Pharmacology & Pharmacy
Vol.4 No.1(2013), Article ID:27006,6 pages DOI:10.4236/pp.2013.41008
Stability of Nafcillin Sodium Solutions in the Accufuser® Elastomeric Infusion Device
![]()
1Department of Nursing, College of Nursing, Yonsei University, Seoul, South Korea; 2Department of Pharmacology & Clinical Pharmacology Lab, College of Medicine and Division of Molecular Therapeutics Development, Hanyang Biomedical Research Institute, Hanyang University, Seoul, South Korea.
Email: jskang@hanyang.ac.kr
Received October 25th, 2012; revised November 30th, 2012; accepted December 18th, 2012
Keywords: Stability; Nafcillin Sodium; Intravenous Elastomeric Infusion Device (Accufuser®)
ABSTRACT
The aim of this study was to investigate the stabilities of two kinds of solutions of nafcillin sodium (2.5 mg/mL) in 0.9% sodium chloride solution (NS, normal saline) and in injectable 5% dextrose water (D5W) in the intravenous elastomeric infusion device (Accufuser®) based on recommended solutions and storage periods. The injectable nafcillin solutions (NSand D5W-nafcillin) in the Accufuser® device were stored and evaluated at controlled temperatures (room temperature, RT, 5˚C ± 2˚C and cold temperature, CT, 4˚C ± 2˚C) during 6 weeks. Effects of the periods of storage (from 0 to 6 weeks) and the temperatures of storage (RT and CT) on the physicochemical appearances and concentrations of active compounds were determined. The visual clarity, pH, and concentrations of nafcillin sodium were determined by stability-indicating high-performance liquid chromatography (HPLC)-ultraviolet (UV) detection. The results showed that in NS and D5W solutions, the amount of nafcillin slightly changed and remained 92.66% and 97.30% of their initial amounts at CT during 6 weeks, respectively. On the other hand, in NS and D5W solutions at RT, the amount significantly decreased with time and reached 27.66% and 31.97% of their initial amounts during 4 weeks, respectively. Slight decrement of pH was observed in CT storage while significant change was observed in the RT storage. Moreover, in CT, no significant changes in physical appearances and colors of the solutions were observed during the study. However, the solutions changed into yellowish color and some particles were detected in both kinds of nafcillin solutions (NS and D5W) after 1.5 weeks in RT conditions. To sum up, under CT two kinds of nafcillin sodium solutions (NS and D5W) were stable with time in Accufuser® without any significant physical changes and retained almost all of the initial concentrations up to 6 weeks. However, the solutions were not stable in RT storage. We suggest that nafcillin sodium solutions in an Accufuser® should be preferentially diluted in NS and D5W while storing in CT condition.
1. Introduction
The disposable silicon balloon infusion device (Accufuser®, Woo Young Medical Co. LTD., Seoul, South Korea) is a well-established simplified silicon-based elastomeric system for the administration of antibiotics and other drugs or nutrients that are suitable for patients as well as healthcare providers. An increasing number of patients are being treated as outpatients, and for them, drugs are often infused using portable pumps or infusion devices outside the hospital [1,2]. The disposable silicon balloon infusion device is advantageous in home health care setting because it is disposable, relatively inexpensive compared to electronic pumps, and it is easy for the patient to fill and manipulate [3]. The increasing popularity of the use of portable infusion devices outside hospital raises the issue of the stability of drugs likely to be administered with such devices. Conditions surrounding the infusion outside hospitals are very different from the conditions of the infusion in hospital settings. Concentrations of drug in solution, temperatures of the environment immediately outside the drug container, bore of the tubing, and fluid flow rates may differ. In contrast to hospital practice, infusion-pump drug reservoirs in temporary clinic or home care setting are routinely loaded with sufficient doses of a drug, which may last for 24 hours or more, and it is not easy to check the solutions for the stability of the drug content [4]. To be suitable for self-administration by home-based patient, the drug should be stable in solution for a number of days under home storage conditions. Therefore, it is necessary to determine the physical and chemical stabilities of the admixtures in the infusion system before they are used. The study was done with nafcillin sodium solutions made with NS and D5W because these are the most available infusion solutions for nafcillin sodium administration. The purpose of this study was to evaluate the physicochemical stability of nafcillin sodium (Figure 1) solutions (2.5 mg/mL, normal saline, NS and 5% dextrose water, D5W) packed in sterile Accufuser® device in which each samples were stored and evaluated at appropriate intervals up to 6 weeks under different storage conditions (room temperature, RT, 25˚C ± 2˚C and cold temperature, CT, 4˚C ± 2˚C).
2. Materials and Methods
2.1. Materials
Nafcillin sodium was purchased from Sigma Co., USA. Normal saline (NS, 0.9% NaCl in water) and 5% dextrose (D5W, injectable 5% dextrose water) were purchased from Choongwae Pharmaceutical Co. Ltd., South Korea. Disposable silicon balloon infusion device (Accufuser®) was obtained from Woo Young Medical Co., Ltd, South Korea. Potassium phosphate monobasic was purchased from Sigma Co., USA, and all the chemicals for HPLC analysis were all HPLC-grade and were prepared immediately before use.
2.2. Preparation and Sampling of Solutions
To prepare the test samples, the appropriate amounts of nafcillin sodium were added to a portion of the infusion solutions and were brought to a final volume of 100 mL with NS and D5W. The test solutions were packed in sterile Accufuser® system for testing. All manipulations were aseptically performed under laminar airflow in a biological safety cabinet. The nominal nafcillin sodium concentration for the sample used in this testing was 2.5 mg/mL. The Accufuser® systems were each filled with 100 mL of drug solution. Triplicate test solutions were prepared and stored in RT and CT conditions. After the Accufuser® systems were filled, an extension-tubing set was attached and primed. About 3 mL of drug solutions was collected in a 4 mL glass vial with a Teflon-lined screw cap and stored in a freezer at −70˚C until the analyzing process. One sample (approximately 3 mL) was obtained from the distal end of the tubing after 48 h, 1, 2, 4, and 6 weeks at RT and CT and stored in the manner described for the time-zero samples (Table 1).
2.3. Methods
The physical stability of the nafcillin sodium solutions were assessed by visual examination and HPLC analysis. Visual examinations were performed in normal diffuse fluorescent room light with unaided eye and high-intensity monodirectional light. The pH of the solutions was measured by a stainless electrode pH meter (Thermo Scientific Co., MA, USA). The drug concentrations were determined using a stability-indicating HPLC assay method based on several references [4-7]. The HPLC system [4, 7] consisted of an isocratic solvent delivery pump (Model 515, Waters Scientific Co. USA) which pumped a mixture (v/v, 65/35, pH 3.0) of acetonitrile (ACN, Sigma Co. USA) in 0.05M potassium phosphate (Sigma Co. USA)

Figure 1. Chemical Structure of nafcillin sodium (C21H22N2NaO5S·H2O), MW = 454.47.
Table 1. Study designs for the stability testing of nafcillin sodium solutions in Accufuser® system.
 *O: processed sample, RT: room temperature, CT: cold temperature, NS: normal saline, D5W: 5% dextrose water.
*O: processed sample, RT: room temperature, CT: cold temperature, NS: normal saline, D5W: 5% dextrose water.
as a mobile phase through a Capsell Pak C18 UG120 (4.6 × 150 mm, 5 μm, Shiseido Co., Japan) column at 1200 µL/min. The ratio of ACN to 0.05 M potassium phosphate (65/35) was held constant during the chromatographic run. The samples of 3.0 µL were injected into the HPLC system using an autosampler (Nanospace SI-2, Shiseido Co., Japan). The effluent from the column was monitored with a variable wavelength ultraviolet detector (Nanospace SI-1, Shiseido Co., Japan) at 220 nm. The integration of the chromatograms was performed by dsCHROM® software (Do-Nam Instrumental Co., Seoul, Korea). The method was validated for linearity, precision (inter-day and intra-day), accuracy and selectivity [7]. The experiment was repeated three times on the same day and additionally on two consecutive days to determine interand intra-day precisions. Assays of control solutions from nafcillin sodium solutions (2.5 mg/mL) were undertaken to calculate the intra-day and inter-day variations using external standard method. Linearity was evaluated by serial dilutions of nafcillin sodium solutions with NS and D5W for analysis. The stability of nafcillin sodium infusion solutions was determined in disposable Accufuser® infusion device during 6 weeks of storage under RT and CT conditions. Periodically, samples were evaluated for appearances, visible particles, pH and chromatographic analysis. We analyzed the concentration of nafcillin sodium in two solutions at 0, 48 h, 1, 2, 4, and 6 weeks after the preparation of solutions by HPLCUV method. On each day, 1.0 mL of samples of nafcillin sodium solution with a nominal concentration were drawn from Accufuser® infusion device for chromatographic analysis and 3.0 µL were directly injected into HPLCUV system. Three aliquots of each solution were processed. Statistical analysis was performed using one-way ANOVA with the level of significance set at 0.05 (PCS, v. 4.0, Springer-Verlag, New York, USA).
3. Results
3.1. Method Validation
The linearity of calibration curves of nafcillin sodium solution was established which showed good linearity over the range concentration of 0.5 ~ 2.8 mg/mL (r2 =
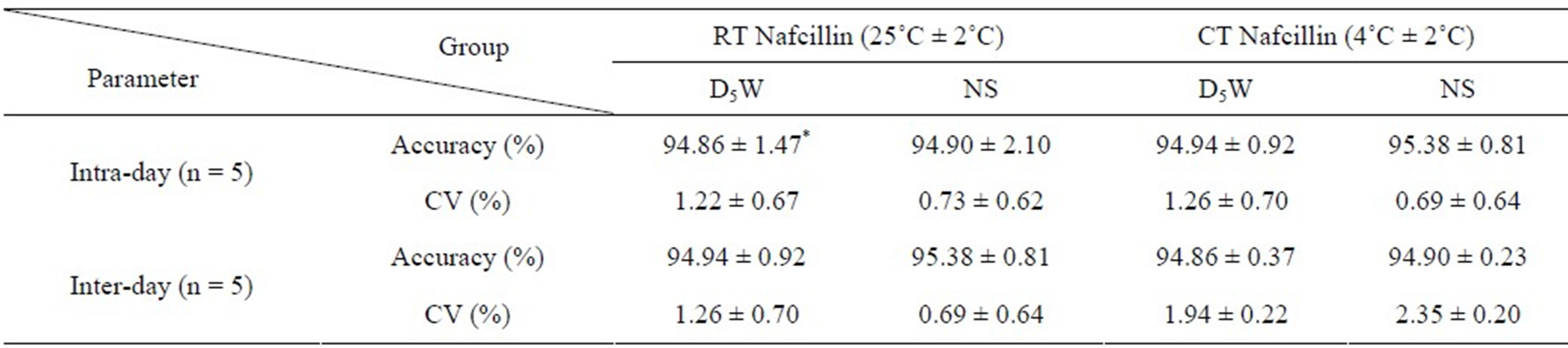
0.9951, Figure 2). Table 2 lists the relative standard deviation (R.S.D.) data obtained from the analysis of the samples on the same day (n = 5) and on consecutive days (n = 5). The R.S.D values were <1.26% and <2.35% for intra-day and inter-day results, respectively, meaning that the method was sufficiently precise.
3.2. Physical Appearance and pH Status
There were no significant changes in physical appearances and clarities of the solutions in CT condition. The color of the samples was transparent with no color discoloration. Particles were not detected. However, the color of NS solutions became opaque after 10 days of study in RT condition. After 2 weeks, solutions changed into yellowish color and particles were detected. On the 4th week, the filter of the silicone balloon infuser was partially clogged, and the injected samples did not flow out with the normal flow rate of 2.0 mL/h. The flow rate was less than the half of the normal flow rate. At the 6th week, the filter of the silicone balloon infuser was completely clogged and the injected samples did not flow out at all. Similarly, the color of D5W solutions in RT condition became opaque after 2 weeks of study and changed into yellowish color at the 3rd week. At the 4th week, particles were detected and at the 6th week, the filter of the silicone balloon infuser was completely clogged and the injected samples did not flow out at all. The pH of D5W and NS solutions slightly decreased from 6.84 to 6.06
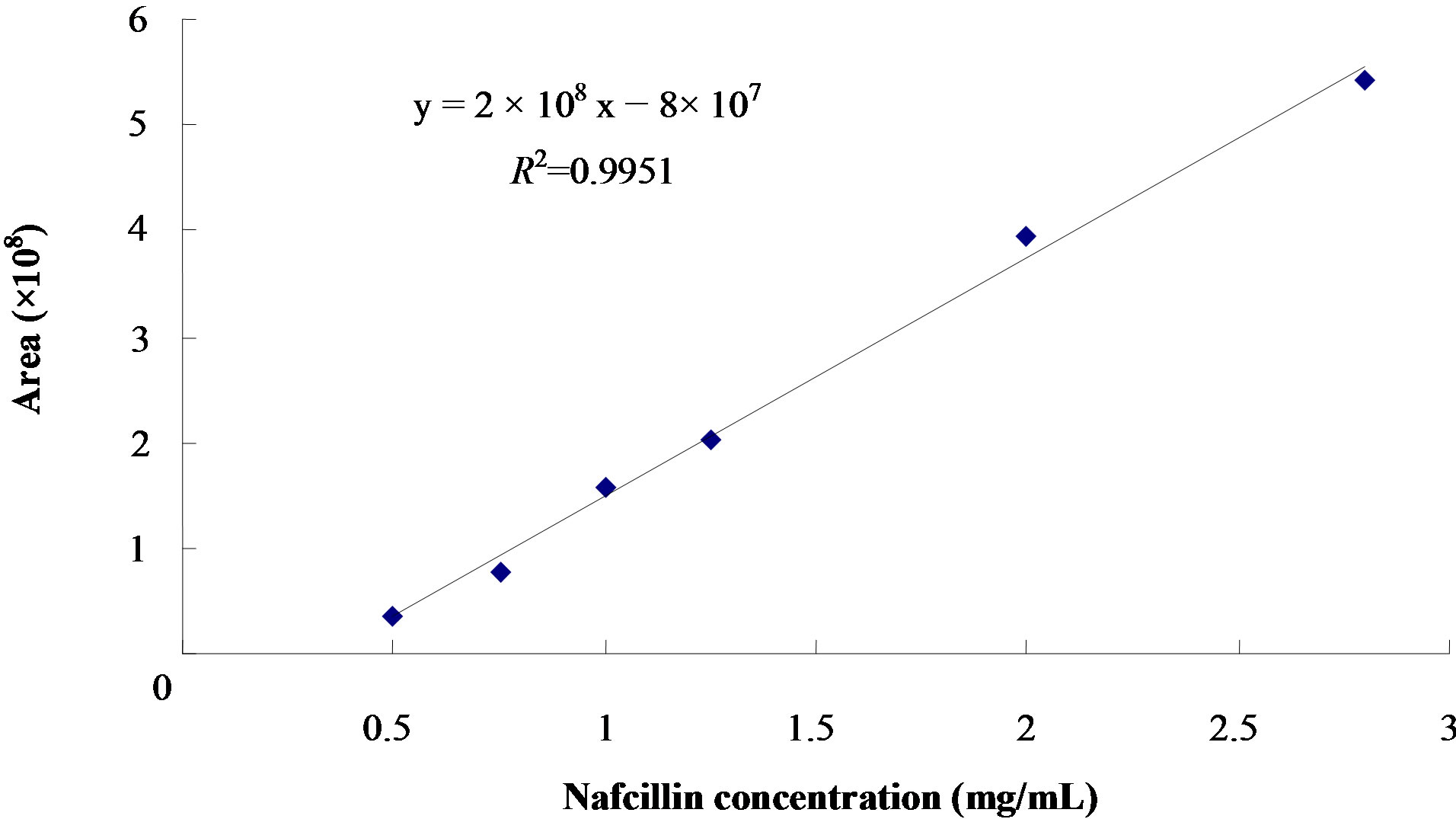
Figure 2. Calibration curve for the determination of nafcillin sodium concentrations in study solutions. Range (0.5 ~ 2.8 mg/mL), Area = Peak area.
Table 2. Intra-day and Inter-day precision studies (n = 5).
and from 6.51 to 5.87 in the CT condition, but significantly (p < 0.05) decreased from 6.85 to 4.49 and from 6.57 to 4.11 in the RT condition during 6 weeks (Table 3).
3.3. Concentration
The typical HPLC chromatogram of nafcillin sodium (2.5 mg/mL) is shown in Figure 3. The retention time for nafcillin sodium was about 10.2 min. The initial concentration and the percentage of the remaining concentration which were observed at analytic time during 6 weeks for each nafcillin sodium solutions and storage conditions are listed in Table 4. The concentration of nafcillin sodium decreased significantly with time and reached 31.97% in D5W and 27.66% in NS after 4 weeks under RT condition. However, 97.30% of initial concentration remained in D5W and 92.66% remained in NS under CT condition.
Table 3. The change of pH (Mean ± SD, %) of nafcillin sodium solutions according to the time of storage.
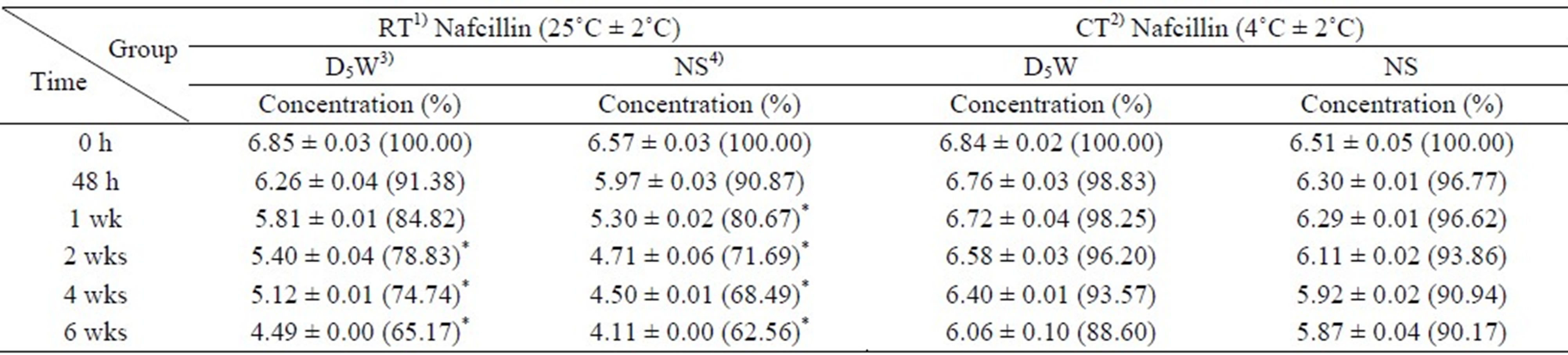
1)RT: room temperature, 2)CT: cold temperature, 3)D5W: 5% dextrose, 4)NS: normal saline, *p < 0.05 vs 0 h.
Table 4. The retention of the amount (%) of nafcillin sodium in solutions (NS and D5W) according to each storage temperature (RT and CT) and time (0 ~ 6 weeks).
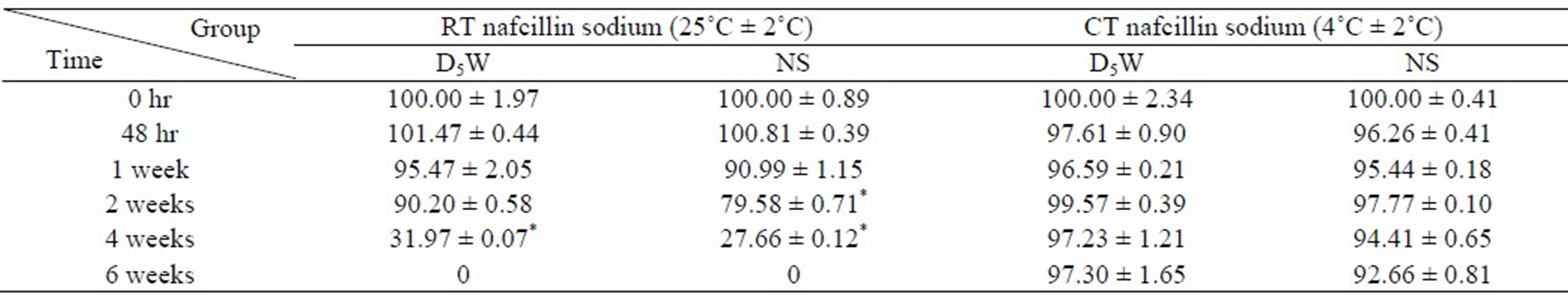
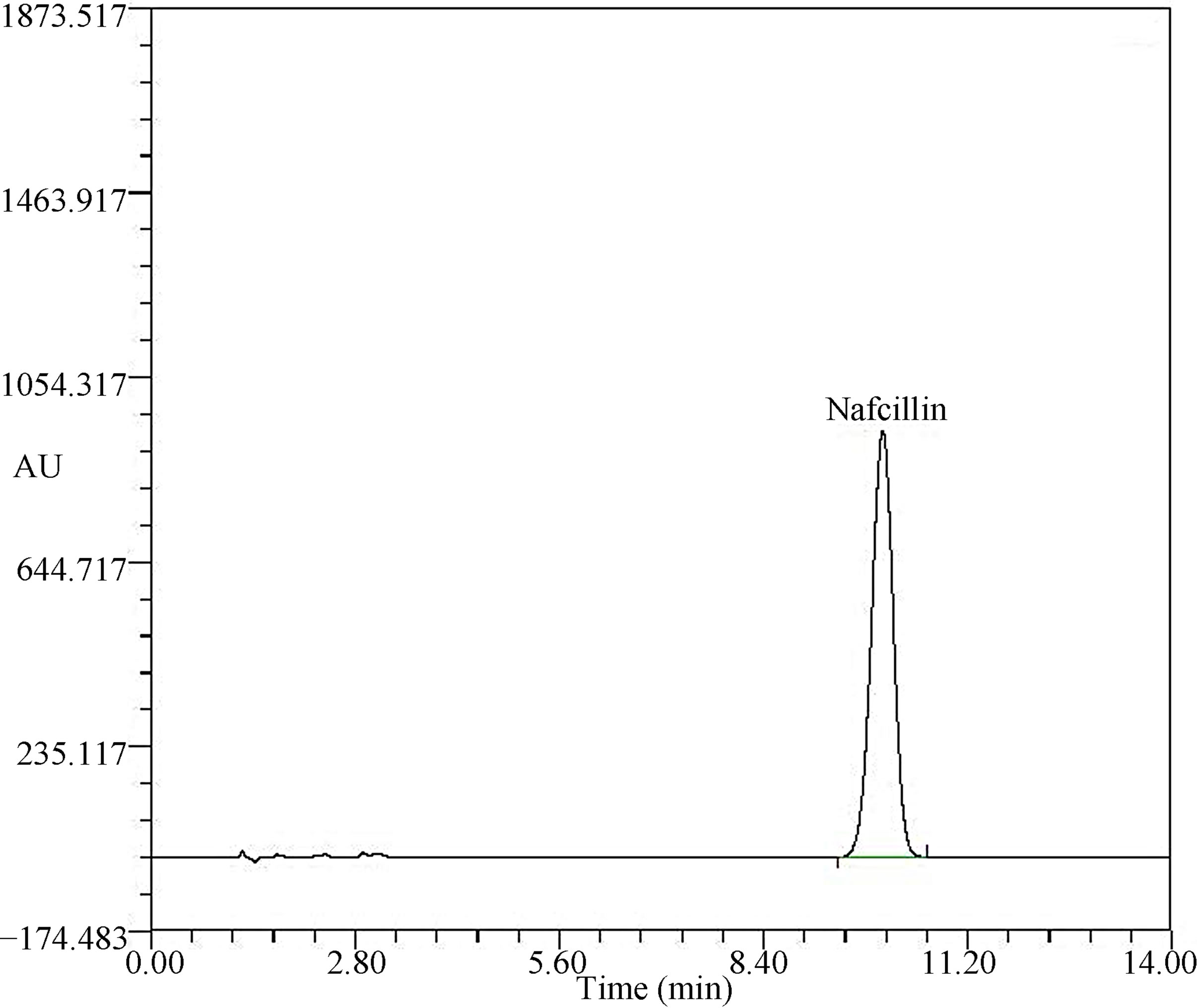
Figure 3. Typical chromatogram of standard solutions (NS and D5W) containing nafcillin sodium of 2.5 mg/mL, Y-axis (Absorbance Units), X-axis (Retention Time, min).
4. Discussion and Conclusion
Drugs that are altered from their original form (e.g. diluted, reconstituted or mixed with other drugs) or aliquoted into a new container do not usually keep their original expiration dates. Diluting, mixing, or transferring to a new container may cause the drugs to lose potency and degrade due to changes in pH, buffering, atmosphere (some drugs are packed under special gases such as argon or nitrogen), or precipitation. In addition, drugs that are kept in a sealed, sterile container may be contaminated and subjected to bacterial degradation [8]. Over the past 20 years, outpatient parenteral anti-microbial therapy has proved to be effective, safe, and economical for patients with a wide range of infectious diseases [9,10]. Outpatient parenteral anti-microbial therapy exemplifies the change of focus from inpatient care to outpatient, ambulatory care. A great number of new modified catheters and infusion devices are available. Therefore, further studies of drug stability under conditions of the usage of various infusion devices are necessary [11]. Stability of drug in solutions is highly influenced by many factors, including temperature, pH, medium and final concentration. Stability is defined as the retention of >90% of the initial drug concentration. If any drug or drug mixture is aliquoted into an unsealed or non-sterile container or if it is contaminated by non-aseptic technique, it would have a maximum of 24-hour expiration period unless it immediately becomes frozen. Frozen aliquots can usually be kept indefinitely (at –90˚C), but once thawed, they have a maximum of 24-hour shelf life. IV fluids (e.g. NS, lactated Ringers) expire after 24 hours of opening if kept unrefrigerated, and may be kept up to 1 week if refrigerated. The stability of nafcillin sodium (20 mg/L) in normal saline solution maintained for 24 hours, 4 days and 12 weeks in the three storage temperature of RT, CT and –20˚C, respectively [3]. Ascertaining the stability not only ensures that the active ingredient(s) are present, but also limits the concentrations of degradation products, which may have toxicological significance [12,13]. Therefore, stability-indicating assays for labeled active ingredients in dosage forms are essential for the safety of such formulations [14]. In this study, we tried to investigate the more various serial periods of storage conditions and to confirm stability of low concentration of nafcillin sodium (2.5 mg/mL) because of improvement of range of options of storage period and concentrations of nafcillin sodium in clinical situations than other papers [3,4,11]. Table 4 presents the better stability of nafcillin sodium in D5W under CT than NS under RT condition. Storage temperature may be the main effect on drug stability. It shows the maintenance of its stability during 2 weeks and 1 week in the NS and D5W solution, respectively under RT condition and 6 weeks in both solutions under CT condition.
5. Acknowledgements
This study was supported by Division of Molecular Therapeutics Development, Hanyang Biomedical Research Institute, Hanyang University and Woo Young Medical Co. LTD., Seoul, South Korea.
REFERENCES
- M. A. Kang and J. S. Kang, “Stability Test of Ampicillin Sodium Solutions in the Accufuser® Elastomeric Infusion Device Using HPLC-UV Method,” Pharmacology & Pharmacy, Vol. 3, No. 4, 2012, pp. 462-467.
- G. A. Lee, M. J. Kim, M. Kang, et al., “Stability of Commonly Used Antibiotics Solutions in the Accufuser® Elastometric Infusion Device under Recommended Storage and Used Conditions,” The Open Nutraceuticals Journal, Vol. 4, 2011, pp. 125-129. doi:10.2174/1876396001104010125
- L. V. Allen Jr., M. L. Stiles, S. J. Prince and J. Smeeding, “Stability of 14 Drugs in the Latex Reservoir of an Elastomeric Infusion Device,” American Journal of HealthSystem Pharmacy, Vol. 53, No. 22, 1996, pp. 2740-2743.
- M. L. Stiles and L. V. Allen Jr., “Stability of Nafcillin Sodium, Oxacillin Sodium, Penicillin G Potassium, Pencillin G Sodium, and Tobramycin Sulfate in Polyvinyl Chloride Drug Reservoirs,” American Journal of HealthSystem Pharmacy, Vol. 54, 1997, pp. 1068-1070.
- Y. Zhang and L. A. Trissel, “Physical Instability of Frozen Pemetrexed Solutions in PVC Bags,” Annals of Pharmacotherapy, Vol. 40, No. 7-8, 2006, pp. 1289-1292.
- L. A. Trissel and Y. Zhang, “Stability of Methylprednisolone Sodium Succinate in Autodose Infusion System Bags,” Journal of American Pharmacists Association, Vol. 42, No. 6, 2002, pp. 868-870.
- V. Kumar, H. Bhutani and S. Singh, “ICH Guidance in Practice: Validated Stability-Indicating HPLC Method for Simultaneous Determination of Ampicillin and Cloxacillin in Combination Drug Products,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 43, No. 2, 2007, pp. 769-773.
- Research Committees of Boston University Research Copmliance, “Conditional Use of Expired Medical Materials,” 2012. http://www.bu.edu/orccommittees/iacuc/policies-and-guidelines/conditional-use-of-expired-medical-materials/
- A. Antoniskis, B. C. Anderson, E. J. van Volkinburg, J. M. Jackson and D. N. Gilbert, “Feasibility of Outpatient Self-Administration of Parenteral Antibiotics,” The Western Journal of Medicine, Vol. 128, 1978, pp. 203-206.
- D. M. Poretz, D. Rich, J. O. Morales, L. V. Behren, et al., “Outpatietns Use of Intravenous Antibiotics,” American Journal of Medicine, Vol. 97, Suppl. 2A, 1994, pp. 1-55. doi:10.1016/0002-9343(94)90280-1
- A. J. J. Wood, “Outpatient Parenteral Antimicrobial-Drug Therapy,” New England Journal of Medicine, Vol. 337, No. 12, 1997, pp. 829-838. doi:10.1056/NEJM199709183371207
- E. Boven, M. M. Nauta, F. P. Schulper, et al., “Secondary Screening of Platinum Compounds in Human Ovarian Cancer Xenografts in Nude Mice,” European Journal of Cancer and Clinical Oncology, Vol. 25, 1995, pp. 276- 283.
- J. P. Fee and G. H. Thompson, “Comparative Tolerability of Profiles of the Inhaled Anesthetics,” Drug Safety, Vol. 16, No. 3, 1997, pp. 157-170.
- D. F. Driscoll, “Stability and Compatibility Assessment Techniques for Total Parental Nutrition Admixtures: Setting the Bar According to Pharmacopeial Standards,” Current Opinion in Clinical Nutrition & Metabolic Care, Vol. 8, 2005, pp. 297-303. doi:10.1097/01.mco.0000165009.24202.64

