Pharmacology & Pharmacy
Vol.5 No.1(2014), Article ID:41887,6 pages DOI:10.4236/pp.2014.51008
Dopamine D1- and D2-Receptors in Immunostimulation under Activation of Mu-Opioid Receptors in Mice with Different Psychoemotional States
![]()
Laboratory of the Mechanisms of Neurochemical Modulation, State Research Institute of Physiology and Basic Medicine, Siberian Branch of the Russian Academy of Medical Sciences, Novosibirsk, Russia.
Email: lalperina@yahoo.com
Received November 15th, 2013; revised December 25th, 2013; accepted January 3rd, 2014
ABSTRACT
The purpose of the present study was to analyze the effect of activation of mu-opioid receptors (mu-OR) on the immune response under blockade of postsynaptic D1- and D2-receptors in mice of the C57BL/6J strain displaying either aggressive or depressive-like behaviors in the social conflict model. It is shown that activation of activation of mu-OR with a highly selective agonist DAGO (100 µg/kg) increased significantly IgM-immune response not only in C57BL/6J mice with an unchanged psychoemotional state but also in mice displaying aggressive or depressive-like behaviors in the social stress model (10 days of agonistic confrontations). Selective blockade of DA receptors of the D1-type with SCH-23390 (1.0 mg/kg with DAGO administration) caused a more pronounced elevation of IgM-immune response than DAGO alone while DAGO effect was completely blocked by prior administration of D2-receptor antagonist haloperidol (1.0 mg/kg). At the same time, both SCH-23390 and haloperidol prevented the immune response increase induced by DAGO injection in mice engaged in aggressive or depressive-like behaviors. Thus, in animals not subjected to social stress DAGO-induced immunostimulation is provided only by D2-receptors, whereas in animals with altered psychoemotional state mu-opioid immunostimulation is mediated by both types of DA receptors—D1 and D2. These data provide evidence for different impacts of the main subtypes of DA receptors in the mediation of immunomodulating effects of mu-opioid system under normal and stressful conditions.
Keywords:Mu-Opioid and Dopamine Receptors; Social Stress; Aggression; Depressive-Like Behavior; Immunomodulation
1. Introduction
At present there is strong evidence for the involvement of central mu-opioid system in immunomodulation [1-5]. A series of neurophysiological and neuropharmacological studies indicate that immunomodulatory effects of this system are mediated by the DAergic mechanisms [4-8] which are known to provide immunostimulation [9,10]. Our previous data have shown that the nigrostriatal (nucleus caudatus) and mesolimbic (nucleus accumbens) DAergic structures are playing an important role in immunostimulation [10] induced by a highly selective [11] agonist of mu-opioid receptors (mu-OR) DAGO [4].
These brain structures have also been found to contain significantly high amounts not only of DA D1- and D2- receptors [12] but also of mu-OR [13]. There is also evidence that DAGO-induced activation of immune reactivity found in mice of the CBA strain not subjected to social stress is realized with the participation of DA receptors of the D2-type [4,7] that is consistent with other studies indicating close interconnections between muopioid and DA systems [14,15].
Recent data have shown that psychoemotional state of animals and humans can significantly affect immune functions [16-19]. At the same time, mu-opioid and DA mechanisms have been found to be implicated in the regulation of psychosocial stress [17,20-22].
In this connection, a role for the main types of DA receptors in DAGO-induced immunostimulation needs to be examined not only in normal healthy animals but also in animals subjected to psychoemotional stress. Therefore, the purpose of the present study was to analyze the effect of activation of mu-OR on the immune response under blockade of postsynaptic D1- and D2-receptors in mice of the C57BL/6J strain displaying either aggressive or depressive-like behaviors in the social conflict model.
2. Materials and Methods
2.1. Animals
The experiments utilized 122 male mice of the C57BL/6J strain weighing 22 - 24 g. The animals were maintained at the State Research Institute of Physiology and Basic Medicine SB RAMS and were housed under standard vivarium conditions and a natural light regime. Food and water were available ad libitum.
The study was performed in compliance with principles of the declaration of Helsinki and was approved by the local Ethics Committee of the State Research Institute of Physiology and Basic Medicine SB RAMS.
2.2. Behavioral Procedure
To produce aggressive and submissive behaviors in C57BL/6J mice, the model of sensory contact was used []">23]. Males were weighed and individually caged for 5 days to abolish group-living effects. Pairs of animals of nearly the same weight were placed in a steel 28 × 14 × 10-cm cage divided in half by a transparent partition with holes. This permitted animals to see and smell each other but prevented physical contact. After 2 days of adaptation to the housing conditions and sensory contact a test started. Every morning (11:00 a.m., local time), a steel cover of cage was replaced by a transparent one and, 5 min later (a period of individual activation) the partition was removed for 10 min, allowing an agonistic interaction between mice. Agonistic interactions were observed in an overwhelming majority of cages (90% - 100%). A daily test of social confrontations continued for 10 days; if animals did not fight, they were excluded from the experiment. Clear superiority of one partner was evident within two or three tests in daily social encounters in the same cage. One partner demonstrated aggression, attacking, biting and chasing the other one which displayed defense behaviors (sideways, upright postures, and “on the back” or “freezing”) during the tests. Submissive mice with experience of defeats during 10 tests of encounters have been shown to display depressive-like behavior and a high level of anxiety [20]. The control for wounding during the aggressive encounters did not show severe injuries in submissive or aggressive mice that could alter the immune parameters.
The group with an unchanged psychoemotional state comprised the group-housed males, after 5 days of individual housing, since, in this case, the submissiveness of grouped C57BL/6J had already disappeared while the repeated experience of aggression had not yet been acquired [20,]">23].
2.3. Drugs
Selective activation of mu-OR was performed by a structural analogue of enkephalin DAGO [D-Ala2, N-Me-Phe4, Gly5-ol]enkephalin (Sigma, Germany) at a dose of 100 µg/kg. To block postsynaptic DA D1- or D2-receptors their highly specific antagonists SCH-23390 [R(+)- 7-hloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro- 1H-3-benzazepinehydrochloride] (Sigma, Germany) and haloperidol (Gedeon Richter A.O., Hungary) respectively were used, both drugs at a dose of 1.0 mg/kg. Drugs were dissolved in saline and injected once intraperitoneally in a final volume of 0.2 ml. DAGO was administered 30 min prior to immunization, SCH-23390 or haloperidol— 5 - 10 min before DAGO. The doses for each drug and the routs of their administration used in the present study were chosen based on those previously reported to affect the corresponding receptor and immune reactivity [9].
Animals not subjected to social stress and mice engaged in aggression or depression were divided into groups receiving: 1) Vehicle (control); 2) DAGO alone; 3) Combination of SCH-23390 + DAGO; 4) Combination of haloperidol + DAGO.
2.4. Immunization
All groups of mice were immunized with sheep red blood cells (SRBC), which were suspended in saline and injected in the tail vein at a dose of 5 × 108 cells in 0.5 ml.
2.5. Immunological Assay
The immune response was assessed by measuring the number of antibody-forming cells (IgM-AFC) [24] in mouse spleen at the peak of the immune response (the fourth day after immunization).
2.6. Data Analyses
The significance of the mean differences between experimental groups (set at p < 0.05) was first analyzed by one-way analysis of variance (ANOVA) with subsequent multiple comparisons by the Student t-test using Statistics for Windows ver.10.0. All data are expressed as the mean ± SEM.
3. Results
3.1. Effect of Mu-Opioid Receptor Activation on the Immune Response under Blockade of Postsynaptic D1- and D2-Receptors in Mice with an Unchanged Psychoemotional State
Activation of mu-OR with a highly selective agonist DAGO (100 µg/kg) produced a significant increase of IgM-immune response (F(1,12) = 79.99; p < 0.001) in C57BL/6J mice having no experience of social encounters when compared to the vehicle-injected group (Figure 1).
As is seen in Figure 1, selective blockade of DA receptors of the D1-type with SCH-23390 (1.0 mg/kg with DAGO administration) caused a more pronounced elevation of IgM-immune response than DAGO alone (F(1,17) = 6.17; p < 0.02). In contrast, effect of DAGO was completely blocked by prior administration of a selective antagonist of D2-receptors haloperidol (1.0 mg/kg). In this case, the number of IgM-AFC did not differ from that of control (F(1,12) = 1.62; p > 0.05) and was significantly lower compared to animals receiving only mureceptor agonist (F(1,12) = 73.7; p < 0.001) (Figure 1).

Figure 1. Effect of the blockade of DA D1- and D2-receptors on immunostimulation caused by mu-opioid receptor (muOR) agonist DAGO in mice having no experience of social encounters. Activation of mu-OR with DAGO at 100 µg/kg (2) increased the number of IgM-AFC in C57BL/6J compared to the vehicle-injected control (1). Blockade of DA of the D1-receptors with SCH-23390 (1.0 mg/kg) enhanced DAGO induced immunostimulation (3). An antagonist of D2-receptors haloperidol (1.0 mg/kg) completely blocked DAGO-induced immunostimulation (4). All drugs were dissolved in saline and injected once intraperitoneally in a volume of 0.2 ml. DAGO was administered 30 min prior to immunization (SRBC 5 × 108), SCH-23390 or haloperidol— 5 - 10 min before DAGO. IgM-AFC number was tested on the 4th day after immunization. Each value indicates the mean ± SEM. Number of animals—7 - 10/group. *p < 0.001—statistical significance compared to the control. op < 0.002, oop < 0.001—compared to the DAGO.
3.2. Effect of Mu-Opioid Receptor Activation on the Immune Response under Blockade of Postsynaptic D1- and D2-Receptors in Aggressive Mice
DAGO at 100 µg/kg administered alone have been also found to increase IgM-immune response in mice conditioned to display aggressive behavior during 10 tests of daily confrontations (F(1,13) = 28.22; p < 0.001) compared to aggressive animals that did not receive the drug (control) (Figure 2(A)). The immune response level was also significantly higher the control values after co-administration of DAGO with either the selective D1-receptor antagonist SCH-23390 (F(1,12) = 23.11; p < 0.001) or D2-receptor antagonist haloperidol (F(1,12) = 14.88; p < 0.01), although the combination of the two drugs caused a less pronounced immunostimulatory effect than that of produced by DAGO (Figure 2(A)). An elevation of IgM-AFC numbers in aggressive mice resulting from combining SCH-23390 and DAGO (F(1,14) = 14.63; p < 0.002) or haloperidol and DAGO (F(1,15) = 15.40; p < 0.001) was two times lower than DAGO alone values.
3.3. Effect of Mu-Opioid Receptor Activation on the Immune Response under Blockade of Postsynaptic D1- and D2-Receptors in Mice with Depressive-Like State
Mu-OR activation with DAGO (100 µg/kg) also led to immunostimulation in mice with experience of 10 defeats in daily confrontations compared to depressive animals not treated with DAGO (control) (F(1,13) = 124.65; p < 0.001) (Figure 2(B)). A similar effect was found in C57BL/6J mice characterized by a high level of depression resulting from a long-term exposure (during 20 days) to the social defeat stress [21].
According to the present data, C57BL/6J mice at early stage of depression (10 days of confrontations) showed increased IgM-immune response when DAGO was combined with one of the DA antagonists: (F(1,12) = 38.4; p < 0.001) for SCH-23390 and (F(1,12) = 19.75; p < 0.001) for haloperidol compared to the control (Figure 2(B)). However, despite the fact that IgM-AFC numbers were elevated above control values after DAGO co-administration with either selective DA antagonist, their levels were significantly lower compared to the group receiving only DAGO (Figure 2(B)).
4. Discussion
It is well established that neuromediator/neuromodulator mu-opioidergic system is playing an important role in psychoneuroimmunomodulation. There is increasing evidence that activation of mu-OR by agonists of different
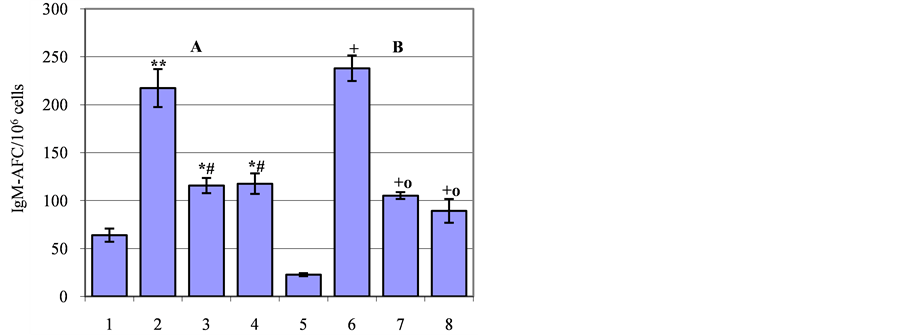
Figure 2. Effect of the blockade of DA D1- and D2-receptors on immunostimulation caused by mu-opioid receptor (muOR) agonist DAGO in aggressive (A) and depressive (B) C57BL/6J mice. Activation of mu-OR with DAGO at 100 µg/kg increased the number of IgM-AFC in aggressive (2) and depressive (6) mice compared to the vehicle-injected control (1 and 5 respectively). The blockade of DA D1-receptors with SCH-23390 at 1.0 mg/kg partially abolished DAGO effect both in aggressive (3) and depressive (7) mice. The blockade of D2-receptors with haloperidol at 1.0 mg/kg also partially abolished DAGO effect in aggressive (4) and depressive (8) mice. All drugs were dissolved in saline and injected once intraperitoneally in a volume of 0.2 ml. DAGO was administered 30 min prior to immunization (SRBC 5 × 108), SCH-23390 or haloperidol—5 - 10 min before DAGO. IgM-AFC number was tested on the 4th day after immunization. Each value indicates the mean ± SEM. Number of animals—7 - 10/group. *p < 0.01, **p < 0.001— statistical significance compared to the group 1; #p < 0.001—compared to the group 2; +p < 0.001—compared to the group 5; op < 0.001—compared to the group 6.
origin may produce a wide variety of effects on immune parameters [2,3,25-7]">27]. It has been shown earlier that administration of the most widely used agonist of mu-OR DAGO significantly increased the intensity of the immune response in mice of the CBA strain and Wistar rats with an unchanged psychoemotional state [1,4,9,26]. According to our previously reported data, the immunostimulatory effect of DAGO is mediated by central mechanisms via the hypothalamus-hypophysis complex [1,4].
In the present study DAGO-induced immunostimulation has been found in C57BL/6J mice having no experience of social confrontations or conditioned to display aggressive or depressive-like behavior during 10 tests of daily social encounters. This effect was more pronounced in depressive mice than that of unconditioned (control) or aggressive animals.
To date, the immunomodulatory effects of mu-agonists are known to be mediated by the DAergic mechanisms with the involvement of brain D1- and D2 receptors [4,5,7,8]. It should be noted that close mu-opioid/DA interconnections in the brain structures have been shown to be particularly important for the regulation of different physiological functions including behavioral [28,9]">29] and immune responses [4,5,7-9].
Our earlier data and results presented here demonstrate a significant role for the DA D2-receptors in mediating DAGO-induced activation of immune responsiveness in mice of the CBA [4,7] and C57BL/6J strains, which were not subjected to social stress. This conclusion is confirmed by the finding that the immunostimulatory effect of DAGO was completely blocked by D2-receptor antagonist haloperidol (1.0 mg/kg) while it remained unaffected after the blockade of D1-receptors with SCH- 23390 (1.0 mg/kg). At the same time, previous animal studies have indicated that both antagonists, which are known to block central DA receptors [9], produced the immune response suppression [4,7,9].
Unlike mice having no experience of social confrontations, the effect of DAGO on immunity was prevented by antagonists for the two receptor types in C57BL/6J mice showing either aggressive or depressive-like behaviors. Although, co-administration of DAGO with either D1- or D2-receptors antagonist (SCH-23390 and haloperidol, respectively) did not completely block the effect of activation of mu-OR on the immune response in animals with aggressive and depressive behaviors, these results indicate the requirement for D1- and D2-receptors in the mediation of DAGO-induced immunostimulation under psychoemotional stress.
Aggressive and depressive-like behaviors are known to be associated with changes in the level and distribution of serotonin (5-HT) and DA and their metabolites over brain structures [17,20,30-32], which appeared to be involved in the mechanisms of immunomodulation [9,10, 17]. There is also evidence for significant differences in immune reactivity of C57BL/6J mice engaged in aggression or depressive-like behavior [17,33]. Numerous data indicate that aggression is characterized by increasing activity of the DAergic system, known to stimulate immune functions, while depressive behavior is accompanied with changes in activity of the 5-HTergic system providing an inhibitory mechanism of immunomodulation [17,31,32].
Consistent with previous results [17,21,33], the present study has demonstrated that aggressive animals showed a higher immune responsiveness compared to animals with depressive-like behavior. At the same time depressivelike behavior is associated with the decreased immune function relative to that of controls and aggressive mice.
Despite the existing facts on the differences of the neurochemical pattern of the brain as well as the immune system functioning in animals displaying aggressive or depressive-like behaviors, our data indicate that the activation of mu-OR produced similar immunostimulatory effect in C57BL/6J mice with opposite types of behavior.
The enhanced immune response induced by the muOR agonist in aggressive animals is likely to be associated with further activation of the DAergic system. Immunostimulation found in mice with depressive-like behavior after DAGO injection seems to be also DAdependent due to the changes in the balance between functionally linked 5-HTand DAergic systems with the domination of the latter. Moreover, as is shown in the present study, DAGO-induced stimulation of immune response observed in mice with the opposite types of behavior is mediated by DA receptors of the D1- and D2-types.
Thus, our results provide evidence for different impact of the main subtypes of DA receptors in the mediation of immunomodulating effects of mu-opioid system under normal and stressful conditions. In animals not subjected to social stress DAGO-induced immunostimulation is provided only by D2-receptors, whereas in animals with altered psychoemotional state mu-opioid immunostimulation is mediated by both types of DA receptors—D1 and D2. These data give a new insight in the receptor mechanisms of the interactions between mu-opioid and DA systems, in which changing activity may contribute to a variety of neurological and psychiatric diseases [34,35], known to be associated with immune dysfunctions [36,37].
REFERENCES
- M. A. Cheido and G. V. Idova, “The Effect of Opioid Peptides on the Immunomodulation Process,” Rossijiskii Fiziologicheskiji Zhurnal imeni I. M. Sechenova, Vol. 84, No. 4, 1998, pp. 385-390.
- C. J. Nelson, G. M. Schneider and D. T. Lysle, “Involvement of Central Mubut not Deltaor Kappa-Opioid Receptors in Immunomodulation,” Brain, Behavior, and Immunity, Vol. 14, No. 3, 2000, pp. 170-184. http://dx.doi.org/10.1006/brbi.1999.0575
- R. T. Rahim, J. J. Meissler Jr., A. Cowan, T. J. Rogerts, E. B. Geller, J. Gaughan, M. W. Adler and T. K. Eisenstein, “Administration of Mu-, Kappaor Delta2-Receptor Agonists via Osmotic Minipumps Suppresses Murine Splenic Antibody Responses,” International Immunopharmacology, Vol. 1, No. 11, 2001, pp. 2001-2009. http://dx.doi.org/10.1016/S1567-5769(01)00128-X
- L. Devoino, M. Cheido, E. Alperina and G. Idova, “Evidence for a Role of Dopaminergic Mechanisms in the Immunostimulating Effect of μ-Opioid Receptor Agonist DAGO,” International Journal of Neuroscience, Vol. 113, No. 10, 2003, pp. 1381-1394. http://dx.doi.org/10.1080/00207450390231437
- T. B. Saurer, K. A. Carrigan, S. G. Ijames and D. T. Lysle, “Suppression of Natural Killer Cell Activity by Morphine Is Mediated by the Nucleus Accumbens Shell,” Journal of Neuroimmunology, Vol. 173, No. 1, 2006, pp. 3-11. http://dx.doi.org/10.1016/j.jneuroim.2005.11.009
- M. A. Cheido and G. V. Idova, “Dopaminergic Mechanisms in Immunostimulating Effect of μ-Opioid Receptors,” Bulletin of Experimental Biology and Medicine, No. 4, 1996, pp. 373-375.
- M. A. Cheido and G. V. Idova, “The Differential Contribution of Dopamine D(1) and D(2) Receptors to MuOpioidergic Immunomodulation,” Neuroscience and Behavioral Physiology, Vol. 37, No. 7, 2007, pp. 721-724. http://dx.doi.org/10.1007/s11055-007-0073-9
- M. A. Cheido and G. V. Idova, “Mu-Opioidergic Immunomodulation: The Neurochemical Basis,” Uspehi Fiziologicheskich Nauk, Vol. 40, No. 2, 2009, pp. 12-25.
- G. V. Idova, E. L. Alperina and M. A. Cheido, “Contribution of Brain Dopamine, Serotonin and Opioid Receptors in the Mechanisms of Neuroimmunomodulation: Evidence from Pharmacological Analysis,” International Immunopharmacology, Vol. 12, No. 4, 2012, pp. 618-625. http://dx.doi.org/10.1016/j.intimp.2012.02.010
- L. Devoino, E. Alperina, O. Galkina and R. Ilyutchenok, “Involvement of Brain Dopaminergic Structures in Neuroimmunomodulation,” International Journal of Neuroscience, Vol. 91, No. 3-4, 1997, pp. 213-228. http://dx.doi.org/10.3109/00207459708986378
- C. Ulens, M. Van Boven, P. Daenens and J. Tytgat, “Interaction of p-Fluorofentanyl on Cloned Human Opioid Receptors and Exploration of the Role of Trp-318 and His-319 in µ-Opioid Receptor Selectivity,” Journal of Pharmacology and Experimental Therapeutics, Vol. 294, No. 3, 2000, pp. 1024-1033.
- C. Missale, S. R. Nash, S. W. Robinson, M. Jaber and M. G. Caron, “Dopamine Receptors: From Structure to Function,” Physiological Reviews, Vol. 78, No. 1, 1998, pp. 189-225.
- A. Mansour, C. A. Fox, S. Burke, H. Akil and S. Watson, “Immunohistochemical Localization of the Cloned MuOpioid Receptor in the Rat CNS,” Journal of Chemical Neuroanatomy, Vol. 8, No. 4, 1995, pp. 283-305. http://dx.doi.org/10.1016/0891-0618(95)00055-C
- Y. Q. Ding, T. Kaneko, S. Nomura and N. Mizuno, “Immunohistochemical Localization of Mu-Opioid Receptors in the Central Nervous System of the Rat,” Journal of Comparative Neurology, Vol. 367, No. 3, 1996, pp. 375- 402. http://dx.doi.org/10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2
- L. M. Ambrose, E. M. Unterwald and E. J. Van Bockstaele, “Ultrastructural Evidence for Co-Localization of Dopamine D2 and μ-Opioid Receptors in the Rat Dorsolateral Striatum,” The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology, Vol. 279A, No. 1, 2004, pp. 583-591. http://dx.doi.org/10.1002/ar.a.20054
- R. Ader, “On the Development of Psychoneuroimmunology,” European Journal of Pharmacology, Vol. 405, No. 1-3, 2000, pp. 167-176. http://dx.doi.org/10.1016/S0014-2999(00)00550-1
- L. V. Devoino, G. V. Idova and E. L. Alperina, “Psychoneuroimmunomodulation: Behavior and Immunity. A Role for ‘The Neuromediator Pattern of the Brain’,” Novosibirsk: Nauka, 2009.
- E. Gomez-Lazaro, A. Arregi, G. Beitia, O. Vegas, A. Azpiroz and L. Garmendia, “Individual Differences in Chronically Defeated Male Mice: Behavioral, Endocrine, Immune, and Neurotrophic Changes as Markers of Vulnerability to the Effects of Stress,” Stress, Vol. 14, No. 5, 2011, pp. 537-548. http://dx.doi.org/10.3109/10253890.2011.562939
- A. R. Prossin, A. E. Koch, P. L. Campbell, M. G. Mclnnis, S. S. Zalcman and J. K. Zubieta, “Association of Plasma Interleukin-18 Levels with Emotion Regulation and µ- Opioid Neurotransmitter Function in Major Depression and Healthy Volunteers,” Biological Psychiatry, Vol. 69, No. 8, 2011, pp. 808-812. http://dx.doi.org/10.1016/j.biopsych.2010.10.014
- D. F. Avgustinovich, O. V. Alekseenko, I. V. Bakshtanovkaia, L. A. Koriakina, T. V. Lipina, M. V. Tenditnik, N. P. Bondar, I. L. Kovalenko and N. N. Kudriavtseva, “Dynamic Changes of Brain Serotonergic and Dopaminergic Activities during Development of Anxious Depression: Experimental Study,” Uspehi Fiziologicheskich Nauk, Vol. 35, No. 4, 2004, pp. 19-40.
- M. A. Cheido and G. V. Idova, “Immune Response of Submissive and Aggressive Mice under Conditions of Opioid Receptor Activation,” Bulletin of Experimental Biology and Medicine, Vol. 148, No. 6, 2009, pp. 886- 888. http://dx.doi.org/10.1007/s10517-010-0843-1
- N. P. Bondar, “Effect of Muand Kappa-Opioid Receptor Antagonists on Aggressiveness of Male Mice with Repeated Experience of Aggression,” Rossijiskii Fiziologicheskiji Zhurnal imeni I. M. Sechenova, Vol. 98, No. 6, 2012, pp. 681-692.
- N. N. Kudryavtseva, “А Sensory Contact Model for the Study of Aggressive and Submissive Behavior in Male Mice,” Aggressive Behavior, Vol. 17, No. 5, 1991, pp. 285-291.
- A. J. Cunningham, “A Method of Increased Sensitivity for Detecting Single Antibody Forming Cells,” Nature, Vol. 207, 1965, pp. 1106-1107. http://dx.doi.org/10.1038/2071106a0
- J. Kowalski, “Immunomodulatory Action of Class Mu-, Deltaand Kappa-Opioid Receptor Agonists in Mice,” Neuropeptides, Vol. 32, No. 4, 1998, pp. 301-306. http://dx.doi.org/10.1016/S0143-4179(98)90052-X
- M. A. Cheido and M. M. Gevorgyan, “Peculiarities of the Distribution of Cellular Subpopulations in the Spleen and Peripheral Blood of CBA Mice under Conditions of Activation of the Muand Delta-Opioid Systems,” Immunology, No. 5, 2011, pp. 886-887.
- S. V. Gein, T. A. Baeva, V. O. Nebogatikov and S. P. Tendryakova, “β-Endorphin Effects on Antibody Production, Proliferation, and Secretion of Th1/Th2 Cytokines in Vivo,” Bulletin of Experimental Biology and Medicine, Vol. 152, No. 5, 2012, pp. 595-599. http://dx.doi.org/10.1007/s10517-012-1584-0
- S. Ito, T. Mori and T. Sawaguchi, “Differential Effects of Micro-Opioid, Delta-Opioid and Kappa-Opioid Receptor Agonists on Dopamine Receptor Agonist-Induced Climbing Behavior in Mice,” Behavioural Pharmacology, Vol. 17, No. 8, 2006, pp. 691-701. http://dx.doi.org/10.1097/FBP.0b013e32801155a1
- E. G. Guy, E. Choi and W. E. Pratt, “Nucleus Accumbens Dopamine and Mu-Opioid Receptors Modulate the Reinstatement of Food-Seeking Behavior by Food-Associated Cues,” Behavioural Brain Research, Vol. 219, No. 2, 2011, pp. 265-272. http://dx.doi.org/10.1016/j.bbr.2011.01.024
- N. N. Kudriavtseva, T. G. Amstislavskaia, D. F. Avgustinovich, I. V. Bakshtanovskaia, T. V. Lipina and O. V. Gorbach, “The Effect of the Repeated Experience of Victories and Defeats in Social Conflicts on the Function of the Brain Serotoninergic System in Male Mice,” Zhurnal Vyssheji Nervnoji Deiatel’nosti imeni I. P. Pavlova, Vol. 46, No. 6, 1996, pp. 1088-1096.
- L. V. Devoino, E. L. Al’perina, E. Podgornaia, O. V. Poliakov, G. V. Idova and RIu. Il’iuchenok, “Involvement of Dopaminergic Systems in the Functional Specialization of Brain Areas during Formation of Aggressive and Submissive Behavior in Mice,” Zhurnal Vyssheji Nervnoji Deiatel’nosti imeni I. P. Pavlova, Vol. 51, No. 2, 2001, pp. 197-205.
- L. Devoino, E. Alperina, E. Podgornaja, R. Ilyutchenok, G. Idova and O. Poljakov, “Regional Changes of Brain Serotonin and Its Metabolite 5-Hydroxyindolacetic Acid and Development of Immunosuppression in Submissive Mice,” International Journal of Neuroscience, Vol. 114, No. 9, 2004, pp. 1049-1062. http://dx.doi.org/10.1080/00207450490450172
- L. Devoino, E. Alperina and T. Pavina, “Immunological Consequences of the Reversal of Social Status in C57BL/ 6J Mice,” Brain, Behavior, and Immunity, Vol. 17, No. 1, 2003, pp. 28-34. http://dx.doi.org/10.1016/S0889-1591(02)00037-5
- L. H. Shen, M. H. Liao and Y. C. Tseng, “Recent Advances in Imaging of Dopaminergic Neurons for Evaluation of Neuropsychiatric Disorders,” Journal of Biomedicine and Biotechnology, Vol. 2012, 2012, pp. 259-349. http://dx.doi.org/10.1155/2012/259349
- E. Scarr, T. T. Money, G. Pavey, J. Neo and B. Dean, “Mu Opioid Receptor Availability in People with Psychiatric Disorders Who Died by Suicide: A Case Control Study,” BMC Psychiatry, Vol. 12, 2012, pp. 126-137. http://dx.doi.org/10.1186/1471-244X-12-126
- N. Müller and M. J. Schwarz, “Immune System and Schizophrenia,” Current Immunology Review, Vol. 6, No. 3, 2010, pp. 213-220. http://dx.doi.org/10.2174/157339510791823673
- J. Blume, S. D. Douglas and D. L. Evans, “Immune Suppression and Immune Activation in Depression,” Brain, Behavior, and Immunity, Vol. 25, No. 2, 2011, pp. 221- 229. http://dx.doi.org/10.1016/j.bbi.2010.10.008

