Pharmacology & Pharmacy
Vol.3 No.1(2012), Article ID:16947,6 pages DOI:10.4236/pp.2012.31008
Antidepressant-Like Activity of Tetramethylpyrazine Measured by Chronic Experimental Method in Rat Model of Depression
![]()
1Laboratory of Marine Materia Medica, Guangdong Ocean University, Zhanjiang, China; 2Depu Biomedical Technology Co., Ltd, Foshan, China; 3Cell Biology Group, Department of Surgery, Department of Pathology, University of Maryland School of Medicine and Baltimore Veterans Affairs Medical Center, Baltimore, USA.
Email: *ywyj9578@sohu.com, *yutingxi@yahoo.com
Received October 4th, 2011; revised November 14th, 2011; accepted December 9th, 2011.
Keywords: Tetramethylpyrazine; Antidepressive-Like Activity; Chronic Mild Stress; Rats
ABSTRACT
The aim of this study was to investigate the potential antidepressive-like effect of tetramethylpyrazine (TMP), one of available blood-activating and stasis-eliminating components from traditional Chinese medicines, in chronic mild stress (CMS)-induced rat model of depression. Male Sprague-Dawley (SD) strain rats were divided into six matched groups (n = 14 in each group) based on their sucrose consumption: control, CMS, CMS + fluoxetine (FLU), and CMS + TMP groups. The rats except control were housed separately in different rooms, and the rat model of depression was established by exposing to an unpredictable sequence of stressors for 28 days; the rats in CMS + FLU were exposed to CMS and received administration of FLU (2.0 mg/kg/d, ig) for 28 days; the rats in CMS + TMP groups were exposed to CMS and received administration of TMP (10, 20, 40 mg/kg/d, ig), respectively, for 28 days. The rats in control group were given ordinary daily care, and the rats in control and CMS groups received ig administration of normal saline instead of FLU or TMP. The body weight, food intake and fluid consumption were measured, and the behaviors were examined by open field test before and after CMS, and forced-swimming test was performed 1 day after last unpredictable stressor. Chronic administration of TMP partially reversed the effects of CMS on consumption of sucrose solution and locomotion and exploration behavior, and potently shortened the immobility time during forced-swimming test following CMS in rats. The results showed that long-term administration of TMP partially reversed the effects of CMS on the consumption of sucrose solution, the squares crossing and rearing in open field test, and the immobility time during forcedswimming test in rats. The present data provide evidences that TMP possesses obvious antidepressant-like activity in CMS-induced rat model of depression.
1. Introduction
Some medicinal plants and traditional Chinese medicines and herbs or their extracts have showed antidepressantlike effects [1-4]. Ligusticum chuanxiong Hort has the action of activating blood, regulating qi and expels wind [5]. Chuanxiongzine, 2,3,5,6-tetramethylpyrazine (TMP) (Figure 1), was isolated from several traditional Chinese medicines, such as Ligusticum Chuanxiong Hort, Curcuma aromatica Salisb, and Jatropya podagrica Hook [6]. TMP is colorless needle crystal, and is soluble in hot water.
TMP can inhibit L-type calcium-channel current and release of intracellular calcium store in hippocampal neuronal cells, and exerts nerve protective effect [7]. Chinese scientists have contributed a lot to its investigation, and as an available blood-activating and stasiseliminating component, it has been extensively applied to the treatment of vascular diseases of heart and brain, and obtained excellent efficiency in China [8]. Our previous experimental results revealed that that TMP possesses potent acute antidepressant-like activity [9]. The present study was to investigate the potential antidepressive-like effect of tetramethylpyrazine (TMP) in chronic mild stress (CMS)-induced rat model of depression.
2. Materials and Methods
2.1. Drugs
TMP was purchased from National Institute for the Con-

Figure 1. Chemical structure of chuanxiongzine.
trol of Pharmaceutical and Biological Products (Beijing, China), and fluoxetine (FLU) hydrochloride purchased from Eli Lilly and Company Limited (USA).
2.2. Animals
Special pathogen free (SPF) adult male Sprague-Dawley (SD) strain rats weighing 220 - 280 g purchased from the experimental animal center of Guangdong Medical College (experimental animal license SCXKyue 2007- 2008A034, No.0001909; Zhanjiang, China), were used across all the experiments. They had free access to tap water and standard laboratory food unless otherwise stated. Housing conditions were controlled, temperature was maintained at 22˚C ± 1˚C with approximately 60% relative humidity. They were kept on a reversed 12/12 h light/dark cycle (light 07:00 - 19:00 h). Animals were acclimated to the animal quarters for 1 week before any experimental procedure. All the animals were treated in compliance with “Guidance Suggestion for the Care and Use of Laboratory Animals” issued by The Ministry of Science and Technology of People’s Republic of China.
2.3. CMS Procedures
The animals were assigned randomly into six matched groups (n = 14 animals in each group) based on sucrose consumption (1% sucrose solution) before onset of CMS: Control, CMS, CMS + FLU, CMS + TMP (10, 20, 40 mg/kg/d) groups. The stressed rats were exposed to CMS for 28 days; The rats in CMS + FLU group were exposed to CMS and received administration of FLU (2.0 mg/kg/d, ig, once-daily) for 28 days; The rats in CMS + TMP groups were exposed to CMS and received administration of TMP (10, 20, 40 mg/kg/d, ig, once-daily) respectively for 28 days. The control rats were given ordinary daily care, and the rats in control and CMS groups received ig administration of normal saline instead of TMP or FLU, for 28 days. The stressed and control rats were kept in different rooms to allow independent manipulation of their environments during the duration of the stress procedure. Control rats were housed together, while the stressed rats were housed singly.
Most of the stressors were adapted from the procedure described by Willner and collaborators [10] and some stressors were included from Moreau and collaborators (e.g. empty water bottle, restricted food) [11]. Each week included 2 h of paired caging, 3 h of tilted cage (45 degrees), 18 h of food deprivation immediately followed by 1 h of restricted access to food (5 micropellets), 2 × 18 h of water deprivation immediately followed by 1 h exposure to an empty bottle, 21 h of wet cage (200 ml water in 100 g sawdust bedding), and 36 h of continuous light. Stressors were presented both during the rats’ active (dark) period and during the inactive (light) period. The same stressors were used in all experiments.
2.3.1. Food and Fluid Consumption, Sucrose Intake and Body Weight
Food and fluid consumption, sucrose intake (1% sucrose solution) and body weight were measured before and after CMS. During a one-hour window after 23 hours of food and water deprivation, the fluid consumption and sucrose intake were measured by comparing bottle weight before and after the one-hour window, and expressed in relation to the animal’s body weight (ml/kg). Baseline was measured five days before the start of CMS. The food and water deprivation period preceding sucrose intake measurement may be considered as a further stress applied on top of the CMS protocol. However, control rats were also exposed to the food and water deprivation, as a part of the sucrose test. A percent preference for sucrose was calculated by determining the percentage of total fluid consumption accounted for by ingestion of the 1% sucrose solution.
2.3.2. Open Field Test during CMS
The open-field test was conducted in a quiet room before and after CMS. The open-field apparatus consisted of a raised plastic platform (80 × 80 × 40 cm). The floor was marked with a grid dividing it into 25 equal-size squares. Each animal was tested in the apparatus once. It was placed in the central square and observed for 3 min. A record was kept of the time each rat, the amount of time it spent rearing (defined as standing upright on its hind legs), and the number of grid lines it crossed with at least three paws [12]. Between animal tests the apparatus was cleaned.
2.3.3. Forced-Swimming Test Following CMS + TMP-Treated in Rats
The rats exposed to CMS and received FLU or TMP for 28 days were subjected to forced-swimming, and the final administration of FLU or TMP was given 24 h prior to testing. The measurement of immobility time was carried out by observing the motoric activity of the rats, which were placed in a pool of water. A glass cylinder 30 cm in diameter, height 40 cm was filled with water to a height of 24 cm. The temperature of water was 25˚C ± 1˚C. Measurement was carried out for five min. Immobility time is the time during which the animal floated on the surface with front paws together and made only those movements which were necessary to keep afloat. Shorter immobility time is an indicator of the stronger antidepressant effect of the tested substance [13].
2.4. Data Analyses
Values are expressed as the means ± SD or means ± SEM of 14 animals per group. Data were analyzed with SPSS 11.0 software. A probability of P < 0.05 was considered significant.
3. Results
3.1. Effects of Long-Term Administration of TMP on Food Consumption and Body Weight Gain in CMS Rat Model of Depression
The mean food consumption and body weight of rats in the six groups did not differ significantly initially. Over the four weeks of the experiment, the mean body weight increased in the six groups. Compared to the control animals, the weight gain was significantly less in the group exposed to CMS (p < 0.05), but the body weight gain in the CMS + TMP (20, 40 mg/kg/d)-treated rats was significantly less than that in the group exposed to CMS. At the end of fourth week, the control rats had gained 168.1 ± 9.1 g, the CMS-treated rats had gained 143.5 ± 32.8 g, while the CMS + TMP (10, 20, 40 mg/ kg/d)- and CMS + FLU (2.0 mg/kg/d)-treated rats had gained 131.7 ± 18.7 g, 124.0 ± 7.8 g, 107.1 ± 23.0 g, and 134.0 ± 34.4 g, respectively (Table 1). The results suggest that administration TMP might retard body weight gain. Over the four weeks of the experiment, the mean food consumption (%) decreased in the six groups. Compared to the control animals, the food consumption was significantly less in the group exposed to CMS (P <
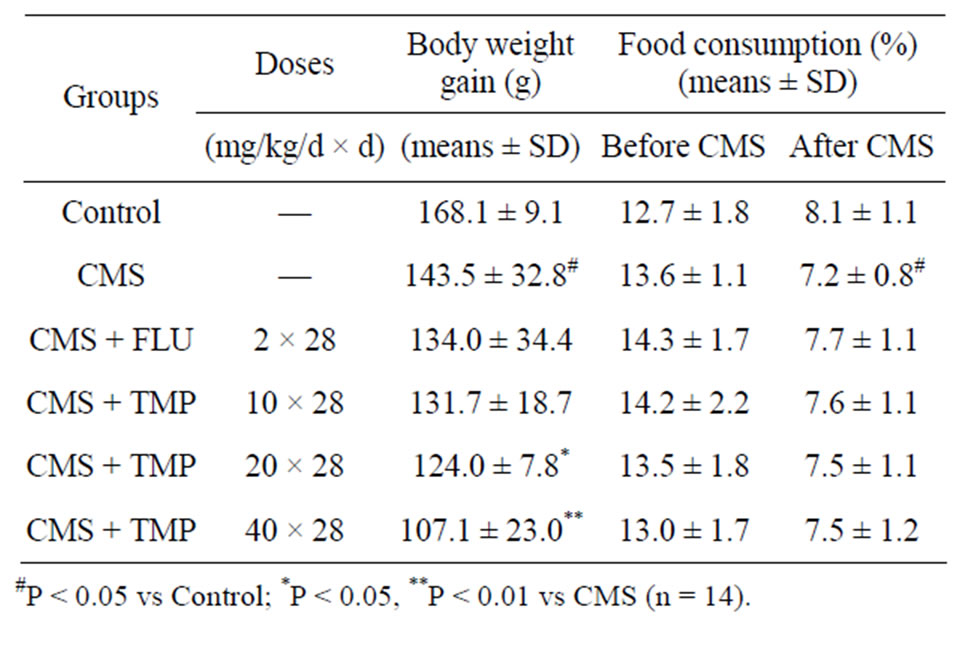
Table 1. Effects of TMP on body weight gain and food consumption in CMS-induced depression-like model rats.
0.05), but food consumption in the CMS + TMP (10, 20, 40 mg/kg/d)- and the CMS + FLU (2.0 mg/kg/d)-treated rats was comparable to that in the group exposed to CMS (P > 0.05). At the end of fourth week, the control rats consumed food of 8.1 ± 1.1 g/100 g/d, the CMS-treated rats consumed food of 7.2 ± 0.8 g/100 g/d, while the CMS + TMP (10, 20, 40 mg/kg/d)- and CMS + FLU (2.0 mg/kg/d)-treated rats consumed food of 7.6 ± 1.1, 7.5 ± 1.1, 7.5 ± 1.2, and 7.7 ± 1.1/100 g/d (Table 1), respectively.
3.2. Effects of Long-Term Administration of TMP on Fluid Consumption, Sucrose Intake and Sucrose Preference in CMS Rat Model of Depression
The mean fluid consumption, sucrose intake and sucrose preference of rats in the six groups did not differ significantly initially. Over the four weeks of the experiment, the stressed animals consumed insignificantly less fluid and sucrose solution (Table 2). However, the consumption of fluid and sucrose solution and sucrose preference by CMS + TMP (40 mg/kg/d)-treated rats was significantly more than that by the rats exposed to CMS (P < 0.05), and was comparable to the control and FLU control (P > 0.05). The results suggest that administration of TMP might attenuate the effects of CMS on fluid consumption, sucrose intake and sucrose preference, and improve the symptom of anhedonia, which is a measure indicative of depression.
3.3. Effects of Long-Term Administration of TMP on Locomotion and Exploration in CMS Rat Model of Depression
Open field testing is used to assess locomotion and exploration behaviors of rats. Compared to stressed rats, the control animals were more active in the open field; compared to the stressed animals, the CMS + TMP (40 mg/ kg/d)- and CMS + FLU (2.0 mg/kg/d)-treated rats were slightly but insignificantly active in the open field (Table 3).
3.4. Immobility Time during Forced-Swimming Test Following CMS
As shown in Figure 2, CMS obviously increased the duration of immobility (P < 0.05), and long-term administration of TMP (40 mg/kg/d, ig, once-daily, for 28 days) markedly decreased the immobility time during forcedswimming test in rat model of depression (P < 0.05), suggesting that TMP has the chronic antidepressant effect.
4. Discussion
TMP is an important available blood-activating and
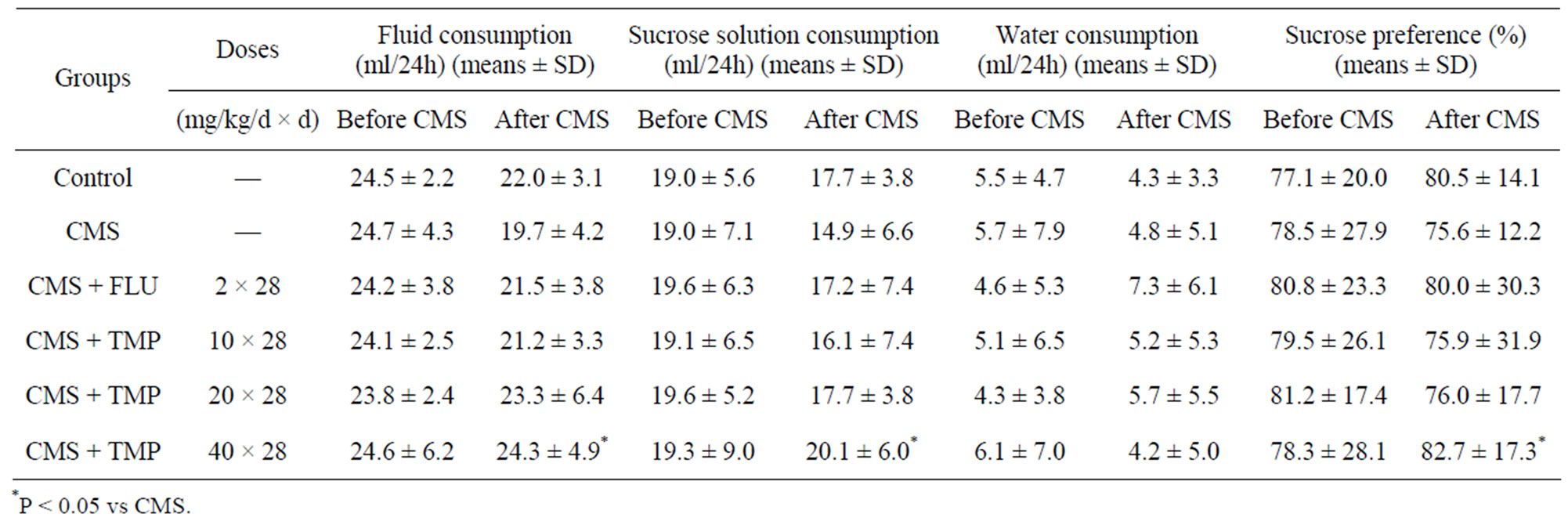
Table 2. Effects of long-term administration of TMP on fluid consumption, sucrose intake and sucrose preference in CMS rat model of depression nd sucrose preference in CMS rat model of depression.
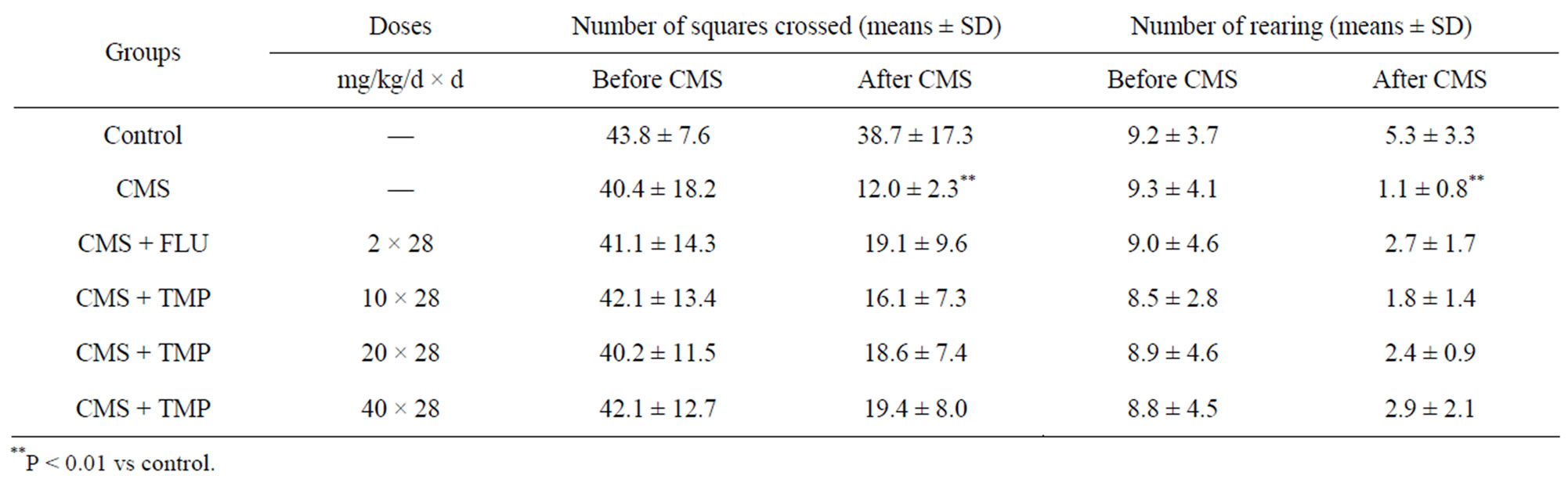
Table 3. Effects of long-term administration of TMP on locomotionand exploration in CMS rat model of depression.
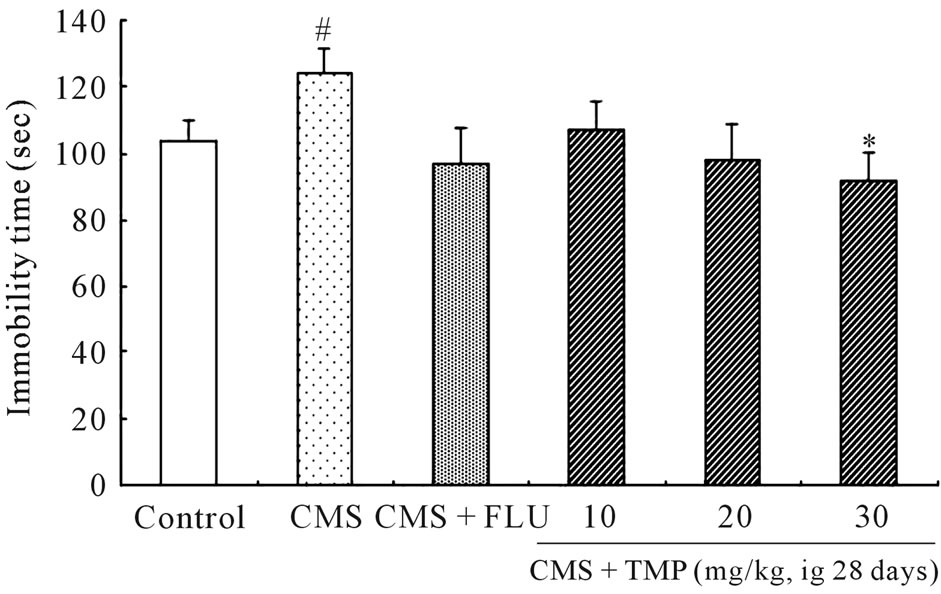
Figure 2. Effect of long-term administration of TMP on the immobility time during the forced-swim test in CMS rat model of depression. The rats exposed to CMS and received TMP (10, 20, 40 mg/kg/d, ig, once-daily, for 28 days) were subjected forced-swimming test, and the measurement of immobility time was carried out by observing the motoric activity of the rats as described in “Materials and methods”. The final administration of FLU or TMP was given 24 h prior to testing. Values are expressed as the means ± SEM of 14 animals per group. Data were analyzed with SPSS 11.0 software. #P < 0.05 vs. control; *P < 0.05 vs. CMS.
stasis-eliminating components from traditional Chinese medicines. Our recent experimental results based on forced-swimming, tail-suspension, reserpine-induced hypothermia, akinesia and ptosis, 5-hydroxytryptophan (5- HTP)-induced head-twitch, and potentiation of noradrenaline (NE) toxicity tests have revealed that TMP possesses potent acute antidepressant-like activity, and it might be an adrenergic component of pharmacological activity, and its mechanism of antidepressant-like action is similar to that of clomipramine, and different from that of fluoxetine [9]. The present study demonstrated that long-term administration of TMP partially reversed effects of CMS on fluid consumption, sucrose intake, sucrose preference, and the immobility time during the forced-swim test, but did not show significant effects on locomotion and exploration behaviors in CMS-induced rat models of depression.
Depression is a highly debilitating disorder that has been estimated to affect up to 21% of the world population. Despite the advances in the treatment of depression with selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs),
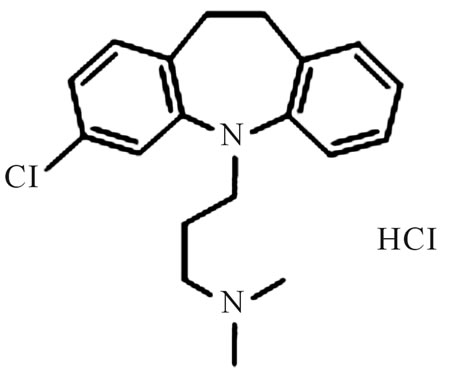
Figure 3. Chemical structure of clomipramine hydrochloride.
there continue to be many unmet clinical needs with respect to both efficacy and side effects. These needs range from efficacy in treatment resistant patients, to improved onset, to reductions in side effects such as emesis or sexual dysfunction. To address these needs, there are numerous combination therapies and novel targets that have been identified. There is tremendous diversity in the types of targets and approaches being taken [14]. From the mentioned-above description, it is understandable that the antidepressant-like action of TMP is similar to that of clomipramine, and different from that of fluoxetine, and that long-term administration of TMP reverses effects of CMS on food consumption, sucrose intake, sucrose preference, and the immobility time during the forced-swim test, but does not show significant effects on locomotion and exploration behaviors in CMS-induced rat models of depression. The findings mean that there are some novel targets of antidepressant-like action of TMP that have not been identified yet.
Despite the advances that have been made in the development of antidepressants, there are clearly still unmet clinical needs that need to be addressed. Indeed there are multiple new approaches in progress to improve current pharmacological means of modulating serotonin or norepinephrine neurotransmission by either combining mechanisms or alternatively selectively stimulating receptor subtypes that may trigger improved efficacy or fewer side effects [14]. Chuanxiongzine consists of four methyls and pyrazine connection, and the chemical structure is simple and quite different from clomipramine (Figure 3). Therefore, it is worthwhile to explore the relationship between the special structure of pyrazine linked to four methyls and antidepressant-like activity, and its relationship with the serotonin and norepinephrine and dopamine neurotransmitter receptors. Probably such a exploration may bring new approaches in progress to improve current antidepressant pharmacological means.
5. Acknowledgements
The authors would like to thank Doctor Depu Yu for his great encouragement.
REFERENCES
- L. Bach-Rojecky, Z. Kalodjera and I. Samarzija, “The Antidepressant Activity of Hypericum Perforatum L. Measured by Two Experimental Methods on Mice,” Acta Pharmaceutica, Vol. 54, No. 2, 2004, pp. 157-162.
- X. Zhong, Q. Mao, Z. Huang and J. Wei, “Anti-Depressive Effect of Suyu Capsules in Mice,” Chinese Journal of Natural Medicines, Vol. 15, No. 15, 2006, pp. 1247- 1249.
- H. B. Zhong, Y. Pan and L. D. Kong, “Antidepressant Effect of Epimedium Brevicornum Extracts,” Chinese Traditional and Herbal Drugs, Vol. 36, No. 10, 2005, pp. 1506-1510.
- Q. Mao, Z. Huang, S. Ip and C. Che, “AntidepressantLike Effect of Ethanol Extract from Paeonia Lactiflora in Mice,” Phytotherapy Research, Vol. 22, No. 11, 2008, pp. 1496-1499.
- G. Liu, “Chinese Herbal Medicine,” Hua Xia Publishing House, Beijing, 2001, pp. 227-228.
- H. B. Li and F. Chen, “Preparative Isolation and Purification of Chuanxiongzine from the Medicinal Plant Ligusticum Chuanxiong by High-Speed Counter-Current Chromatography,” Journal of Chromatography A, Vol. 1047, No. 2, 2004, pp. 249-253.
- M. Su, Y. Zhou and G. Yang, “Effects of Tetramethylpyrazine on L-Type Calcium-Channel Current and the Intracellular Calcium Concentration in Primary Cultured Hippocampal Neuronal Cells,” Chinese Journal of Rehabilitation, Vol. 23, No. 1, 2008, pp. 17-19.
- Y. B. Ji, “Pharmacological Action and Application of Blood-Activating and Stasis-Eliminating Available Composition of Traditional Chinese Medicine,” Heilongjiang Science and Technique Press, Harbin, 1999, pp. 118-121.
- L. Yu, X. Jiang, M. Liao, R. Ma and T. Yu, “Antidepressant-Like Effect of Tetramethylpyrazine in Mice and Rats,” Neuroscience & Medicine, Vol. 2, No. 6, 2011, pp. 142-148.
- P. Willner, A. Towell, D. Sampson, S. Sophokleous and R. Muscat, “Reduction of Sucrose Preference by Chronic Unpredictable Mild Stress, and Its Restoration by a Tricyclic Antidepressant,” Psychopharmacology, Vol. 93, No. 3, 1987, pp. 358-364. doi:10.1007/BF00187257
- J. L. Moreau, F. Jenck, J.R. Martin, P. Mortas and W. E. aefely, “Antidepressant Treatment Prevents Chronic Unpredictabl Mild Stress-Induced Anhedonia as Assessed by Ventral Tegmentum Self-Stimulation Behavior in Rats,” European Neuropsychopharmacology, Vol. 2, No. 1, 1992, pp. 43-49. doi:10.1016/0924-977X(92)90035-7
- K. T. Hallam, J.E. Horgan, C. McGrath and T. R. Norman, “An Investigation of the Effect of Tacrine and Physostigmine on Spatial Working Memory Deficits in the Olfactory Bulbectomised Rat,” Behavioural Brain Research, Vol. 153, No. 2, 2004, pp. 481-486. doi:10.1016/j.bbr.2004.01.005
- R. D. Porsolt, G. Anton, N. Blavet and M. Jalfre, “Behaviour Despair in Rats: A New Model Sensitive to Antidepressant Treatments,” European Journal of Pharmacology, Vol. 47, No. 4, 1978, pp. 379-391. doi:10.1016/0014-2999(78)90118-8
- L. E. Schechter, R. H. Ring, C. E. Beyer, Z. A. Hughes, X. Khawaja, J. E. Malberg and S. Rosenzweig-Lipson, “Innovative Approaches for the Development of Antidepressant Drugs: Current and Future Strategies,” NeuroRx, Vol. 2, No. 4, 2005, pp. 590-611. doi:10.1602/neurorx.2.4.590
NOTES
*Corresponding author.
#The authors contributed equally to this work.

