Pharmacology & Pharmacy
Vol.5 No.4(2014), Article ID:44678,9 pages DOI:10.4236/pp.2014.54046
Cefprozil versus Amoxicillin/Clavulanate for the Treatment of Acute Otitis Media in Children: Meta-Analysis of Efficacy and Safety
Rafael Bolaños-Díaz1,2, María Calderón-Cahua3
1Unidad de Análisis y Generación de Evidencias en Salud Pública (UNAGESP), Instituto Nacional de Salud, Lima, Perú
2Organización Médica para el Desarrollo de la Salud, ODDS PERU, Lima, Perú
3Universidad Peruana Cayetano Heredia (UPCH), Lima, Perú
Email: rbolanos@ins.gob.pe, rbolanosd@oddsperu.com, maria.calderon@upch.pe
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 25 February 2014; revised 18 March 2014; accepted 1 April 2014
Abstract
Introduction: The efficacy of amoxicillin combined with clavulanic acid in Acute Otitis Media (AOM) is not debatable, but studies report a high frequency of gastrointestinal adverse effects related to this intervention. In this scenario, several studies about the antibiotic cefprozil report that it has the same efficacy as amoxicillin/clavulanate for the treatment of AOM with significantly fewer side effects. The aim of our study was to compare the efficacy and safety of both treatments in AOM. Methodology: We searched for clinical trials and systematic reviews with or without meta-analyses in the Cochrane Group Register and the MEDLINE database up to November 2013, comparing the two interventions mentioned. Results were expressed as a rate of treatment failures or favorable response rates (clinical and microbiological in both cases), and as the rate of adverse events. The analysis was carried out considering the fixed and random effects models. The significance level used in the test for heterogeneity was 0.05. Funnel plot was used to search for publication bias. Results: 7 clinical trials were included in the analysis and no significant difference was found for both interventions regarding clinical and bacteriological response (RR = 1.02, 95% CI, 0.97 - 1.06, p = 0.780 and RR = 1.02, 95% CI, 0.99 - 1.07, p = 0.228, respectively). However, a significant difference was found in the comparison of adverse effects, showing that amoxicillin/clavulanate has a significantly higher risk of developing side effects than cefprozil (RR = 0.52, 95% CI, 0.45 - 0.59, p = 0.000). Conclusion: Both treatments demonstrated equal clinical and microbiological efficacy. However, the use of cefprozil is significantly associated with a lower risk of adverse effects.
Keywords
Cefprozil; Amoxicillin/Clavulanate; Acute Otitis Media

1. Introduction
Acute otitis media (AOM) is one of the most common childhood diseases as well as the major cause of antibiotic prescribing for outpatients in this age group [1] . Despite a decline in the trend of antibiotic prescriptions for AOM, it is still observed with significant frequency in comparison to other diseases with probable bacterial cause [2] . The lack of proper treatment can lead to serious consequences such as recurrent AOM, tympanic perforation, ventilation tube insertion or hearing loss [3] . Thereby, a US study estimated that the annual socioeconomic costs of AOM exceed 3.5 billion US dollars between direct and indirect costs [4] .
The etiology of AOM is polymicrobial. It’s often a result of viral infections which alter the properties of adhesion and colonization of the bacteria found in the nasopharynx and eustachian tube in addition to causing inflammation in the area [5] . Streptococcus pneumoniae, non-typeable Haemophilus influenzae and Moraxella catarrhalis are the most common bacteria that develop AOM and are considered as a part of the normal flora until a viral infection occurs [5] [6] . Amongst the most common viruses isolated in AOM, there are the respiratory syncytial virus, adenovirus, influenza, parainfluenza and others [7] . AOM is diagnosed by a physical examination in patients when there is a presence of middle ear effusion with sudden onset of inflammation and symptoms such as pain, irritability or fever [8] .
The treatment of AOM begins with an adequate analgesia. The recommended antibiotic therapy is high-dose amoxicillin (80 to 90 mg per kg per day) if the patient is not allergic to penicillin. If the child’s symptoms persist for more than 48 to 72 hours, amoxicillin with clavulanic acid is recommended [8] . In the Clinical Practice Guidelines (CPG), developed by the American Academy of Pediatrics in 2013, amoxicillin is also recognized as a first-line treatment, but other possible antibiotics are contemplated as effectively as a amoxicillin/clavulanate combination due to the high prevalence of resistance to amoxicillin [9] .
The efficacy of the amoxicillin/clavulanate association is not debatable, but several studies have reported gastrointestinal effects associated with the drug intake so a search for alternatives to this treatment was started [10] -[13] . In this scenario, the cumulative evidence would suggest that cefprozil has the same efficacy as amoxicillin/clavulanate for the treatment of AOM, but with a significant reduction in adverse effects such as dyspepsia or diarrhea and other gastrointestinal complaints [11] [14] -[18] .
For all the above, the objective of the study was to compare the efficacy and safety of both antibiotics in the treatment of childhood AOM.
2. Material and Methods
2.1. Criteria for the Systematic Search
Only published studies were considered. They had to be randomized with comparative designs and with a minimum duration of 4 weeks. Participants were children <14 years with the clinical diagnosis of AOM. The interventions evaluated were cefprozil and amoxicillin/clavulanate. Results were expressed as number of treatment failures or clinical response rate, microbiological response rate and the rate of adverse events.
2.2. Search Strategy for Identifying Studies
Filters: clinical trials, age < 18 years Search#1: (“cefprozil”) AND (“acute otitis media”)
Search#2: (“cefprozil”) AND (“amoxicillin/clavulanate) AND (“acute otitis media”)
The research question for this systematic review was: are cefprozil and the amoxicillin/clavulanate combination equally effective and safe for the treatment of acute otitis media in children?
The search was limited to clinical trials and systematic reviews with or without meta-analysis in the pediatric population. Language was restricted to English and Spanish. The search was conducted in the Cochrane Group Register, MEDLINE, SCOPUS and EMBASE databases (until November 2013).
The complete reference list of the found publications was neatly reviewed. Unpublished studies were not considered.
2.3. Review Methods
In each study, the results were expressed as the rate of therapeutic response (clinical and bacteriological) in addition to the rate of adverse events. The analysis was carried out considering the model of fixed effects and random effects. The significance level (p-value) used in the heterogeneity test was 0.05. A funnel plot was performed to search for publication bias. We used Stata software (version 11, StataCorp, TX, USA) for calculations.
Two reviewers independently analyzed the clinical trials to assess their quality and a third reviewer resolved possible discrepancies. The Jadad scale was used. This scale comprises five basic criteria: 1) presence of randomization, 2) description and appropriateness of the randomization, 3) presence of blinding, 4) description and appropriateness of blinding, and 5) a description of losses and withdrawals from the study. According to the original Jadad study [19] , every experimental clinical trial that meets 3 or more criteria can be considered of sufficient quality.
3. Results
3.1. Description of the Studies
During the systematic review, 10 studies that met the eligibility criteria specified for this analysis were identified. After peer review in the form of an assessment of the quality of the full text, 7 clinical trials were selected for inclusion in the meta-analysis. The flowchart for the inclusion of studies is presented in Figure 1. The selected studies were analyzed with the funnel plot test without finding evidence of publication bias (Figure 2).
Three of the selected articles have been analyzed by subgroups according to the presentation of data from the original study. Hedrick et al. [16] divide the results of clinical response into two age groups: 6 months - 2 years old and 2 - 7 years old, while Aronovitz et al., Gehanno et al. and Stutman et al. divided the results according to the frequency of adverse effects in a group younger than 3 years and a group older than 3 years [12] [13] [20] .
Characteristics and results of the included studies are shown in Table 1. The studies were conducted in the United States, the Dominican Republic, Greece, Holland and France. The total number of participants in all the meta-analysis was 2304. Treatment duration was 7 to 10 days. Only interventions with amoxicillin/clavulanate and cefprozil were evaluated. Usually, cefprozil dose was 30 mg/kg/day divided into two daily doses, and amoxicillin/clavulanate was 40 mg/kg/day in three daily doses. This pattern was repeated in all trials except in the study of Hedrick et al., where high-dose amoxicillin and cefprozil (45 mg/kg/day) were used [16] .
3.2. Clinical Response
Clinical response was assessed by the number of treatment failures. In this case the 7 selected clinical trials were included, including subgroup analysis presented by Hedrick et al. This meta-analysis had a total of 2304 participants for which RR = 1.02 (95% CI, 0.97 - 1.06, p = 0.780) was obtained with a heterogeneity coefficient of 51.9% (I2 = 51.9%), proving thus not there is a significant difference in the likelihood of therapeutic failure therapy between cefprozil and amoxicillin/clavulanate. These results are presented in Figure 3. Using meta-regression we could not find any significant source to explain heterogeneity and Harbord’s modified test with Galbraith plot confirmed this result (data not shown).
3.3. Microbiological Response
Six trials assessed clinical and microbiological responses at the same time. This analysis yielded an RR = 1.02 (95% CI, 0.99 - 1.07, p = 0.228) with I2 = 0.0%. This result demonstrates that there is no significant difference in the microbiological response between the two drugs. The study of Hedrick et al. was the only selected article that did not present results for microbiological response (Figure 4).
3.4. Adverse Effects
When comparing the adverse effects, a significant difference in favor of cefprozil was found, with RR = 0.52
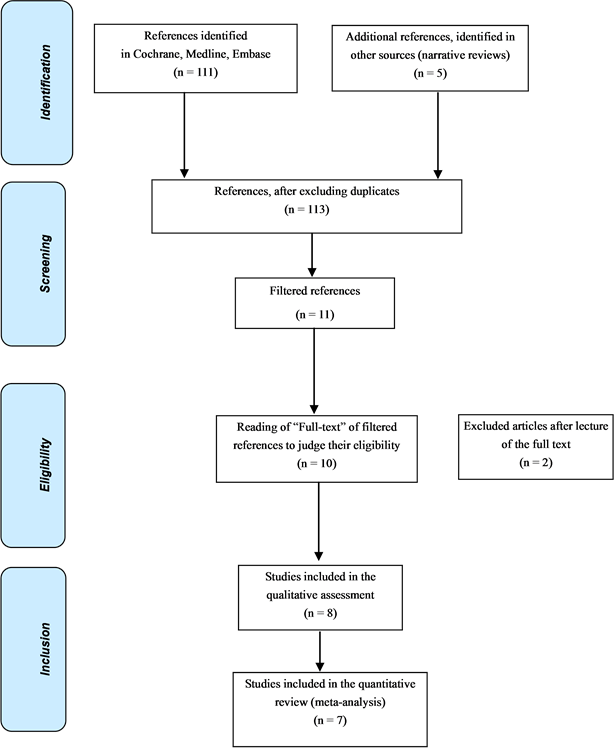
Figure 1. Flowchart of the systematic review.
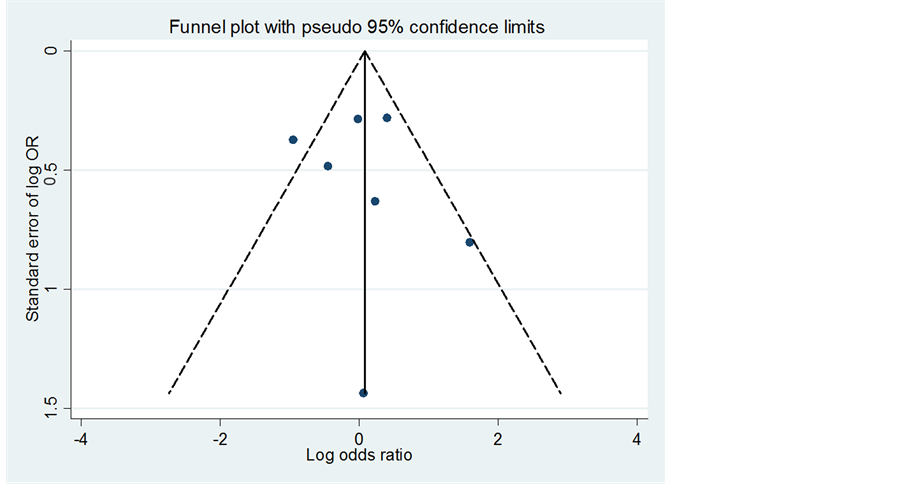
Figure 2. Funnel plot of the trials included in the analyses.
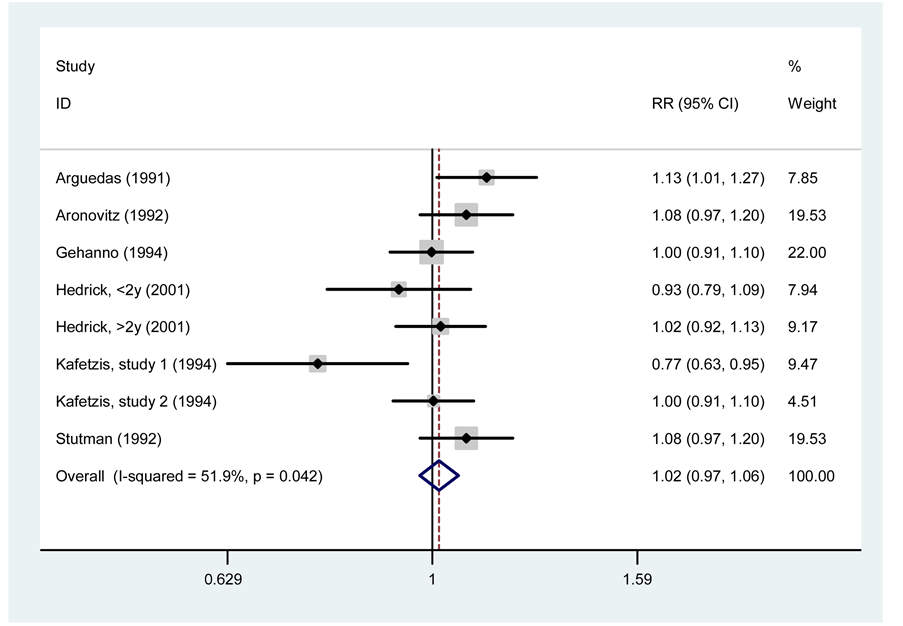
Figure 3. Meta-analysis of clinical response between cefprozil and amoxicillin/clavulanate.
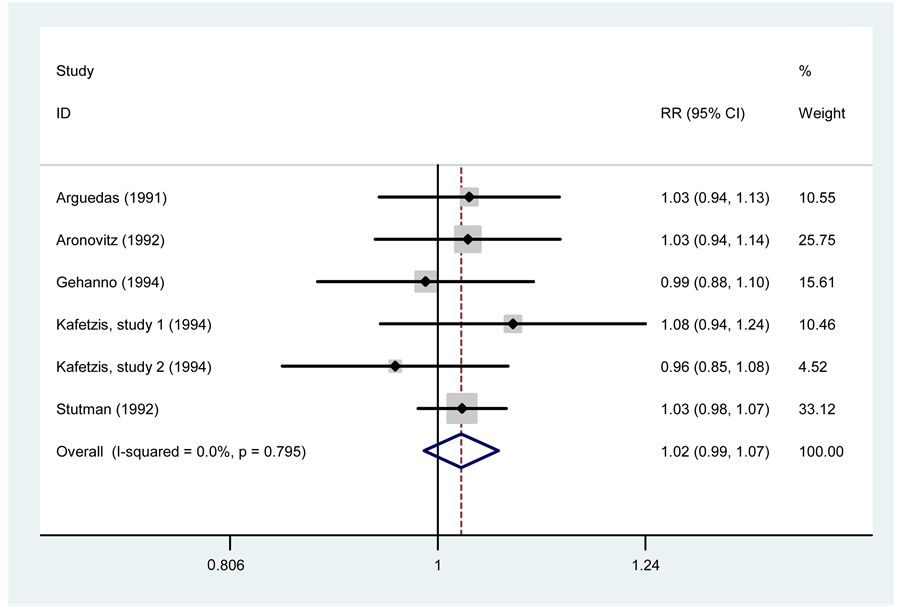
Figure 4. Meta-analysis of microbiological response between cefprozil and amoxicillin/clavulanate.
Table 1. Comparative clinical trials between cefprozil and amoxicillin/clavulanate in acute otitis media.
(95% CI, 0.45 - 0.59, p < 0.000) and I2 = 7.4%. This is interpreted as a reduced risk of adverse effects for cefprozil in approximately 48%, compared with the use of amoxicillin/clavulanate.
Of all the adverse effects reported in different studies, the most common are related to the gastrointestinal area, such as loose stools and diarrhea. The results are shown in Figure 5.
4. Discussion
According to the presented results, it is clear that there is no significant difference in clinical and microbiological efficacy of both treatments, whereas there was a statistically significant difference with regard to the safety of the two drugs. Analysis of the data showed that cefprozil has a lower risk of adverse effects compared with amoxicillin/clavulanate.
Before discussing the results in more detail it is important to note that the greatest strength of the study lies in the inherent design features. The fact that it was done through a systematic review and also included the use of statistical methods such as meta-analysis, allows us to say that until November 2013 this is the synthesis of the evidence regarding the treatment of AOM with cefprozil and amoxicillin/clavulanate. To our knowledge, this is the first meta-analysis that is done on both efficacy and safety of these drugs based on randomized clinical trials.
However, it is important to highlight some limitations of this analysis: 1) although all studies underwent a quality assessment to control the overall technical standardization, they are individually exposed to methodlogical weaknesses, 2) not all studies have a standard dose, although most studies uses the recommended doses for each drug (cefprozil 30 mg/kg/day and amoxicillin/clavulanate dose 40 mg/kg/day). Exceptionally, the study of Hedrick et al. used higher doses of amoxicillin (45 mg/kg/day).
Regarding the results of the clinical and bacteriological efficacy of this meta-analysis, the literature findings were confirmed, i.e., cefprozil is a good alternative to the use of antibiotic amoxicillin/clavulanate in AOM cases. The finding of a significant difference in the risk of adverse effects is also consistent with the observations
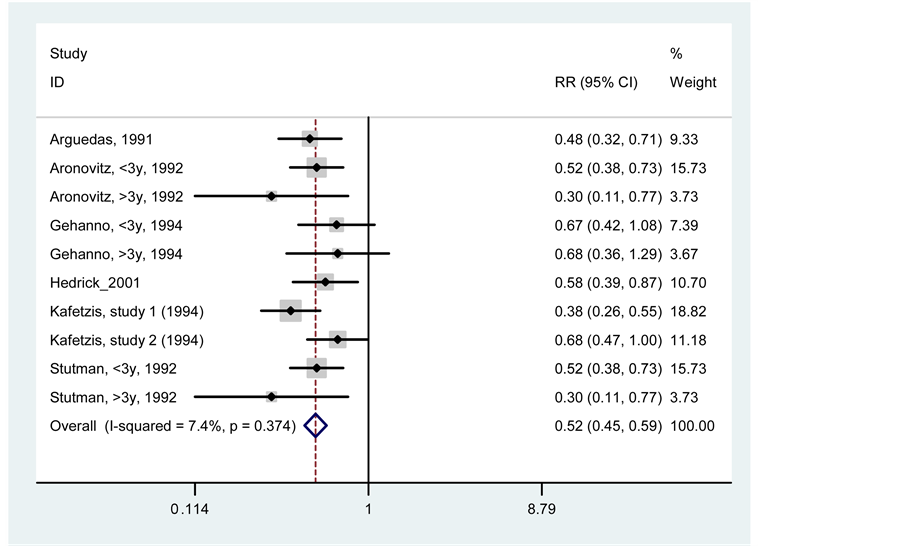
Figure 5. Meta-analysis of adverse effects between cefprozil and amoxicillin/clavulanate.
made in isolated studies [10] [15] [21] [22] . The most frequent adverse effects found were gastrointestinal distress (loose stools or diarrhea) and skin rash. This advantage is most likely related to the rate of intestinal absorption of both drugs. It is well known that antibiotics that are absorbed almost entirely have a lower risk of causing alteration of intestinal microbiota (dysbiosis), which explains the best intestinal tolerance of cefprozil in contrast with amoxicillin/clavulanate. In relation to this, previous studies have observed that cefprozil has a lower impact on commensal bacterial flora on the adenoids, suggesting a more selective antimicrobial effect for this antibiotic [15] . With respect to the treatment compliance, cefprozil has the advantage of frequency of administration, being administered twice daily compared with three daily doses for amoxicillin/clavulanate [19] -[21] [23] .
The better tolerability of cefprozil and its similar efficacy with amoxicillin/clavulanate makes this cephalosporin an important option to consider in the treatment of AOM in children [24] . As for the duration of treatment, no difference between the shortened and the standard therapy of 10 days was observed, which means cefprozil could be used in a short 5 day treatment, in contrast to the traditional 10-day treatment [25] for cases of acute bronchitis, tonsillitis, pharyngeal and skin infections [14] [25] -[27] . Finally, in vitro studies demonstrate an adequate penetration of cefprozil in tissues and fluids of the ear, features that support its benefit in more complicated scenarios as otitis media with effusion of the middle ear. In this clinical condition, concentrations up to 4.4 ug/ml can be quickly reached and maintained for up to 6 hours after administration of 15 mg/kg, and so, levels are higher than the minimum inhibitory concentration for S. pneumoniae (MIC90) [28] -[31] .
5. Conclusion
Considering the results of this meta-analysis, cefprozil is a good therapeutic option compared with amoxicillin/clavulanate by presenting similar clinical and microbiological efficacy with less risk of adverse effects.
Acknowledgements
Nina van der Stuyft1 for the valuable assistance in the implementation of this meta-analysis.
References
- McCaig, L.F. and Hughes, J.M. (1995) Trends in Antimicrobial Drug Prescribing among Office-Based Physicians in the United States. JAMA, 273, 214-219. http://dx.doi.org/10.1001/jama.1995.03520270048030
- McCaig, L.F., Besser, R.E. and Hughes, J.M. (2003) Antimicrobial Drug Prescription in Ambulatory Care Settings, United States, 1992-2000. Emerging Infectious Disease, 9, 432-437. http://dx.doi.org/10.3201/eid0904.020268
- Marom, T., Tan, A., Wilkinson, G.S., Pierson, K.S., Freeman, J.L. and Chonmaitree, T. (2013) Trends in Otitis Media-Related Health Care Use in the United States, 2001-2011. JAMA Pediatrics, 168, 68-75. http://dx.doi.org/10.1001/jamapediatrics.2013.3924
- Stool, S.E. and Field, M.J. (1989) The Impact of Otitis Media. The Pediatric Infectious Disease Journal, 8, S11-S14. http://dx.doi.org/10.1097/00006454-198901001-00006
- Marom, T., Nokso-Koivisto, J. and Chonmaitree, T. (2012) Viral-Bacterial Interactions in Acute Otitis Media. Current Allergy and Asthma Reports, 12, 551-558. http://dx.doi.org/10.1007/s11882-012-0303-2
- Faden, H., Duffy, L., Wasielewski, R., Wolf, J., Krystofik, D. and Tung, J. (1997) Relationship between Nasopharyngeal Colonization and the Development of Otitis Media in Children. Journal of Infectious Diseases, 175, 1440-1445. http://dx.doi.org/10.1086/516477
- Posfay-Barbe, K.M. (2012) Infections in Pediatrics: Old and New Diseases. Swiss Medical Weekly, 142, w13654.
- Harmes, K.M., Blackwood, R.A., Burrows, H.L., Cooke, J.M., Harrison, R.V. and Passamani, P.P. (2013) Otitis Media: Diagnosis and Treatment. American Family Physician, 88, 435-440.
- Lieberthal, A.S., Carroll, A.E., Chonmaitree, T., Ganiats, T.G., Hoberman, A., Jackson, M.A., Joffe, M.D., et al. (2013) The Diagnosis and Management of Acute Otitis Media. Pediatrics, 131, e964-e999. http://dx.doi.org/10.1542/peds.2012-3488
- Ball, P. (1994) Efficacy and Safety of Cefprozil versus Other Beta-Lactam Antibiotics in the Treatment of Lower Respiratory Tract Infections. European Journal of Clinical Microbiology & Infectious Diseases, 13, 851-856. http://dx.doi.org/10.1007/BF02111352
- Arguedas, A.G., Zaleska, M., Stutman, H.M., Blumer, J.L. and Hains, C.S. (1991) Comparative Trial of Cefprozil vs. Amoxicillin Clavulanate Potassium in the Treatment of Children with Acute Otitis Media with Effusion. Pediatric Infectious Disease Journal, 10, 375-380. http://dx.doi.org/10.1097/00006454-199105000-00006
- Stutman, H.R. and Arguedas, A.G. (1992) Comparison of Cefprozil with Other Antibiotic Regimens in the Treatment of Children with Acute Otitis Media. Clinical Infectious Diseases, 14, S204-S208. http://dx.doi.org/10.1093/clinids/14.Supplement_2.S204
- Kafetzis, D.A. (1994) Multi-Investigator Evaluation of the Efficacy and Safety of Cefprozil, Amoxicillin-Clavulanate, Cefixime and Cefaclor in the Treatment of Acute Otitis Media. European Journal of Clinical Microbiology & Infectious Diseases, 13, 857-865. http://dx.doi.org/10.1007/BF02111353
- Pichichero, M.E. and Casey, J.R. (2005) Acute Otitis Media: Making Sense of Recent Guidelines on Antimicrobial Treatment. Journal of Family Practice, 54, 313-322.
- Brook, I. and Foote Jr., P.A. (2004) Effect of Antimicrobial Therapy with Amoxicillin and Cefprozil on Bacterial Interference and Beta-Lactamase Production in the Adenoids. Annals of Otology, Rhinology, and Laryngology, 113, 902-905.
- Hedrick, J.A., Sher, L.D., Schwartz, R.H. and Pierce, P. (2001) Cefprozil versus High-Dose Amoxicillin/Clavulanate in Children with Acute Otitis Media. Clinical Therapeutics, 23, 193-204. http://dx.doi.org/10.1016/S0149-2918(01)80002-3
- Powers, J.L., Gooch 3rd, W.M. and Oddo, L.P. (2000) Comparison of the Palatability of the Oral Suspension of Cefdinir vs. Amoxicillin/Clavulanate Potassium, Cefprozil and Azithromycin in Pediatric Patients. Pediatric Infectious Disease Journal, 19, S174-S180. http://dx.doi.org/10.1097/00006454-200012001-00008
- Quach, C., Collet, J.P. and LeLorier, J. (2005) Effectiveness of Amoxicillin, Azithromycin, Cefprozil and Clarithromycin in the Treatment of Acute Otitis Media in Children: A Population-Based Study. Pharmacoepidemiology and Drug Safety, 14, 163-170. http://dx.doi.org/10.1002/pds.991
- Moher, D., Jadad, A.R., Nichol, G., Penman, M., Tugwell, P. and Walsh, S. (1995) Assessing the Quality of Randomized Controlled Trials: An Annotated Bibliography of Scales and Check-Lists. Controlled Clinical Trials, 16, 62-73. http://dx.doi.org/10.1016/0197-2456(94)00031-W
- Gehanno, P., Berche, P., Boucot, I., Lambert-Zechovskvy, N., Bingen, E., Gres, J.J. and Rollin, C. (1994) Comparative Efficacy and Safety of Cefprozil and Amoxycillin/Clavulanate in the Treatment of Acute Otitis Media in Children. Journal of Antimicrobial Chemotherapy, 33, 1209-1218. http://dx.doi.org/10.1093/jac/33.6.1209
- Barriere, S.L. (1992) Pharmacology and Pharmacokinetics of Cefprozil. Clinical Infectious Diseases, 14, S184-S188. http://dx.doi.org/10.1093/clinids/14.Supplement_2.S184
- Barriere, S.L. (1993) Review of in Vitro Activity, Pharmacokinetic Characteristics, Safety, and Clinical Efficacy of Cefprozil, a New Oral Cephalosporin. Annals of Pharmacotherapy, 27, 1082-1089.
- Kafetzis, D.A., Astra, H. and Mitropoulos, L. (1997) Five-Day versus Ten-Day Treatment of Acute Otitis Media with Cefprozil. European Journal of Clinical Microbiology and Infectious Diseases, 16, 283-286. http://dx.doi.org/10.1007/BF01695632
- Gupta, N., Bagga, V., Parmar, B.J., Kar, K., Mukherjee, A., Mehta, S. and Moharana, A.K. (2004) Efficacy and Tolerability Assessment of Cefprozil in Children with Acute Otitis Media. Indian Journal of Pediatrics, 71, 319-324. http://dx.doi.org/10.1007/BF02724098
- Brook, I., Aronovitz, G.H. and Pichichero, M.E. (2001) Open-Label, Parallel-Group, Multicenter, Randomized Study of Cefprozil versus Erythromycin in Children with Group A Streptococcal Pharyngitis/Tonsillitis. Clinical Therapeutics, 23, 1889-1900. http://dx.doi.org/10.1016/S0149-2918(00)89084-0
- Brook, I. and Foote, P.A. (1997) Effect of Penicillin or Cefprozil Therapy on Tonsillar Flora. Journal of Antimicrobial Chemotherapy, 40, 725-728. http://dx.doi.org/10.1093/jac/40.5.725
- Gupta, N., Mukherjee, A. and Moharana, A.K. (2004) Pediatric Tonsillopharyngitis—An Evaluation of Cefprozil in Indian Patients. Indian Journal of Pediatrics, 71, 601-605. http://dx.doi.org/10.1007/BF02724119
- Barbhaiya, R.H., Shukla, U.A., Gleason, C.R., Shyu, W.C., Wilber, R.B. and Pittman, K.A. (1990) Comparison of Cefprozil and Cefaclor Pharmacokinetics and Tissue Penetration. Antimicrobial Agents and Chemotherapy, 34, 1204-1209. http://dx.doi.org/10.1128/AAC.34.6.1204
- Wilber, R.B., Doyle, C.A., Durham, S.J., Conetta, B.J., DeGraw, S.S. and Leigh, A. (1992) Safety Profile of Cefprozil. Clinical Infectious Diseases, 14, S264-S271. http://dx.doi.org/10.1093/clinids/14.Supplement_2.S264
- Wiseman, L.R. and Benfield, P. (1993) Cefprozil. A Review of Its Antibacterial Activity, Pharmacokinetic Properties, and Therapeutic Potential. Drugs, 45, 295-317. http://dx.doi.org/10.2165/00003495-199345020-00008
- Nicolau, D.P., Sutherland, C.A., Arguedas, A.G., Dagan, R. and Pichichero, M.E. (2007) Pharmacokinetics of Cefprozil in Plasma and Middle Ear Fluid: In Children Undergoing Treatment for Acute Otitis Media. Pediatric Drugs, 9, 119-123. http://dx.doi.org/10.2165/00148581-200709020-00005


