American Journal of Plant Sciences
Vol.4 No.2A(2013), Article ID:28464,9 pages DOI:10.4236/ajps.2013.42A055
Physiological Response of Halophyte (Suaeda altissima (L.) Pall.) and Glycophyte (Spinacia oleracea L.) to Salinity
![]()
Plant Physiology Department, Faculty of Biology, M. V. Lomonosov Mosow State University, Moscow, Russia.
Email: meychik@mail.ru
Received December 7th, 2012; revised January 8th, 2013; accepted January 15th, 2013
Keywords: Suaeda altissima (L.) Pall.; Spinacia oleracea L.; Salinity; Ions; Praline; Nitrogen; Protein
ABSTRACT
We have done a comparative study of ion status, growth and biochemical parameters in shoots and roots of seablite (Suaeda altissima (L.) Pall.) and spinach (Spinacia oleracea L.) grown with different salinity levels in the medium (0.5 - 750 mМ). A distinctive feature of the halophyte was a high Na+ content in tissues at its low concentration in the medium (0.5 mM). In these conditions, Na+ accumulation in seablite roots was four-fold higher than in spinach roots, and Na+ content in seablite leaves was almost 20-fold higher than in spinach. Together with an increase in sodium concentration in the medium, K+ content decreased six-fold in seablite leaves, while in spinach it did not decrease so drastically. We can suppose that in the halophyte, some processes occur only in the presence of sodium, and these functions of sodium cannot be fully fulfilled by potassium. Analysis of protein and total nitrogen content in tissues shows that at high salinity, the ability to synthesize non-protein nitrogen-containing compounds increases in the halophyte and decreases in the glycophyte. Data on proline content dynamics show that its increase in tissues of spinach (salinity levels 150 and 250 mМ) and seablite (salinity levels 0.5 and 750 mМ) is an indicator of plant injury. In seablite and spinach, proline is not a major osmoregulator. Its concentration both in roots and leaves was no more than 2.5 µmol/g fresh weight. The data presented in this work concern the accumulation and distribution of Na+, Cl−, K+ and  ions, as well as growth and biochemical parameters. Our data show that the development of adaptation reactions in the whole plants in the conditions of high salinity is determined by morphofunctional systems and their interaction.
ions, as well as growth and biochemical parameters. Our data show that the development of adaptation reactions in the whole plants in the conditions of high salinity is determined by morphofunctional systems and their interaction.
1. Introduction
Overcoming the negative effects of high soil salinity on plants is a serious problem that is being tackled by professionals from various fields. We can assume that overall, the biochemical and physiological mechanisms of plant salt tolerance on the cellular level are deciphered. It is currently known that one of the main strategies for plant adaptation to high salt concentrations on the cellular level is to maintain low concentrations of Na+ and Cl− ions in the cytoplasm. This is reached through the selectivity of transport systems in the plasma membrane that transfer K+ and Na+ into the cell, and due to the export of sodium from the cytoplasm. The activity of these transport systems contributes significantly to determining the cytoplasmic K+/Na+ ratio, which in turn determines salt tolerance [1,2]. Also, due to osmolyte synthesis in the cytoplasm and compartmentation of Na+ and Cl− ions into the vacuole, cells maintain the level of osmotic potential needed to absorb water in high salinity conditions [3].
However, plant resistance to high salt concentrations is largely determined by the efficiency of mechanisms functioning on the whole plant level. High salinity of soils causes osmotic stress and ion imbalance, which leads to a decrease in growth and productivity of plants susceptible to high concentrations of NaCl [4]. Osmotic stress manifests itself in a decreased availability of water and its absorption by the plant, and thus disrupts the transport of water and nutrients [5]. Ion toxicity is associated with an increase in the sodium and potassium concentration ratio in the cytoplasm (ion stress) and consequently causes a potassium deficit in plants; it leads to a drastic change in the direction of metabolic processes [6].
We can claim that salt tolerance in plants depends on the distribution of Na+ and Cl− in organs and tissues, the activity of ion transport systems in different tissues, and on the transport pathways of Na+ and Cl− ions in the whole plant [5]. On the level of the whole plant, salt tolerance manifests itself in the ability to maintain the water potential gradient in the soil-root-shoot system and low sodium and chloride concentrations in young, active plant parts, mainly in the meristem and reproductive organs [7].
The tolerance strategies described above are, to some degree, characteristic of halophytes as well as glycophytes [5]. Some authors suppose that the difference in physiological functions and biochemical processes between these two groups of plants is rather quantitative than qualitative: Halophytes, as compared to glycophytes, have more efficient systems of maintaining the water and ion homeostasis (osmolyte synthesis, ion balance support, detoxication) [8].
High salt tolerance in dicotyledonous halophytes, for example, from the Chenopodiaceae family, is due to their ability to accumulate up to 1 - 1.5 M of sodium ions in shoot vacuoles in high salinity conditions, as well as their ability to use Na+ to maintain turgor and act instead of K+ [9,10]. In high salinity conditions, the halophytes show increase in growth rate, caused by the effect of sodium on cell extension growth and plant water balance. Monocotyledonous halophytes absorb less sodium, as compared to dicotyledonous halophytes, and are able to maintain a high concentration of K+ in shoot tissues [11]. In this case, the needed osmotic pressure in the cells is reached by sugar synthesis.
A major mechanism of salt tolerance in the glycophytes is the limitation of inward flow of sodium and chloride into the roots and their transport into shoots [12]. It has been shown for a number of crops that the more salt-tolerant species are more efficient at excluding Na+ from leaf cells and maintaining a high level of K+ in them [13]. In salinity conditions, the leaf growth rate of most grain crops shows a negative correlation with Na+ concentration in leaf cells. However, unlike monocotyledons, dicotyledons do not always show this correlation. For example, a salt-tolerant wild tomato species, Lycopersicon peruvianum, accumulates more Na+ in shoot tissues than the salt-sensitive L. esculentum [13].
It is supposed that the main distinction between halophytes and glycophytes is that the halophytes are capable of withstanding salt shock. This allows them to reach a new metabolically stable state faster than glycophytes, and continue growth in salinity conditions [14]. However, the response to salt stress, or at least the ability to reach a new stable state, is not unique to halophytes. Glycophytes also show high tolerance on the level of the cell and the whole plant, if the salt concentration in the environment increases gradually [5]. Probably the main advantage of halophytes, as compared to glycophytes, is a more efficient regulation of sodium flow in coordination with growth-controlling processes both on the level of the cell and the whole plant [14].
Proline plays a major role in reactions to stress, including salt stress. It has a polyfunctional physiological effect: It plays a role as an osmoregulator, a protector compound, and performs other functions that help maintain cell homeostasis [15]. It is supposed that this amino acid stabilizes proteins and membranes, protects the cell from salt and oxidative damage, binding reactive oxygen species. However, the correlation between proline accumulation and salt tolerance has been shown to be both positive and negative in different plants [2].
In spite of the high number of works on salt tolerance, further study is needed to evaluate the role of different protective systems and their interaction on the whole plant level in the formation of salt tolerance.
Presented here is a comparative study of the effects of salinity on the accumulation and distribution of ions, proline, protein, and total nitrogen in roots and leaves of seablite and spinach. Seablite and spinach differ in their salt tolerance: Seablite is a succulent euhalophyte [10], and spinach is a glycophyte.
2. Materials and Methods
Experiments were performed on 50 - 60-day-old seablite (Suaeda altissima (L.) Pall.) and spinach (Spinacia oleracea L., cultivar “Matador”) plants of the Chenopodiaceae family.
Plants were grown with solution culture, as described in [9,16], with the use of Robinson and Dounton mineral solution [17]. The growth solution contained 4 mM Ca(NO3)2, 6 mM KNO3, 1 mM KH2PO4, 2 mM MgSO4·7H2O, 50 mM H3BO3, 50 mM FeNaEDТА, 10 mM MnCl2·4H2O, 1 mM ZnSO4, 0.5 mM CuSO4, 0.1 mM Na2MoO4·2H2O. On the forth week the sodium chloride was added to the growth medium every 2 - 3 days so that NaCl concentration in a pot increased by no more than 50 mM. Finish sodium chloride concentration in the solution was 0.5, 150, 250 mM for spinach and 0.5, 250, 750 mM for seablite. Plants were grown at a constant ambient temperature of 23˚C, illuminated with fluorescent lamps and a light intensity of 46 µmol∙m−2∙s−2 photons at the level of the upper leaf.
All the parameters were determined separately for roots and leaves.
2.1. Determining Ion Content in Plant Tissues
100 - 150 mg of dry plant material were ground in a porcelain mortar and transferred into a glass tube. To determine cation content, 5 - 10 ml of 0.0025 N HCl were added to the tube, to determine anion content 5 - 10 ml of distilled water were added; the tubes were then placed into a thermostat. Extraction was performed for 4 - 5 hours at a temperature of 80˚C. Potassium, sodium and nitrate content in the extracts were determined potentiometrically (pH Meter, Model 3320, “Jenway”, England) with ion-selective electrodes (“NIKO”, Russia).
2.2. Determining Chloride Ion Content
An aliquote of the water extract was placed into a titration flask. Then, 5 ml of isopropyl alcohol, 0.05 ml of 1 N HNO3, 0.05 ml of α-nitroso-β-naphthol, and 0.2 ml of diphenyl carbosone were added to the titration flask. The mixture was titrated with 0.01 N Hg(NO3)2 until the solution had become purple. The normality of the Hg(NO3)2 solution was determined with a 0.01 N NaCl solution.
2.3. Extraction of Free Proline and Determining Its Content
Extraction of free proline and determining its content was performed according to the method of Bates et al. [18]. 100 - 200 mg of dry plant material were ground in a porcelain mortar and transferred into a glass tube. After adding 5 - 10 ml of distilled water, the tube was closed and placed into a boiling-water bath. Extraction was performed for one hour. The water extract was quantitatively transferred into a 25 ml volumetric flask, washing it multiple times with water and filtering through a paper filter. One ml of filtrate was reacted with 1 ml of ninhdrin reagent and 2 ml of glacial acetic acid in a test tube for 1 hour at 100˚C, and the reaction terminated in an ice bath. Proline content was determined spectrophotometrically (SmartSpec 3000, USA) at 440 nm. The concentration was determined from a standard curve. Proline from “Sigma” (USA) was used for calibration.
Preparation of ninhdrin reagent: 1.25 g of ninhdrin (Sigma, USA) was reacted with 20 ml of 6 M phosphoric acid and 30 ml of glacial acetic acid in a glass-stoppered flask for 0.5 hour at 100˚C.
2.4. Total Nitrogen Content
Total nitrogen content was determined in dried (55˚C - 60˚C) and ground samples with a semiautomated CNHanalyzer (“Carlo-Erba”, Italy).
2.5. Protein Isolation
200 mg of dry plant material was ground in a porcelain mortar and transferred into a glass tube. After adding 5 - 10 ml of distilled water, the tube was placed into a water bath (80˚C). Extraction was performed for one hour. The water extract was quantitatively transferred into a 25 ml volumetric flask, washing it multiple times with water and filtering through a paper filter. 15 ml of the extract was used to determine protein content.
To each 5 ml of water extract, placed into centrifugation tubes, 2 ml of 50% TCA was added, and the mixture was then incubated for 30 - 40 minutes with constant stirring. The content of the tubes was then centrifuged for 15 minutes at 12,000 - 15,000 rpm. The supernatant was discarded, and the pellet was washed successively with 5% and 1% TCA solutions. In the leaf extracts, after treatment with 1% TCA, the protein pellet was washed with 80% acetone. After each stage of washing, centrifugation was repeated. The final protein pellet was slightly dried with air and dissolved in 1 ml of 1% NaOH solution. To completely dissolve the protein, the tubes were placed into a thermostat and kept at 40˚C for 12 hours. The protein solution was then used to determine protein content with the Lowry’s method [19].
After extracting water-soluble proteins, alkali-soluble proteins were extracted from the plant material [20]. To the plant material left after water extraction, 5 - 7 ml of 0.2% alkali solution was added; the mixture was incubated for 20 - 30 minutes with constant stirring and decanted into a 25 ml volumetric flask. The extraction was repeated no less than 3 - 4 times. After the extraction with alkali, the plant material was washed with water, and the filtrates were transferred into the volumetric flask containing the alkali extracts. Protein precipitation, washing, and content determination were performed as described above.
After extraction with water and alkali, 70% ethanol was added to the plant material in portions of 3 - 5 ml and the mixture was incubated for 30 minutes with constant shaking [20]. The liquid was poured into a weighing bottle and left until complete evaporation of ethanol; then 5 ml of 1% alkali solution was added to dissolve the pellet. Protein precipitation, washing, and content determination were performed as described above.
Total protein content was calculated as a sum of its content in water, alkali, and ethanol extracts.
2.6. Statistical Analysis
Statistical Analysis was performed in Excel 7.0. The tables and figures represent the average values of 3 - 10 individual experiments and their standard deviations.
3. Results and Discussion
Under stress, growth parameters reflect the physiological state of the plant. Data on biomass accumulation by seablite and spinach plants show that upon an increase in salt content up to 250 mM, fresh and dry mass of all organs in the halophyte increases more than two-fold. When the salt concentration in the medium is further increased to 750 mM, organ mass decreases 4 - 5-fold (Figures 1(a) and (c)). These results support the known data that NaCl concentration of 250 mM in the medium is optimal for seablite growth and development [9]. In the glycophyte, an increase in NaCl concentration in the medium up to 250 mM leads to a slight decrease in fresh biomass (Figure 1(b)), while dry mass does not change significantly (Figure 1(d)).
Measurement of K+ and Na+ accumulation in seablite and spinach organs (Figures 2 and 3) distinctly shows
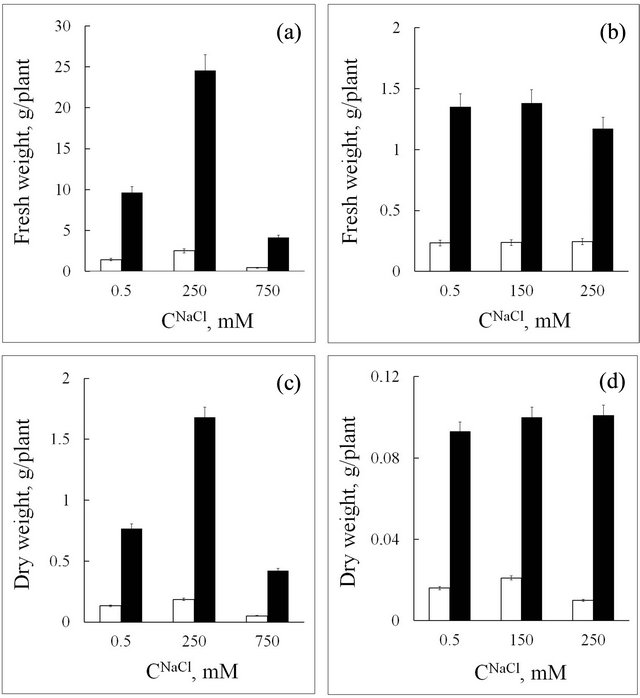
Figure 1. Fresh and dry weight of roots (white rectangle) and leaves (black rectangle) in seablite (a, c) and spinach (b, d) at different NaCl concentrations (CNaCl) in the nutrition medium. Means ± standard deviation are shown, n = 8 - 10.
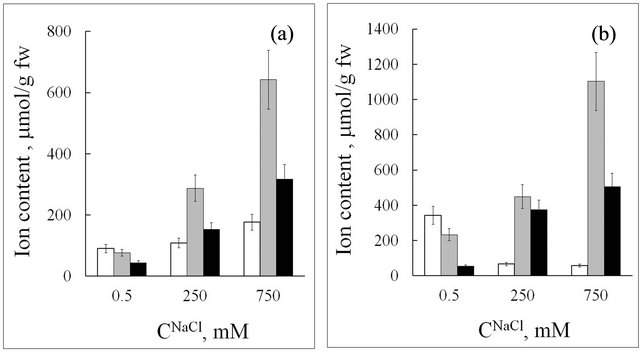
Figure 2. Ion content in roots (a) and leaves (b) of seablite at different NaCl concentrations (CNaCl) in the nutrition medium. Means ± standard deviation are shown, n = 5 - 8. White rectangle—K+; grey rectangle—Na+; black rectangle—Cl−.
different reactions in the halophyte and glycophyte. Upon an increase in NaCl concentration in the nutrition medium, sodium content in seablite and spinach tissues increases. However, in all experiments seablite tissues accumulated substantially more sodium ions than spinach. Upon an increase in NaCl content in the nutrition medium from 0.5 to 250 mM, Na+ content in seablite roots increased from 80 to 300 µmol/g fresh weight, while in spinach roots it increased from 20 to 120 µmol/g fresh
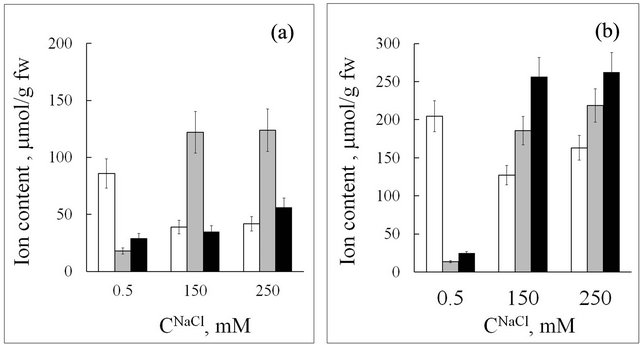
Figure 3. Ion content in roots (a) and leaves (b) of spinach at different NaCl concentrations (CNaCl) in the nutrition medium. Means ± standard deviation are shown, n = 5 - 8. White rectangle—K+; grey rectangle—Na+; black rectangle—Cl−.
weight. In leaves, the difference in sodium content between the halophyte and the glycophyte is even greater, and reach 500 µmol/g fresh weight for seablite. Seablite is a salt-accumulating halophyte. It adapts to high salinity by accumulating high quantities of ions in vacuoles of shoot cells in order to create a gradient of water potential along the axis of the plant [9]. These data indicate the presence of an efficient mechanism of Na+ loading into the xylem in the halophyte. This is in according with data acquired by other authors [10].
Upon an increase in sodium concentration in the medium from 0.5 to 250 mM, K+ content in seablite roots did not change, while in spinach roots it decreased twofold (Figures 2(a) and 3(a)). At the same time, in seablite leaves K+ content drastically decreased (from 350 to 60 µmol/g fresh weight, six-fold), and did not decrease so strongly in spinach (Figures 2(b) and 3(b)). A possible reason for this is that, contrary to the glycophyte, in the halophyte Na+ can replace a large amount of K+ as an osmotic in leaves. It is known that high NaCl concentrations in the medium cause potassium deficit in salt-sensitive plants [4]. In high salinity conditions, a substantial decrease in potassium concentration in glycophyte roots, as compared to the halophyte, is probably due to a decrease in ion uptake or a direct effect of Na+ on transport proteins of root plasma membrane (for example, on K+- selective channels) [6].
A distinct feature of the halophyte is a high Na+ content in its tissues at low concentrations of this ion in the medium (СNa+ = 0.5 mM; CK+/CNa+ ~ 14, where CK+ and CNa+-K+ and Na+ concentration in the medium). In these conditions, Na+ content in seablite roots is four-fold higher than in spinach roots, and Na+ content in leaves is more than 20-fold higher than in spinach leaves (Figures 2 and 3).
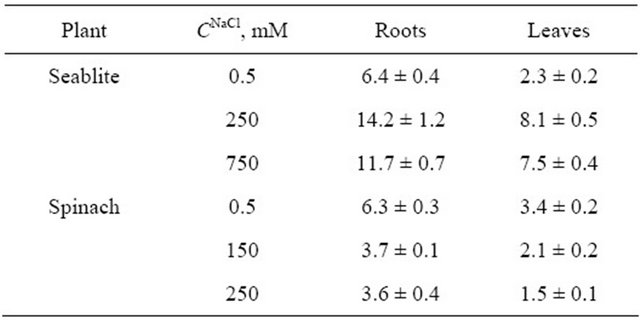
A qualitative parameter characterizing the accumulation of an ion may be its concentration coefficient (ki), which is equal to Cin/Cout, where Cin is the ion concentration in the tissue, mM, and Cout is the ion concentration in the medium, mM (Table 1). It is important to note that at low Na+ concentrations in the medium, the kNa+ parameter in seablite leaves and roots is nearly an order of magnitude higher than in spinach. In these conditions, Na+ concentration in spinach tissues also occurs (Table 1).
Sodium is an important element for halophytes of the Chenopodiaceae family. An important question is, what processes benefit from the significant concentration of this ion in seablite organs, when its concentration in the environment is low? Works concerned with studying salt
Table 1. Ion concentration coefficients (ki) in roots and leaves of the halophyte S. altissima and glycophyte S. oleracea, grown with nutrition medium containing 0.5 mM NaCl.
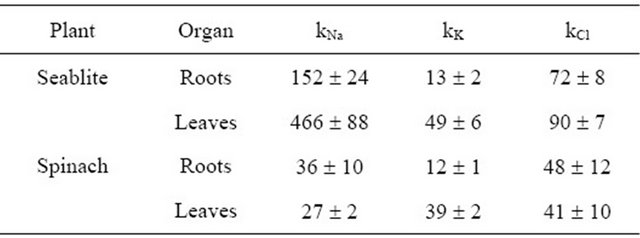
Note. Concentration coefficient ki = Cin/Cout, where Cin-ion concentration in the tissues, mM per 1 kg fresh weight; Cout-ion concentration in the medium, mM. Presented are average ki values and their relative errors.
tolerance mechanisms in halophytes are mainly aimed at finding the possible functions for sodium. For example, it has been found that in C4-plants, high concentrations of NaCl stimulated oxygen evolution by PSII, while the same concentrations of kCl inhibited this process [21]. It has also been shown that in some halophytes, sodium takes part in phosphoenolpyruvate regeneration for CO2 fixation, plays a role in osmoregulation and can partially replace potassium in protein synthesis [12]. However, the most important role of sodium in the growth of halotolerant plants is considered to be its role as an osmotic [11].
The preference for Na+ accumulation over K+ in plant tissues at low NaCl concentrations in the medium may be due to different hydration of these ions in water solutions. Hydration is currently viewed not as a process of binding a certain number of water molecules of the solution to ions, but as the effect of ions on translational motion of water molecules [22]. It is known that sodium ions show positive hydration and thus decrease the mobility of water molecules. Alternatively, potassium ions show negative hydration, i.e. water molecules near the ions become more mobile than in pure water. Based on this, we can suppose that plants need to accumulate sodium at low concentrations of ions in the environment in order to bind a part of water molecules or decrease its activity, and thus decrease transpirational water loss.
The results and considerations stated above give reasons to conclude that some processes in the halophyte probably occur only in the presence of sodium, and these functions of sodium cannot be fully fulfilled by potassium.
In previous studies of ion-exchange properties of the cell wall in seablite and spinach, we have shown that cell walls are a compartment which can hold 10% to 20% of cations absorbed by the plant [23,24]. Cell walls of roots and shoots show different capacity in different conditions of salt nutrition during plant growth [16]. Calculations done from data acquired in this and previous works show, for example, that at NaCl concentration in the medium of 250 mM, the cell walls of seablite roots may contain up to 20%, and cell walls of leaves-up to 10% of sodium ions present in the tissue. Thus, the reaction of the whole plant to high salinity is much more complex than previously viewed and involves more structures and mechanisms in the plant.
Cl− is an element necessary for all plants, but the amount of this ion that is needed for plant nutrition is rather low and reaches only 3 - 10 µmol/g dry weight. However, all plants accumulate chloride in concentrations higher than the required level. The reasons for this phenomenon are yet unknown [7].
Upon an increase in NaCl concentration in the medium (CNaCl) in the medium, chloride content increases in both leaves and roots of the halophyte (Figure 2), while in the glycophyte it does not change significantly in roots but increases by more than an order of magnitude in leaves (Figure 3). In seablite in all salinity conditions studied Na+ accumulation is two-fold higher than Cl− accumulation both in roots and shoots. In spinach, at 150 mM NaCl in the medium chloride content in leaves is 30% higher than sodium content, and at 250 mM NaCl in the medium the content of these two ions is nearly equal. Many salt-sensitive species are able to effectively remove Na+ from cells, but cannot do the same with Cl− [11]. Thus, in these plants chloride toxicity is the main reason for impaired growth even at low salinity levels [6]. It needs to be noted that even at low Cl− concentrations in the environment, Chenopodiaceae plants accumulate a significant amount of this ion in their tissues, which can be illustrated by its concentration coefficients kCl (Table 1). We can suppose that chloride is an efficient osmoregulator in these plants.
Nitrate is another anion that plays an important physiological role. In tissues of the halophyte, the functional relation between nitrate content and salinity level has a distinct minimum at 250 mM NaCl in the nutrition medium (Figure 4(a)). At optimal growth conditions (СNaCl = 250 mM) nitrate concentration in seablite shoots is equal to that in roots and is only 6 mM (Figure 4(a)). It is known that in halophytes, high concentrations of sodium stimulate nitrate uptake by roots and its assimilation in leaves due to an increase in nitrate reductase activity [11]. Based on these data, we can suppose that in plants grown at 250 mM NaCl, low endogenous content of  should be accompanied by an increase in protein and non-protein nitrogen-containing compound concentration in the tissues. However, analysis of protein, proline and total nitrogen content in seablite organs shows that the functional dependence between their concentrations in leaf and root tissues and salinity level has a minimum at СNaCl = 250 mM (Figures 5(a)-(c)). One hypothesis based on these results may have been that in these plants, chloride inhibits nitrate uptake and replaces it as an osmoregulator. However, the observed increase in nitrate concentration in tissues upon an increase in chloride concentration in the medium to 750 mM contradicts this supposition (Figure 4(a)).
should be accompanied by an increase in protein and non-protein nitrogen-containing compound concentration in the tissues. However, analysis of protein, proline and total nitrogen content in seablite organs shows that the functional dependence between their concentrations in leaf and root tissues and salinity level has a minimum at СNaCl = 250 mM (Figures 5(a)-(c)). One hypothesis based on these results may have been that in these plants, chloride inhibits nitrate uptake and replaces it as an osmoregulator. However, the observed increase in nitrate concentration in tissues upon an increase in chloride concentration in the medium to 750 mM contradicts this supposition (Figure 4(a)).
Data on protein and total nitrogen content in tissues of the halophyte (Figures 5(a) and (b)) show that an increase in CNaCl in the medium leads to an increase in nitrogen content per unit of protein mass (Table 2). Based on these data, we can suppose that in seablite, upon an increase in salt concentration in the medium, a higher portion of assimilated nitrogen is included into non-protein compounds, probably into “compatible” organic compounds or osmoprotectors. It is now known that in Chenopodiaceae, the majority of such compounds are glycine betaines [25]. An increase in the total nitrogen to protein content ratio upon an increase in СNaCl in the medium may indicate an increase in osmoprotector concentration in the tissues of the halophyte.
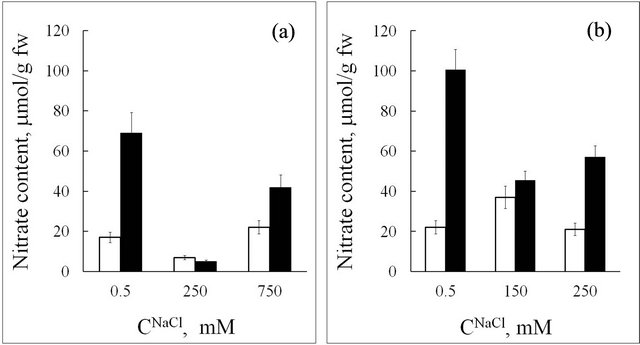
Figure 4. Nitrate content in seablite (a) and spinach (b) tissues at different NaCl concentrations (CNaCl) in the nutrition medium. White rectangle-roots, black rectangle-leaves. Means ± standard deviation are shown, n = 3 - 5.
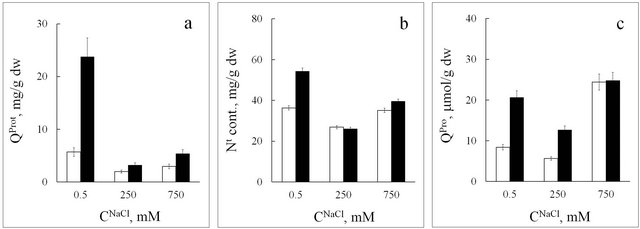
Figure 5. Protein (QProt, (a)), total nitrogen (Nt, (b)), and proline (QPro, (c)) content in seablite tissues at different NaCl concentrations (CNaCl) in the nutrition medium. White rectangle-roots, black rectangle-leaves. Means ± standard deviation are shown, n = 3 - 5.
In the glycophyte, upon an increase in NaCl concentration (from 0.5 to 150 mM) nitrate content decreases in leaves and does not change much in roots (Figure 4(b)). At the same time, protein content in roots and leaves increases (Figure 6(a)), and the amount of nitrogen per unit of protein mass decreases (Table 2). These results show that upon an increase in salinity, the content of nonprotein nitrogen-containing compounds in spinach tissues decreases.
Upon an increase in salinity level, the amount of nitrogen per unit of protein mass increases in seablite and decreases in spinach (Table 2). This shows that upon an increase in NaCl concentration the ability to synthesize non-protein nitrogen-containing compounds increases in the halophyte, while in the glycophyte this parameter decreases.
It is widely known that in response to stress, various proteins are synthesized in plant cells: Regulatory and protective proteins, enzymes that carry out cell metabolism under stress, including hydrolases that are response
Table 2. The total nitrogen to protein content ratio in seablite and spinach organs, at different NaCl concentrations in the medium (CNaCl).
Note: The ratio of total nitrogen and protein content was calculated by dividing the average total nitrogen content (mg nitrogen per 1 g tissue dry weight) by the average protein content (mg protein per 1 g tissue dry weight). Presented are average values and their relative errors.
ble for degrading damaged macromolecules [4]. It needs to be noted that low salinity conditions, which are “normal” for the glycophyte, are not normal for the halophyte [4]. Our results on protein content changes in tissues of the halophyte support this statement (Figure 5(a)). Upon a decrease or an increase in NaCl concentration (from 250 to 0.5 or from 250 to 750 mM), the amount of protein in root and leaf tissues drastically increases, which indicates that seablite is under stress at both 0.5 and 750 mM NaCl (we should note again that 250 mM NaCl is optimal for seablite growth and development). In spinach, in growth conditions other than “normal” (an increase in salinity level from 0.5 to 150 mM NaCl) protein content in tissues also increases, but not so strongly as in the halophyte. We believe that these data confirm the previous supposition that the difference in physiological functions and biochemical processes between halophytes and glycophytes is rather quantitative than qualitative [8].
In response to salt stress cells of many plants accumulate proline [2]. This amino acid is viewed as a “compatible” organic compound which stabilizes proteins and membranes, protects the cell from extreme temperatures, salt and oxidative damage. It is currently proposed that an increase in proline content is probably a symptom of osmotic damage, rather than an indication of tolerance [26].
The data obtained in this work show that in response to an increase in NaCl concentration to 250 mM, proline content increases in roots and leaves of the glycophyte (Figure 6). However, we can suppose that at 250 mM NaCl in the medium, proline in spinach does not play a major role as an osmoregulator, because its concentrations in roots and leaves are 0.56 and 2.6 µmol per 1 g fresh weight, respectively. In high salinity conditions, the increase in proline content in spinach tissues is probably an indicator of damage to the plant.
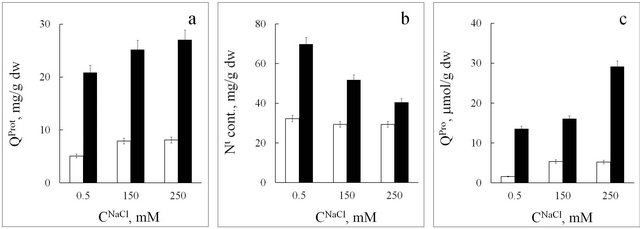
Figure 6. Protein (QProt, (a)), total nitrogen (Nt, (b)), and proline (QPro, (c)) content in spinach tissues at different NaCl concentrations (CNaCl) in the nutrition medium. White rectangle-roots, black rectangle-leaves. Means ± standard deviation are shown, n = 3 - 5.
In seablite, an increase in NaCl concentration from 0.5 to 250 mM leads to a two-fold decrease in proline content in both roots and leaves; further increase in salinity level to 750 mM leads to an increase in this parameter in both organs: five-fold in roots and two-fold in leaves (Figure 5(c)). These results indicate that plants grown with 0.5 mM and 750 mM NaCl (both non-optimal growth conditions) show symptoms of damage due to osmotic imbalance; in the case with 0.5 mM NaCl it is due to insufficient amounts of NaCl in the medium, and in the case with 750 mM NaCl—due to excess NaCl. These results are biochemical proof for the statement that NaCl concentration of 250 mM is the optimal salinity level for this halophyte. At the same time, it has been shown that in seablite, upon an increase in salinity level over the optimum, lignin content in shoot tissues significantly decreases, and extensin content in cell walls increases [27]. Based on this, we can suppose that an increase in proline content in halophyte tissues at 750 mM NaCl may be due to its role in the synthesis of hydroxyproline-rich glycoproteins.
Numerous data from biochemistry, physiology and molecular biology provide a good understanding for the structure and dynamics of the processes of adaptation to salinity on the cellular level [4,6,28]. However, they do not explain the diversity of reactions in whole organisms from different ecological groups. Studies on the physiological functions in the whole plant conducted previously [9,10], as well as data presented in this work on the accumulation and distribution of Na+, Cl−, K+ and  ions, growth and biochemical parameters, show that the development of adaptation reactions in plants in the conditions of high salinity is determined by morphofunctional systems and their interaction.
ions, growth and biochemical parameters, show that the development of adaptation reactions in plants in the conditions of high salinity is determined by morphofunctional systems and their interaction.
The work was supported by the Russian Foundation for Basic Research (grants No. 04-04-49379-a and 08- 04-01398-a).
REFERENCES
- F. Aleman, M. Nieves-Cordones, V. Martınez and F. Rubio, “Potassium/Sodium Steady-State Homeostasis in Thellungiella halophila and Arabidopsis thaliana under LongTerm Salinity Conditions,” Plant Science, Vol. 176, No. 6, 2009, pp. 768-774. doi:10.1016/j.plantsci.2009.02.020
- M. A. Ghars, E. Parre, A. Debez, M. Bordenave, L. Richard, L. Leport, A. Bouchereau, A. Savoureґ and C. Abdelly, “Comparative Salt Tolerance Analysis between Arabidopsis thaliana and Thellungiella halophila, with Special Emphasis on K+/Na+ Selectivity and Proline Accumulation,” Journal of Plant Physiology, Vol. 165, No. 6, 2008, pp. 588-599. doi:10.1016/j.jplph.2007.05.014
- I. S. Møller and M. Tester, “Salinity Tolerance of Arabidopsis: A Good Model for Cereals?” Trends in Plant Science, Vol. 12, No. 12, 2007, pp. 534-540. doi:10.1016/j.tplants.2007.09.009
- R. Munns and M. Tester, “Mechanisms of Salinity Tolerance,” Annual Review of Plant Biology, Vol. 59, No. 1, 2008, pp. 651-681. doi:10.1146/annurev.arplant.59.032607.092911
- M. Tester and R. Danenport, “Na+ Tolerance and Na+ Transport in Higher Plants,” Annals of Botany, Vol. 91, No. 5, 2003, pp. 503-527. doi:10.1093/aob/mcg058
- T. J. Flowers and T. D. Colmer, “Salinity Tolerance in Halophytes,” New Phytologist, Vol. 179, No. 4, 2008, pp. 945-963. doi:10.1111/j.1469-8137.2008.02531.x
- P. J. White and M. R. Broadley, “Chloride in Soils and Its Uptake and Movement within the Plant,” Annals of Botany, Vol. 88, No. 6, 2001, pp. 967-988. doi:10.1006/anbo.2001.1540
- A. R. Yeo, “Molecular Biology of Salt Tolerance in the Context of Whole-Plant Physiology,” Journal of Experimental Botany, Vol. 49, No. 323, 1998, pp. 915-929. doi:10.1093/jxb/49.323.915
- Yu. V. Balnokin, A. A. Kotov, N. A. Myasoedov, G. F. Khailova, E. B. Kurkova, R. V. Lun’kov and L. M. Kotova, “Involvement of Long-Distance Na+ Transport in Maintaining Water Potential Gradient in the MediumRoot-Leaf System of a Halophyte Suaeda altissima,” Russian Journal of Plant Physiology, Vol. 52, No. 4, 2005, pp. 489-496. doi:10.1007/s11183-005-0072-z
- Yu. V. Balnokin, N. A. Myasoedov, Z. Sh. Shamsutdinov and N. Z. Shamsutdinov, “Significance of Na+ and K+ for Sustained Hydration of Organ Tissues in Ecologically Distinct Halophytes of the Family Chenopodiaceae,” Russian Journal of Plant Physiology, Vol. 52, No. 6, 2005, pp. 779-787. doi:10.1007/s11183-005-0115-5
- H. Marschner, “Mineral Nutrition of Higher Plants,” 2nd Edition, Academic Press, San Diego, 1995.
- E. Blumwald, “Sodium Transport and Salt Tolerance in Plants,” Current Opinion in Cell Biology, Vol. 12, No. 4, 2000, pp. 431-434. doi:10.1016/S0955-0674(00)00112-5
- J.-K. Zhu, “Plant Salt Tolerance,” Trends in Plant Science, Vol. 6, No. 2, 2001, pp. 66-71. doi:10.1016/S1360-1385(00)01838-0
- P. M. Hasegawa, R. A. Bressan, J.-K. Zhu and H. J. Bohnert, “Plant Cellular and Molecular Responses to High Salinity,” Annual Review of Plant Physiology and Plant Molecular Biology, Vol. 51, 2000, pp. 463-499. doi:10.1146/annurev.arplant.51.1.463
- Vl. V. Kuznetsov and N. I. Shevyakova, “Proline under Stress: Biological Role, Metabolism and Regulation,” Russian Journal of Plant Physiology, Vol. 46, No. 2, 1999, pp. 274-287.
- N. R. Meychik, I. P. Yermakov, S. D. Khonarmand and Yu. I. Nikolaeva, “Ion-Exchange Properties of Cell Walls in Chickpea Cultivars with Different Sensitivities to Salinity,” Russian Journal of Plant Physiology, Vol. 57, No. 5, 2010, pp. 620-630. doi:10.1134/S1021443710050043
- S. P. Robinson and S. D. Dountov, “Potassium, Sodium and Chloride Concentrations in Leaves and Isolated Chloroplasts of the Halophyte Suaeda australius R. Br,” Australian Journal of Plant Physiology, Vol. 12, No. 5, 1985, pp. 471-479. doi:10.1071/PP9850471
- L. S. Bates, R. P. Waldren and I. D. Teare, “Rapid Determination of Free Proline for Water Stress Studies,” Plant & Soil, Vol. 39, No. 1, 1973, pp. 205-207. doi:10.1007/BF00018060
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275.
- A. I. Yermakov, V. V. Arasimov and N. P. Yarosh, “Methods of Biochemical Analysis of Plants,” Agropromizdat, Leningrad, 1987.
- J.-L. Zhang, T. J. Flowers and S.-M. Wang, “Mechanisms of Sodium Uptake by Roots of Higher Plants,” Plant and Soil, Vol. 326, No. 1-2, 2010, pp. 45-60. doi:10.1007/s11104-009-0076-0
- O. Ya. Samoilov, “A New Approach to the Study of Hydration of Ions in Aqueous Solutions,” Discussions of the Faraday Society, Vol. 24, 1957, pp. 141-146. doi:10.1039/df9572400141
- N. R. Meychik, J. I. Nikolaeva and I. P. Yermakov, “Ion Exchange Properties of the Root Cell Walls Isolated from the Halophyte Plants (Suaeda altissima L.) Grown under Conditions of Different Salinity,” Plant & Soil, Vol. 277, No. 1-2, 2005, pp. 163-174. doi:10.1007/s11104-005-6806-z
- N. R. Meychik, J. I. Nikolaeva and I. P. Yermakov, “IonExchange Properties of Cell Walls of Spinacia oleracea L. Roots under Different Environmental Salt Conditions,” Biochemistry (Moscow), Biokhimiya, Vol. 71, No. 7, 2006, pp. 781-789. doi:10.1134/S000629790607011X
- S. D. McNeil, M. L. Nuccio and A. D. Hanson, “Betaines and Related Osmoprotectants. Targets for Metabolic Engineering of Stress Resistance,” Plant Physiology, Vol. 120, No. 4, 1999, pp. 945-949. doi:10.1104/pp.120.4.945
- S. Shabala, O. Babourina and I. Newman, “Ion-Specific Mechanisms of Osmoregulation in Bean Mesophyll Cells,” The Journal of Experimental Botany, Vol. 51, No. 348, 2000, pp. 1243-1253. doi:10.1093/jexbot/51.348.1243
- L. W. Wang, A. M. Showalter and I. A. Ungar, “Effect of Salinity on Growth, Ion Content and Cell Wall Chemistry in Atriplex prostata (Chenopodiaceae),” American Journal of Botany, Vol. 84, No. 9, 1997, pp. 1247-1255. doi:10.2307/2446049
- J.-K. Zhu, “Salt and Drought Stress Signal Transduction in Plants,” Annual Review of Plant Biology, Vol. 53, 2002, pp. 247-273. doi:10.1146/annurev.arplant.53.091401.143329

