American Journal of Plant Sciences
Vol.4 No.4(2013), Article ID:29832,6 pages DOI:10.4236/ajps.2013.44102
Response of Explants of Calli Rice (Oryza sativa L.) Japonica cv. “Ilmi” to Gene Transformation Using Agrobacterium tumefaciens-Mediated
![]()
1Agrotechnology Study Program, Faculty of Agriculture, Jember University, Jember, Indonesia; 2Division of Plant Biosciences, School of Applied Biosciences, College of Agriculture & Life Science, Kyungpook National University, Daegu, South Korea.
Email: fika.pika_piko19@yahoo.com, moh_ubaedellah@yahoo.com, miswar_musni@yahoo.com, *kkm@knu.ac.kr
Copyright © 2013 Fika Ayu Safitri. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 23rd, 2013; revised March 27th, 2013; accepted April 3rd, 2013
Keywords: AtBI-1; Agrobacterium-Mediated; GFP; Transformation; Rice
ABSTRACT
The AtBI-1 gene encoding the Arabidopsis thaliana Bax inhibitor was introduced into Japonica cultivars of rice (“Ilmi”) by Agrobacterium-mediated transformation, and a large number of transgenic plants were produced. The neomycin phosphotransferase II (NPTII) gene was used as a selectable marker. The activity of neomycin phosphotransferase could be successfully detected in transgenic rice calluses. Introduction of the AtBI-1 gene was also confirmed by PCR using AtBI-1 specific oligonucleotide primers in regenerated plants. Stable integration and expression of the AtbI-1 gene in plants were confirmed by GFP analysis.
1. Introduction
Rice is the one of the most important cereal crops in the world because it has a more complex nutritional content than other cereals, including proteins, carbohydrates, vitamins and minerals. According to Kiple and Ornelas [1], rice paddies can produce more energy and protein per hectare than the same area of land used for wheat and corn cultivation. Moreover, rice is easily prepared and has a good taste [2]. There are three types of rice, javanica (tropical japonica), japonica, and indica. Each type of rice has several diverse varieties with their own advantages and disadvantages. In this study, “Ilmi” was used as the explant because it has resistance to leaf blast as well as bacterial blight pathotype K1 and stripe virus disease, which are commonly found in South Korea. “Ilmi” originated from a cross of Milyang’96//Milyang’95//Seomjinbyeo. In addition, based on a panel test, “Ilmi” has a delicious taste. This variety has a short stature, with plants being an average of 79 cm long, short seeds and high adaptability to direct seed cultivation [3]. The population of the world is expected to increase to 9.2 million in 2050, which will result in an increase in food demand of up to 50% [4]. Such an increase would surpass current production capabilities. Rice production is limited by many factors, including biotic or abiotic stresses that can disturb metabolic pathways in plant cells. Biotic and abiotic stress can cause damage in the cell stage and influence programmed cell death (PCD), leading to death of the plant if it cannot be managed. Watanebe and Lam [5] reported that PCD (also known as apoptosis) is connected to the defense response to stress. Cell apoptosis in any system can occur in a short time, so it is necessary to reduce and control plant stress. The BI (Bax Inhibitor) gene is known to suppress the induction of PCD [6]. Gene Bax Inhibitor, which is recognized byAtBI-1 (Arabidopsis Bax Inhibitor) [7], is constructed by pBIN19 for preparation fortransformation into plant genome. In this study, we used Agrobacterium tumefaciens, LBA4404, for transformation into plants. This method was selected because Agrobacterium is a simple and inexpensive medium for transformation. Furthermore, Agrobacterium has high efficiency for transfer of the target gene into plant chromosomes. Use of transformation mediated by Agrobacterium will allow genetic improvement of diverse varieties of “Ilmi”. Therefore, this study was conducted to develop a proper method for the transformation of rice genes in japonica “Ilmi” mediated by Agrobacterium. The results of this study will provide valuable information to rice researchers, especially those studying the japonica “Ilmi” variety.
2. Materials and Methods
2.1. Explant Preparation
Japonica rice (Oryza sativa L.) cv. “Ilmi” was used as the explant in this study. Dehulled mature seeds were soaked in 70% ethanol with vigorous shaking for approximately 1 min, washed in sterile distilled water three times, and then incubated in sterilization liquid (sterile distilled water containing 1% NaOCl) for 30 minutes with 120 rpm. The samples were then washed with sterile water three times, after which they were dried on sterile filter paperfor an hour in room temperature and pre-cultured on PCI (Table 1) at 28˚C under continuous dark conditions for 25, 35 or 40 days. The composition of media used in this study is described in Table 1.
2.2. Transformation Mediated Agrobacterium
Agrobacterium tumefaciens strain LBA 4404:pBin-AtBI- 1-GFP was cultured in PB (Table 1) at 28˚C in a shaker incubator for one day then adjusted to 0.1 OD600 by dilution in PIL medium. For infection of explants, the endosperm was removed from the calli (Figure 1(H)), after which the explants were referred to as ready-calli. Ready-Calli were inoculated with Agrobacterium suspension in PIL and then incubated in a shaker incubator at 28˚C for 30 minutes, after which they were dried on sterilized filter paper for 1 hour. The Calli were then cocultivated on PCo media (Table 1) for one day at 30˚C, then at 28˚C for 3 days under continuous dark conditions. After 4 days, calli were rinsed with sterile water fourtimes, then treated with carbenecillin (Sigma, USA) to kill the Agrobacterium. The samples were subsequently dried on sterilized paper for 15 minutes, after
Table 1. Composition of medium used during various transformation stages.

which they were grown on PSRmedia at 30˚C ± 2˚C for 1 month under continous light. Aftershoot sappeared from the calli, the samples were (hereafter referred to as putative transformants) transferred to test tubes containingat 28˚C PSR (Table 1) for a week. Putative transformant would appear from calli and it was transferred to test tubes containing PSR for growth leaves and root. After 11 days on PSR in a test tube, the Putative transformants grew to complete plants. The samples were transferred into PA (Table 1) and grown for oneweek at room temperature (30˚C ± 2˚C) under continous light conditions.
2.3. PCR Confirmation of Transformation
Leaves of putative transformants were harvested and transferred into acclimatization medium, after which genomic DNA was extracted using a genomic Dneasy Plant kit (Qiagen). Polymerase Chain Reaction (PCR) analysis was then performed usingspecific primersfor the kanamycin resistance gene (forward: 5’-ATGGATGCG TTCTCTTCCTT-3’; reverse: 5’-AACAGGATTATCAT TTTCCTT-3’). PCR was conducted by subjecting the samples to the following conditions: predenaturation at 94˚C for 2 minutes, followed by 30 cycles of denaturation at 94˚C for 30 seconds, annealing at 56˚C for 30 seconds and extension at 72˚C for 30 seconds and then post-extension at 72˚C for 4 minutes. The PCR products were then subjected to electrophoresis on 1% agarose gel and visualized by ethidium bromide staining.
2.4. Gene-Expression Analysis of Selected Rice Sequences Involved in AtBI-1 Using GFP
The target protein, AtBI-1, was observed by confocal laser scanning microscopy (LSM700, Carl Zeiss, German). Visualization of GFP in leaves following the induction of AtBI-1 expression revealed that GFP showed cytoplasmic localization that was indistinguishable from that observed for the GFP-only control. In contrast, AtBI-1 expression was predominantly in the cell.
3. Results and Discussion
Callus induction is an important stage in the transformation process to produce embryogenic callus as explant transformation. Callus formation in rice (Oryza sativa L.) cv Japonica “Ilmi” cannot be distinguished from callus formation in other plants. This process begins with the formation of an embryogenic callus, after which globular somatic embryogenesis occurs. At this stage of callus induction, the embryogenic callus is very important as an effective medium for genetic transformation either through particles or Agrobacterium [8]. The results of the present study revealed that the embryogenic callus was yellow with high rigidity and good for transformation. The embryogenic calli appeared from the embryo inside of the seed, after which the callus was induced during elongation of the root for a week (Figures 1(B) and (C)) and then formed white clumps on the base of the root (Figure 1(D)). The clump then became yellow (Figures 1(E)-(G)) and developed high rigidity and a larger size.
It is important to optimize infection of plant cells by Agrobacterium to ensure transformation of the callus. The optical density of the Agrobacterium was used to ensure stable and efficient transformation potency. This is because high concentrations of Agrobacterium can produce overgrowth on medium during co-cultivation, while low concentrations can reduce transformation efficiency. In this study, we used 0.1 OD600 for infection. Rice is not a dicotyledone plant, so it cannot produce phenolic compounds as stimuli in response to infection. In the mechanism of infection, phenolic compounds act as the signaling mechanism by which bacteria express virulence genes (vir A and vir G) and sugar molecules serve as coinducers. Sugar was responded by proteins, ChvE, which is encoded by a gene on chromosome Agrobacterium. In the presence of sugar, low concentrations of phenol are more effective for induction of genomic plants [9]. In several studies, the phenolic compound was acetosyringone. In this study, this concentration of acetosyringone was 0.1 mM and the time of infection was 30 minutes at 28˚C in a shaker incubator. The endosperms on the calli were then removed to expand the embryogenic calli area so that putative transformed plants could be produced.
Following infection, co-cultivation was conducted for 4 days at 30˚C and then at 28˚C for 3 days under continuous dark conditions. Incubation temperature was shown to play an important role in management of the Agrobacterium growth and optimizing infection of the plant genome. Agrobacterium can grow well at 28˚C; however, in this study, the use of 30˚C for the first temperature did not impact the infection. Nijmona and Lamparter [10] reported that the ability of Agrobacterium to infect plants at 35˚C or 40˚C is drastically lower than at 25˚C. Conversely, dark conditions were optimal for the expression of Agrobacterium flagella [11]. In this case, dark conditions were more suitable for infection. The appearance of a callus a day after co-cultivation at 30˚C indicated that there was no overgrowth of Agrobacterium and after 3 days on co-cultivation medium at 28˚C the Agrobacterium grew well and was not characterized by overgrowth (Figure 2).
During the selection and regeneration stage it was important to kill Agrobacterium tumefaciens that had infected the callus. Therefore, we rinsed infected calluses with sterilized water and added carbenecilin to kill the Agrobacterium. Figure 3 is a development of callus. In this study, we observed albino (Figure 4(A)), discolored (Figure 4(B)), half grown plants (Figure 4(C)), and green (Figure 4(D)) plants. We assumed that these differences were caused by genetic mutationsin chlorophyll producing genes and by the impact of varying light conditions. Specifically, the light levels were insufficient to help proto-chlorophyll become chlorophyll-a, which resulted in albino plants dying in the PA medium (Table 1) after approximately one week.
In general, the results showed that shoots appeared approximately one month after washing, and it would be occur the amount of putative plant, but, the total of callus infected would decrease because kanamycin resistance.
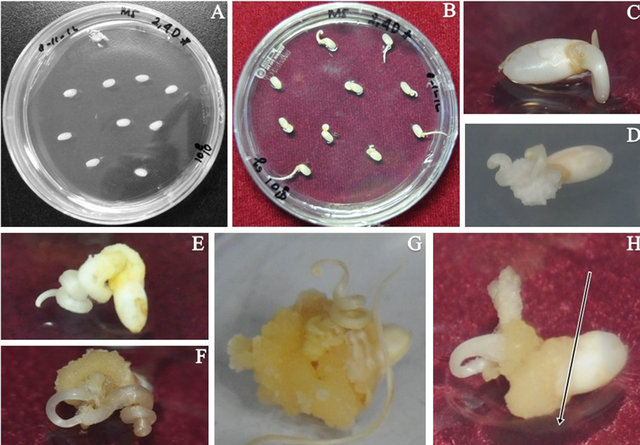
Figure 1. Development of callus. (A) 1st day of callus induction; (B) and (C) 7th day of callus induction; (D) 11th day of callus induction; (F) 18th day of callus induction; (G) 28th day of callus induction; (H) 35th day of callus induction (Callus was ready as an explant transformation; thelong arrow shows the cutting side).

Figure 2. Co-cultivation stage. (A) Before co-cultivation; (B) Oneday after co-cultivation; (C) 4 days after co-cultivation.

Figure 3. Development of rice callus on PSR medium. (A) Callus explant after 4 days of co-cultivation; (B), (C) and (D) 10th day of infection of callus seed explant; (E), (F) and (G) 17th day of infection callus seed explant; (H) and (I) 24th day of infection callus seed explant; (J) 31st day of infection callus seed explant; (K) 45th day of infection callus seed explant; (L) Transgenic plants transplanted in a pot.
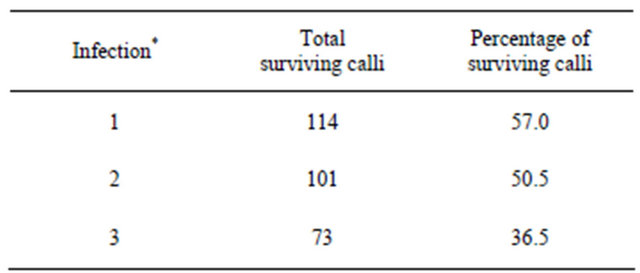
The uninfected plants also died because kanamycin antibiotic could attack the plant cells (Table 2). The survival of the callus was highest after 3 weeks of co-cultivation following the first infection; therefore, we assumed the ability for callus regeneration was better than at 35 and 40 days. Moreover, albino and white-green plants appeared after one month of co-cultivation, and the first infection produced more white-green and albino plants than the second and third infections (Table 3).
The leaves were harvested from 4 putative plants following the first infection, one putative plant following the
Table 2. Total surviving calli and regeneration after 3 weeks.
*Infection 1: 25 days inducing callus; Infection 2: 35 days inducing callus; Infection 3: 45 days inducing callus.

Figure 4. Development of callus regeneration. (A) Albino plant; (B) Discolored plant; (C) Half plant; (D) Green plant.
second infection and one putative plant after the third infection. And that, we seperated the plant contained more than one plant and we got 20 putative plants, consist of: 16 putative plants first infection, a putative plant second infection and 3 putative plants third infection. PCR analysis showed that among 20 putative plants, there were three transformed plants (Figure 5), all of which were generated from the first infection. We supposed that the other plants, kanamycin antibiotic just blocked in a spoot area, so that the plant didn’t be transferred by gene.
To investigate the potential effects of theAtBI-1-GFP gene, we constructed the vector pBin-AtBI-1-GFP and introduced it into rice. GFP (Green Fluorescent Protein)
Table 3. The development of albino and white-green plants after one month of co-cultivation.
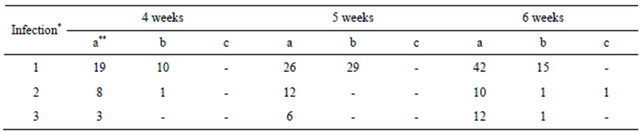
*Infection 1: 25 days inducing callus; Infection 2: 35 days inducing callus; Infection 3: 45 days inducing callus. **a: green plant, b: albino plant, c: white-green plant.
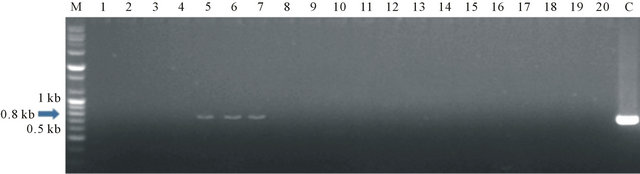
Figure 5. The results of PCR analysis. M: DNA size marker, C: DNA of AtBI-1 plamid, 1-20: samples plants estimated transformant.

Figure 6. Luminescent images of a leaf by expression of AtBI-1 in transgenic plants. The luminescent images of a leaf loaded with green fluorescent protein (GFP). GFP imaging was achieved at 480 nm by confocal laser scanning microscopy (LSM700, Carl Zeiss, German). Barsindicate 100 μm.
is a common maker of transformation; therefore, the leaves of three transformant plants were tested for GFP expression. The results revealed that the leaves were loaded with GFP, and GFP imaging showed a stronger signal in transgenic plants than non-transgenic plants (Figure 6).
4. Acknowledgements
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ00950503), Rural Development Administration, Republic of Korea. We are grateful to Dr. Hirofumi Uchimiya in Institute of Molecular and Cellular Biosciences, The University of Tokyo.
REFERENCES
- K. F. Kiple and K. C. Ornelas, “The Cambrigde World: History of Food,” Cambridge University Press, Cambridge, 2000.
- M. Kubo and M. M. Purevdorj, “The Future of Rice Production and Consumption,” Journal of Food Distribution Research, Vol. 35, No. 1, 2004, pp. 128-142.
- S. J. Lim, H. G. Hwang, S. J. Yang, B. G. Oh, U. S. Yoe, N. B. Park, H. Y. Kim, G. H. Yi, B. Y. Jun, S. C. Kim, S. K. Lee, Y. J. Oh and G. S. Chung, “High Eating Quality and Medium-Late Maturing Rice Variety with Resistance to Diseases and Lodging ‘Ilmi’,” Korean Journal of Breeding Science, Vol. 28, No. 4, 1996, pp. 481-481.
- Y. Duan, C. Zhai, H. Li, J. Li, W. Mei, H. Gui, D. Ni, F. Song, L. Li, W. Zhang and J. Yang, “An Efficient and High through Protocol for Agrobacterium Mediated Transformation Based on Phosphomannose Isomerase Positive selection in Japonica Rice (Oryza sative L.),” Plant Cell Reports, Vol. 31, No. 9, 2012, pp. 1611-1624. doi:10.1007/s00299-012-1275-3
- N. Wanatabe and E. Lam, “Bax Inhibitor-1, Conversed Call Death Suppressor, Is a Key Molecular Switch Downstream from a Variety of Biotic and Abiotic Stress Signals in Plants,” International Journal of Molecular Sciences, Vol. 10, No. 7, 2009, pp. 3149-3167. doi:10.3390/ijms10073149
- N. Wanatabe and E. Lam, “Arabidopsis Bax Inhibitor-1 Arheostat for ER Stress-Induced Programmed Cell Death,” Plant Signaling & Behaviour, Vol. 3, No. 8, 2008, pp. 564-566. doi:10.4161/psb.3.8.5709
- M. Kawai, L. Pan, J. C. Reed and H. Uchimiya, “Evolutionally Conserved Plant Homologue of the Bax Inhibitor-1 (BI-1) Gene Capable of Suppressing Bax-Induced Cell Death in Yeast,” FEBS Letters, Vol. 464, No. 3, 1999, pp. 143-147. doi:10.1016/S0014-5793(99)01695-6
- P. D. Kasi, Sumaryono, “Development of Embryogenic Callus Sago (Metroxylon sagu Rottb.) on Three in Vitro Culture System,” Tower Estates, Vol. 76, No. 1, 2008, pp. 1-10.
- W. T. Peng, Y. W. Lee and E. N. Nester, “The Phenolic Recognition Profiles of the Agrobacterium tumefaciens Vir Aprotein Are Broadened by a High Level of the Sugar Binding Protein ChvE,” Journal of Biotechnology, Vol. 190, No. 21, 1998, pp. 5632-5638.
- I. Nijmona and T. Lamparter, “Temperature Effects on Agrobacterium Phytochrome Agp1,” PLoS ONE, Vol. 6, No. 10, 2011, p. e25977. doi:10.1371/journal.pone.0025977
- I. Oberpichler, R. Rosen, A. Rasouly, M. Vugman, E. Z. Ron and T. Lamparter, “Light Affects Motolity and Infectivity of Agrobacterium tumefaciens,” Environmental Microbiology, Vol. 10, No. 8, 2008, pp. 2020-2029. doi:10.1111/j.1462-2920.2008.01618.x
NOTES
*Corresponding author.

