflammatory response particularly deep in connective tissue and scant new vessel blood; arrows indicate the epithelial wound marginin which the continuity of epithelial layer was interrupted (original magnification 10×).
cells accumulation particularly in lamina properia directly below the gingival epithelium and deep in the connective tissue with moderate tissue disruption as well as edema, weak scaffold of collagen fibers which generally indicated relatively poor healing that seemed insignificant compared with day 4 (Figure 4). In addition, wounds treated with periodontal dressing A presented are latively mild persisting inflammatory response with moderate repair parameters in comparison with those surgical sites which periodontal dressing B were applied (Figure 5).
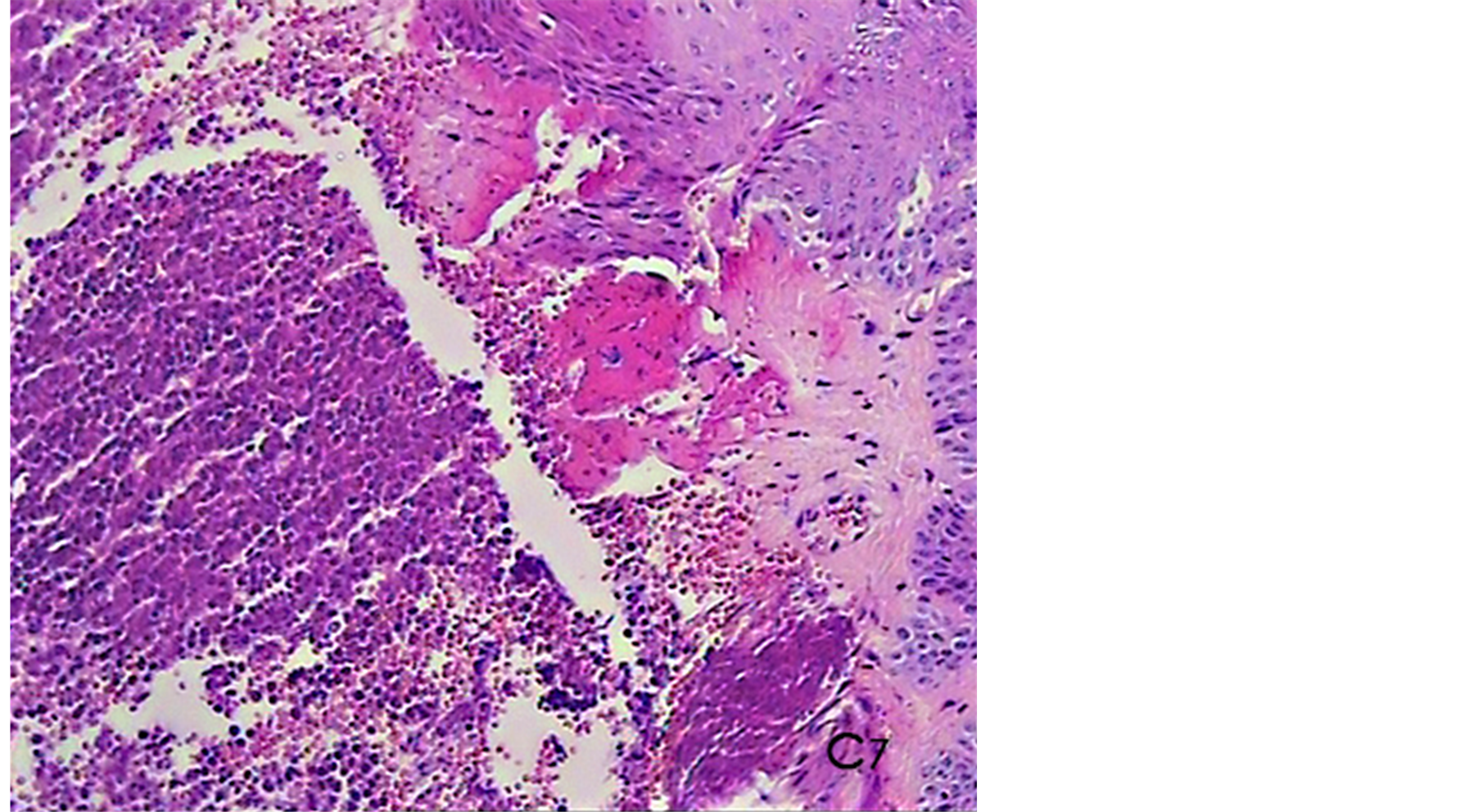
Figure 4. A histological photomicrograph of hematoxylin and eosin stained sections of surgical wound one week post-operatively. (Control, C7) Gingival untreated sites showing moderate to severe persisting inflammatory response particularly in connective tissue with insignificant signs of repair parameters that reveal relatively poor healing (original magnification 10×).
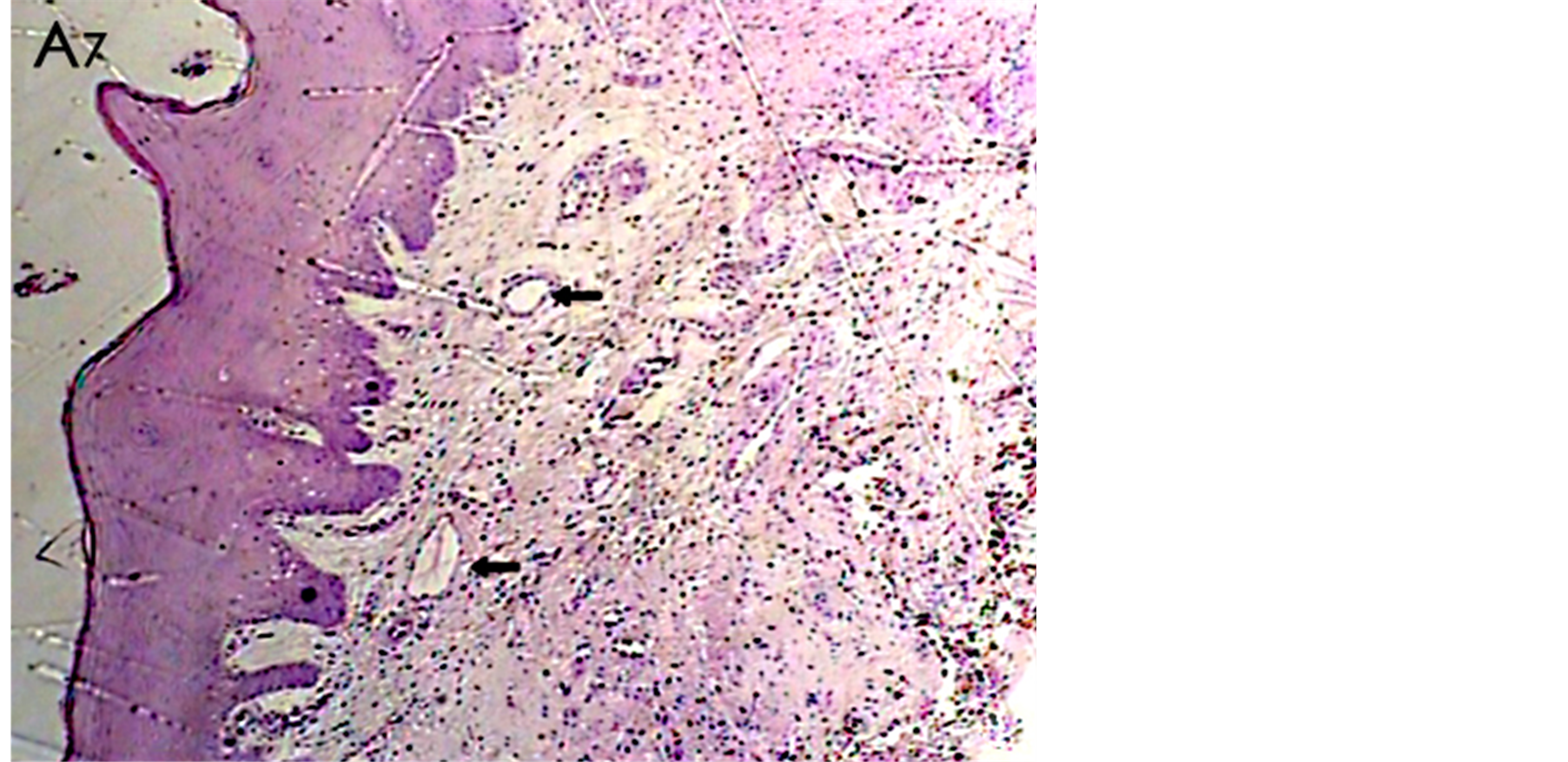
Figure 5. A histological photomicrograph of hematoxylin and eosin stained sections of surgical wound one week post-operatively. (A7) Mild persisting inflammatory response with moderate repair parameters,new blood vessels (arrows) observed in periodontal dressing Agroup (original magnification 4×).
In the group B, we observed poor signs of inflammatory response in the connective tissue with sparsely scattered chronic inflammatory cells infiltration, dense accumulation of relatively well-aligned new collagen fibers and relatively complete integrity between the epithelial cells with moderate thickness (Figure 6). Our result suggested that wounds treated with this novel periodontal dressing in general terms has all the hallmarks of relatively complete healing one week post operatively.
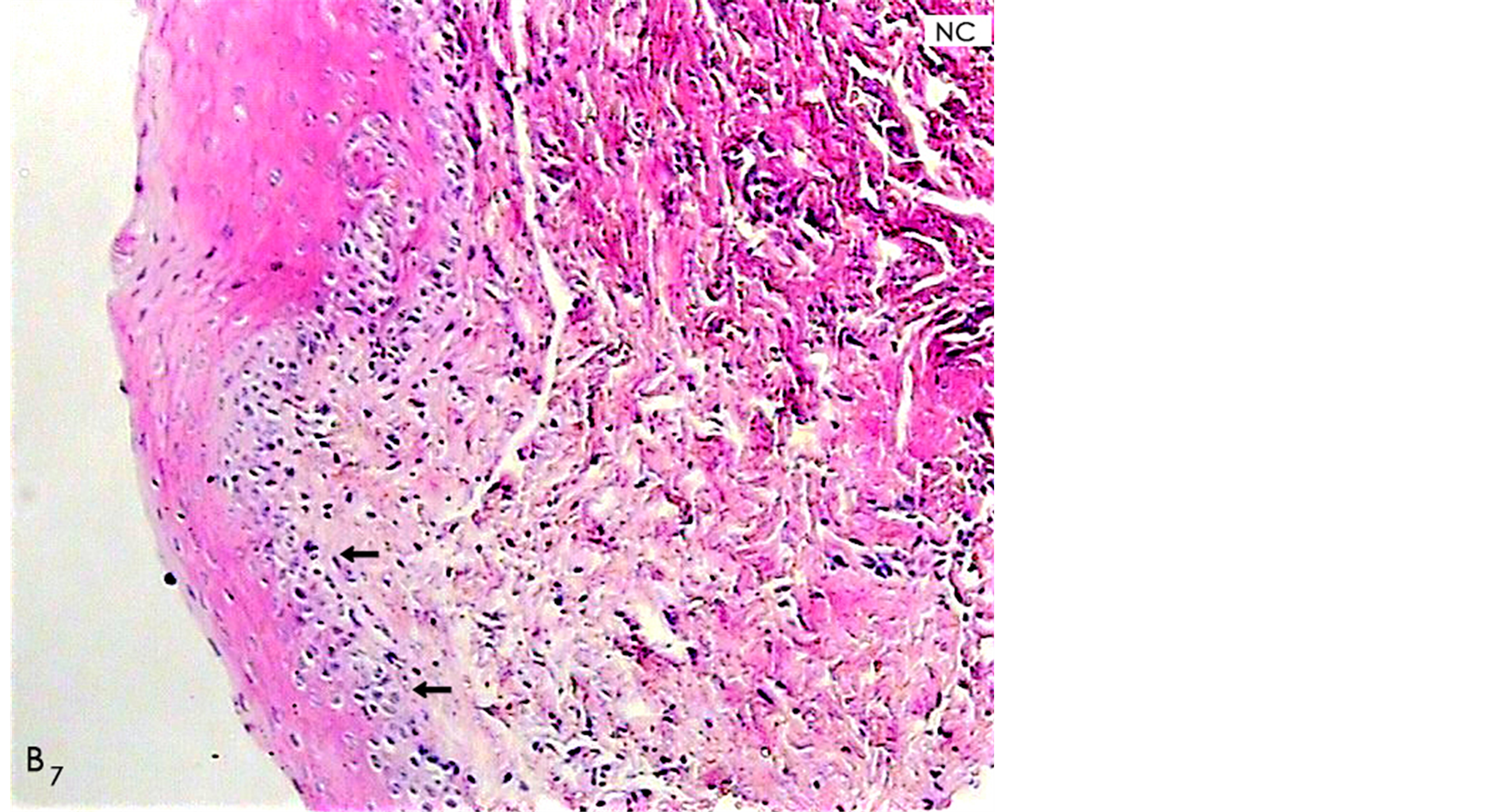
Figure 6. A histological photomicrograph of hematoxylin and eosin stained sections of surgical wound one week post-operatively. (B7) Wounds treated with periodontal dressing B shows poor signs of inflammatory response, well-aligned new collagens (NC) and particularly formation of Epithelial layer with relatively normal thickness as a moderate epithelializationthat in general terms has all the hallmarks of complete healing (original magnification 10×).
4. Discussion
Wound healing involves a sequence of molecular and cellular events including inflammation, cell migration, angiogenesis, extracelluler matrix synthesis, and re-epithelialization. Principally, inflammation is a protective response to eliminate the initial cause of cell injury as well as necrotic cells and tissues resulting from the original insult; however, inflammation is an integral stage of wound healing process. The inflammatory response is terminated when the injuries stimulus is removed and the inflammatory mediators have been dissipated, catabolized or inhibited. Recruited inflammatory cells not only clean up the necrotic debris but also elaborate mediators that drive the synthesis of new extracellular matrix (ECM). Thus, healing begins very early in the process of inflammation [19] .
In the other hand, it is important to explore biomaterials and dressings to promote wound healing in the shortest time possible. However, the early strength of the gingival wounds and tissue function restore may be critical to the treatment outcome following surgery. Since significant finding of pervious investigations revealed a relevant role of silver NPs in wound enhancement, we designed a study to make and evaluate our dressing’s effects on gingival wound healing in animal model.
Oral wound regeneration compared to other sites has distinct problems due to its high potency to bacterial infection and frequent damage by mastication. Therefore, the controlling of oral cavity microbial activity should be emphasized to enhance the wound repair and regeneration particularly following surgery. Interestingly, the silver ion Ag+ is the active antimicrobial entity; it can interfere with thiol (-SH) groups and provoke the generation of reactive oxygen species (ROS), a major contributor to its antibacterial efficacy. Recently, silver nanoparticles have gained considerable interest in wound bio burden reduction, as they can release Ag+ ions at a greater rate than bulk silver, by virtue of their large surface area [20] .
Furthermore, following periodontal surgeries, the area usually is covered by current periodontal pack which decrease postoperative infection and bleeding and are usually without therapeutic effects [15] . However, the findings of several researches suggested that the packs caused aggregation of plaques [16] , stimulation of regenerated tissues [17] [21] and insertion of small injury to periodontitis tissue due to increase of inflammation [22] which its efficacy currently is questionable. However, a case study conducted by Tropl et al. investigated the absorption of silver NPs from a burn wound dressing and its findings revealed that the wounds healed rapidly with treatment [23] . Thus, on grounds of pervious studies’ results, the advantageous of the application of silver NPs in periodontal dressing may outweigh the demerits.
We observed a remarkable decrease in the amount of the inflammatory parameters within the first 4 days after surgery in the test group comparing to those of the control group suggested that silver NPs component dressing altered or compressed the inflammatory events in the wounds and facilitated the early phases of wound healing. This result confirms finding of the Woodward et al. who indicated that silver ions caused neutrophil apoptosis so the inflammatory events were suppressed and repair process were increased [24] . In addition, the ability of silver NPs to modulate cytokine production, and thereby diminish the inflammatory response following burn injury (confirmed due to decreased neutrophil infiltration within the wound), was thought to contribute to their ability to promote, and accelerate, wound healing and reduce scar appearance [25] . On the other hand, the amount of inflammatory parameters particularly edema was relatively higher in sites treated with periodontal dressing B in comparison with periodontal dressing A which probably results from the lower percentage of silver NPs in dressing B.
In the present work, our finding suggested that wounds treated with this dressing had a poor signs of inflammatory response in the connective tissue with sparsely scattered chronic inflammatory cells infiltration one week post-operatively; thus, provided proper basis for emigration and proliferation of epithelial and connective tissue cells, synthesis of extracellular matrix and remodeling of paranchymal elements and connective tissue which leads to restoring the tissue function and achieving wound strength particularly at the first days of the healing process. Besides, the regions where this dressing were applied showed a marked increase in the amount of repair parameters, wound re-epithelialization with relatively normal thickness, and compact and well-aligned collagen fibers, suggesting that the wound was essentially healed at day 7 following operation. The results of our study are in agreement with other studies showing that the application of different therapeutic forms of nanocrystalline silver particles enhanced wound healing [26] [27] . The wound-healing capabilities of silver NPs have been demonstrated, and found to be better than traditional treatments. Tian et al. used a thermal injury mouse model to determine the wound-healing capabilities of a silver NPs-containing wound dressing. The healing of burn wounds occurred more quickly when topically treated with silver NPs as compared to silver sulfadiazine (a standard burn treatment) [28] . Although the application of silver NPs within wound dressings has obvious advantages, Hollinger reported that silver delayed wound healing [29] , and Hidalgo and Dominguez have also reported that silver nitrate is toxic to fibroblasts and endothelial cells, both of which are required for wound healing [30] . There was marginally more chronic inflammatory cell count in sites treated with periodontal dressing A in comparison with periodontal dressing B, suggesting that a relatively mild persisting inflammatory response may related to the higher volumetric density of silver NPs in periodontal dressing A that may keep the inflammatory response. We applied this periodontal dressing with two different NPs concentrations to determine the dose-dependent histopathological and biological effects of silver NPs on wound healing and ensure the optimal utilization of this periodontal dressing in a clinical setting.
Ip M et al. on evaluation of antimicrobial activities of silver dressings found the similar results. They suggested that performing the silver NPs dressing or other silver pharm products for wound protection should be used by care [31] . So it might be claimed that high amount of silver particles caused mild cytotoxic effects, even acted as foreign bodies and kept the inflammatory responses.
5. Conclusion
The results presented here therefore indicated a relevant role for this new formulation of periodontal dressing in wound healing, which led to improvement of therapeutic resources in the treatment of surgical wounds. From a clinical standpoint, the significant differences of the inflammatory and repair stage of healing treated with this periodontal dressing in comparison with control group, indicated a positive potential therapeutic effects on acceleration of surgical wound healing particularly improvement of periodontal treatment consequences after surgery. In addition, it could play an important role in the treatment of open wound, such as skin burns, where faster wound healing would be beneficial for the patient. Moreover, in diabetic patients who frequently present difficulties in wound healing, it could be useful in modulating the inflammatory response and better repair. Finally, if further studies support its efficacy, this novel periodontal dressing might be clinically utilized as alternative to current periodontal dressing.
Acknowledgements
This experiment is supported by the Dental Research Center of the Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of Interest and Source of Funding Statement
The authors declare that they have no conflict of interests.
References
- Cheng, D., Yang, J. and Zhao, Y. (2004) Antibacterial Materials of Silver Nanoparticles Application in Medical Appliances and Appliances for Daily Use. Chinese Medical Equipment Journal, 4, 26-32.
- Zhang, Z., Yang, M., Huang, M., Hu, Y. and Xie, J. (2007) Study on Germicidal Efficacy and Toxicity of Compound Disinfectant Gel of Nanometer Silver and Chlorhexidineacelate. Chinese Journal of Health Laboratory Technology, 17, 1403-1406.
- Cohen, M.S., Stern, J.M., Vanni, A.J., Kelley, R.S., Baumgart, E., Field, D., Libertino, J.A. and Summerhayes, I.C. (2007) In Vitro Analysis of a Nanocrystalline Silver-Coated Surgical Mesh. Surgical Infections, 8, 397-403. http://dx.doi.org/10.1089/sur.2006.032
- Chaloupka, K., Malam, Y. and Seifalian, A.M. (2010) Nanosilver as a New Generation of Nanoproduct in Biomedical Applications. Trends in Biotechnology, 28, 580-588. http://dx.doi.org/10.1016/j.tibtech.2010.07.006
- Sharma, V.K., Yngard, R.A. and Lin, Y. (2009) Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Advances in Colloid and Interface Science, 145, 83-96. http://dx.doi.org/10.1016/j.cis.2008.09.002
- Newman, M. and Carranza, F. (2006) Carranza’s Clinical Periodontology. 10th Edition, Saunders Co., Lindo.
- Fong, J. and Wood, F. (2006) Nanocrystalline Dressings in Wound Management, a Review. International Journal of Nanomedicine, 1, 441-449. http://dx.doi.org/10.2147/nano.2006.1.4.441
- Alt, V., Bechert, T., Steinrücke, P., Wagener, M., Seidel, P., Dingeldein, E., Domann, E. and Schnettler, R. (2004) An in Vitro Assessment of the Antibacterial Properties and Cytotoxicity of Nanoparticulate Silver Bone Cement. Biomaterials, 25, 4383-4391. http://dx.doi.org/10.1016/j.biomaterials.2003.10.078
- Fuj, J.I.J., Fan, D. and Shen, J. (2007) Construction of Antibacterial Multilayer Films Containing Nanosilvervia Layer-by-Layer Assembly of Heparin and Chitosen-Silver Ions Complex. Journal of Biomedical Materials Research, 790, 665-674.
- Castellano, J.J., Shafii, S.M., Ko, F., Donat, G., Wright, T.E., Mannavi, R.J., Payne, W.G., Smith, D.J. and Robson, M.C. (2007) Comparative Evaluation of Silver-Containing Antimicrobial Dressings and Drugs. International Wound Journal, 4, 114-122. http://dx.doi.org/10.1111/j.1742-481X.2007.00316.x
- Min, S., Gao, X., Han, C., Chen, Y., Yang, M., Zhu, L., Zhang, H., Liu, L. and Yao, J. (2012) Preparation of a Silk Fibroin Spongy Wound Dressing and Its Therapeutic Efficiency in Skin Defects. Journal of Biomaterials Science, Polymer Edition, 23, 97-110.http://dx.doi.org/10.1163/092050610X543609
- Zhao, L., Zhang, W.X., Chen, D.L., Qiu, H. and Li, M. (2013) Function Model Construction on the Question of Defining Optimal Ag-Concentration in Silver-Containing Biological Wound Dressing. Composite Interfaces, 20, 139-153. http://dx.doi.org/10.1080/15685543.2013.764510
- Singh, R. and Singh, D. (2012) Radiation Synthesis of PVP/Alginate Hydrogel Containing Nanosilver as Wound Dressing. Journal of Materials Science: Materials in Medicine, 23, 2649-2658. http://dx.doi.org/10.1007/s10856-012-4730-3
- Sachs, H.A., Farnoush, A., Checchi, L. and Joseph, C.E. (1984) Current Status of Periodontal Dressing. Journal of Periodontology, 55, 689-696. http://dx.doi.org/10.1902/jop.1984.55.12.689
- Smeekens, J.P., Maltha, J.C. and Renggli, H.H. (1992) Histological Evaluation of Surgically Treated Oral Tissues after Application of Photocuring Periodontal Dressing Material: An Animal Study. Journal of Clinical Periodontology, 19, 641-645. http://dx.doi.org/10.1111/j.1600-051X.1992.tb01712.x
- Wampole, H.S., Allen, A.L. and Gross, A. (1978) The Incidence of Transient Bacteremia during Periodontal Dressing Change. Journal of Periodontology, 49, 462-468. http://dx.doi.org/10.1902/jop.1978.49.9.462
- Nezwek, R.A., Caffesse, R.G., Bergenholtz, A. and Nasjleti, C.E. (1980) Connective Tissue Response to Periodontal Pack. Journal of Periodontology, 51, 521-529. http://dx.doi.org/10.1902/jop.1980.51.9.521
- Scardino, M.E., Swaim, S.F., Strain, E.A., Hoffman, C.E., Oligive, G.K., Hanson, R.A., et al. (1999) The Effect of Omega-3 Fatty Acid Diet Enrichment on Wound Healing. Veterinary Dermatology, 10, 283-290. http://dx.doi.org/10.1046/j.1365-3164.1999.00148.x
- Martin, P., Hopkinson-Wooley, J. and McClusky, J. (1992) Growth Factor and Cutanous Wound Repair. Progress in Growth Factor Research, 4, 25-44. http://dx.doi.org/10.1016/0955-2235(92)90003-Z
- Wilkinson, L.J., White, J.K. and Chipman, J.K. (2011) Silver and Nanoparticles of Silver in Wound Dressings: A Review of Efficacy and Safety. Journal of Wound Care, 20, 543-549.
- Pesson, G. and Thilander, H. (1968) Experimental Studies of Surgical Packs. In Vitro Experiments on Antimicrobial Effect. Odontol Tidskr, 76, 147-155.
- Heaney, T.G. and Appleton, J. (1976) The Effect of Periodontal Dressings on the Healthy Periodontium. Journal of Clinical Periodontology, 3, 66-76. http://dx.doi.org/10.1111/j.1600-051X.1976.tb01852.x
- Trop, M., Novak, M., Rodl, S., Hellbom, B., Kroell, W. and Goessler, W. (2006) Silver Coated Dressing Acticoat Caused Raised Liver Enzymes and Argyria-Like Symptoms in Burn Patient. Journal of Trauma-Injury Infection & Critical Care, 60, 648-652. http://dx.doi.org/10.1097/01.ta.0000208126.22089.b6
- Woodward, M. (2005) Silver Dressings in Wound Healing: What Is the Evidence? Primary Intention, 13, 153-160.
- Johnston, H.J., Hutchison, G., Christensen, F.M., Peters, S., Hankin, S. and Stone, V. (2010) A Review of the in Vivo and in Vitro Toxicity of Silver and Gold Particulates: Particle Attributes and Biological Mechanisms Responsible for the Observed Toxicity. Critical Reviews in Toxicology, 40, 328-346. http://dx.doi.org/10.3109/10408440903453074
- Tredget, E.E., Shankowsky, R., Groenveld, A. and Burrell, R. (1998) A Matched-Pair, Randomized Study Evaluating the Efficacy and Safety of Acticoat Silver-Coated Dressing for the Treatment of Burn Wounds. Journal of Burn Care & Rehabilitation, 19, 531-537. http://dx.doi.org/10.1097/00004630-199811000-00013
- Voight, D. and Paul, C. (2001) The Use of Acticoat as Silver Impregnated Telfa Dressings in a Regional Burn and Wound Care Center: The Clinicians View. Wounds, 13, 11-20.
- Tian, J., Wong, K.K., Ho, C.M., Lok, C.N., Yu, W.Y., Che, C.M., Chiu, J.F. and Tam, P.K. (2007) Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem, 2, 129-136. http://dx.doi.org/10.1002/cmdc.200600171
- Hollinger, M.A. (1996) Toxicological Aspects of Topical Silver Pharmaceuticals. Critical Reviews in Toxicology, 26, 255-260. http://dx.doi.org/10.3109/10408449609012524
- Hidalgo, E. and Dominguez, C. (1998) Study of Cytotoxicity Mechanisms of Silver Nitrate in Human Dermal Fibroblasts. Toxicology Letters, 98, 169-179. http://dx.doi.org/10.1016/S0378-4274(98)00114-3
- Ip, M., Lui, S.L., Poon, V.K., Lung, I. and Burd, A. (2006) Antimicrobial Activities of Silver Dressings: An in Vitro Comparison. Journal of Medical Microbiology, 55, 59-63. http://dx.doi.org/10.1099/jmm.0.46124-0
NOTES

*Corresponding author.


