Advances in Aging Research
Vol.3 No.1(2014), Article ID:42936,5 pages DOI:10.4236/aar.2014.31008
Relationship between bone mineral density, coronary risk factors, C-reactive protein, and Insulin growth factor in frail elderly
![]()
1Geriatrics and Gerontology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt; *Corresponding Author: hebageasaber@yahoo.co.uk
2Clinical Pathology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Copyright © 2014 Moatassem S. Amer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Moatassem S. Amer et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 19 November 2013; revised 19 December 2013; accepted 26 December 2013
KEYWORDS
Frailty; Bone Mineral Density; Coronary Risk Factors
ABSTRACT
Objective: This study aimed to explore the relation between bone mineral density (BMD), inflammation, coronary risk factors, and Insulin growth factor (IGF) in frail elderly. Design: A casecontrol study. Setting: Ain Shams University Hospital Cairo, Egypt. Participants: Ninety aged 60 years and above; sixty frail (30 males and 30 females; mean age 70.03 ± 8.10) and thirty matched controls (15 males and 15 females; mean age 69.07 ± 5.93). Measurements: History, examination, comprehensive geriatric assessment, measurement of BMD, and laboratory investigations including; high sensitivity C-reactive protein (hs-CRP) glycated haemoglobin, IGF, lowdensity lipoprotein (LDL), high-density lipoprotein, total cholesterol, and triglycerides. Results: The frail group had significantly lower levels of triglycerides and LDL, hs-CRP and glycated haemoglobin levels were significantly higher (P < 0.001), and BMD and IGF levels were significantly lower when compared with the control group (P < 0.00*, P = 0.05) respectively. We also found that BMD had a significant positive linear correlation with IGF and negative correlation with age. Conclusion: Frailty was associated with low BMD, lower triglycerides & LDL levels, and higher hsCRP levels. A borderline significance was found between IGF levels and frailty. A positive correlation was found between BMD and IGF in frail.
1. INTRODUCTION
The cornerstone of geriatric medicine concerns the identification, evaluation, and treatment of frail older adults and prevention of loss of independence and other outcomes for which they are at risk. The proportion of frail within the older population is high and will increase with the aging of society [1].
Central to the frailty definition has been the concept that multiple systems must be involved. The most commonly suggested multisystem impairments involve dysregulation of neuromuscular, endocrine, and immune systems with aging. Low-level inflammation, sarcopenia, osteopenia, and nutritional changes are diagnosed, and often the presence of other contributing factors of chronic and acute illness, and environmental stresses could be found [2]. Frailty is associated with an increased risk of osteoporosis, as it involoves chronic undernutrition, sarcopenia, and reduced total energy expenditure [3]. Osteoporosis inturn increases morbidity and dependence, which in turn has a negative impact on the quality of life [4].
Low gonadal hormones and low insulin growth factor (IGF-1), combined with high peripheral levels of inflammatory mediators, cytokines, low vitamin D and pro-coagulation state enhance the risk of sarcopenia and frailty [5].
Frailty and cardiovascular disease (CVD) share a common biological pathway, and CVD may accelerate its development. Infact, frailty is identified in up to half of patients with CVD [6]. Newman et al. suggested that the relationship between frailty and CVD may be explained by two different pathways. The first explanation might be that pathological processes part of disease can lead to subclinical end organ damage, thus decreasing total physiologic reserve. The second possibility is that atherosclerosis, as a state of chronic inflammation, may result in a catabolic state with its systemic manifestations contributing to frailty [7]. The purpose of this article is to further explore the relation between bone mineral density, inflammation, IGF-1, and coronary risk factors in frail elderly Egyptians.
2. MATERIALS AND METHODS
2.1. Study Design and Setting
This was a case control study. The study sample comprised 90 participants aged 60 years and above. They were selected from Ain Shams University Hospital from inpatient wards and outpatient clinics. Cases included thirty frail elderly females and thirty frail elderly males diagnosed by Fried’s criteria [8] as applied by AvilaFunes et al. [9]. Controls included thirty healthy elderly subjects (15 males and 15 females) age matched with no apparent evidence of disease after full medical history and physical examination.
Any patient who refused to participate in the study, patients who were suffering from acute infection, and any patients who were taking drugs that have anti-inflammatory effects as steroids, statins and aspirin were excluded from this study.
2.2. Data Collection
Each patient then underwent comprehensive geriatric assessment in the form of detailed history and physical examination, cognitive function assessment by Minimental status examination (MMSE) [10] (The Arabic version used in this study was done by El-Okl et al. [11]), functional assessment by Activities of daily living (ADL) [12], Arabic version [13], and Instrumental activities of daily living (IADL) [14] (An Arabic version of the test was applied [15]), and Geriatric depression scale 15 items (GDS-15) [16] was used to screen for depression (the Arabic version of the test was applied by Shehta et al.) [17].
Frailty was defined according to the construct previously validated by Fried et al. in the Cardiovascular Health Study [8]. All five components from the original phenotype were retained; however, the metrics used to characterize the frailty criteria were slightly different and defined as follows [9]:
• Shrinking—Recent and unintentional weight loss of ≥3 kg in the prior year was identified and body mass index calculated. Participants who answered ‘‘yes’’ for weight loss or had a body mass index < 21 kg/m2 were considered to be frail for this component.
• Poor endurance and energy—As indicated by selfreport of exhaustion, identified by two questions from the Center for Epidemiological Studies-Depression scale (CES-D) [18]: ‘‘I felt that everything I did was an effort’’ and ‘‘I could not get going.’’ Participants were asked: ‘‘How often, in the last week, did you feel this way?’’ 0 = rarely or none of the time; 1 = some or a little of the time; 2 = a moderate amount of the time; or 3 = most of the time. Participants answering ‘‘2’’ or ‘‘3’’ to either of these questions were considered as frail by exhaustion.
• Slowness—Meets criteria for frailty if time to walk 6 m was ≥8 seconds for height ≤ 173 cm or >7 seconds for height > 173 cm in males, and ≥8 seconds for height ≤ 159 cm or >7 seconds for height > 159 cm in females.
• Weakness—Participants answering ‘‘yes’’ to the following question were categorized as frail for this component: ‘‘Do you have difficulty rising from a chair?’’
• Low physical activity—A single response was used to estimate physical activity. Individuals who denied doing daily leisure activities such as walking or gardening and/or denied doing some sport activity per week were categorized as physically inactive. Those who reported doing them were considered to be active.
2.3. Laboratory Investigations
Five mL whole blood was drawn from each participant after 12 hours fasting, and was divided into 2 tubes: 1 mL in EDTA-anticoagulated tube for glycated haemoglobin (HBA1C) assay, and 4 mL allowed to cloting in a plain tube. The resulting serum was used for quantitative c-reactive protein (CRP), and the remaining serum was frozen to −70˚C till assay of IGF-1. HBA1c was assayed by ion-exchange chromatographic separation and colorimetric detection kit (Biosystems, SA, Barcelona, Spain). Quantitation of CRP was performed by immunoturbidimetric assay using Biosystems CRP-hs kit (Biosystems, SA, Barcelona, Spain). IGF-1 was measured by ELISA using kits supplied by DRG (DRG international, New Jersey, USA). Lipid profile was done in the central laboratory in Ain Shams University teaching hospital.
2.4. Bone Mineral Density
Measured by dual-energy x-ray absorptiometry, at the left femoral neck and lumbar spine, with the use of a Lunar DPX-L densitometer (Lunar).
2.5. Statistical Analysis
Analysis of data was performed by using the 19th version of Statistical Package of Social Science (SPSS). Description of all data in the form of mean (M) and standard deviation (SD) for all quantitative variables.
Frequency and percentage for all qualitative variables. Comparison between quantitative variables was done using t-test to compare two groups. Comparison of qualitative variables was done using Chi square test. Correlation coefficient was also done to find linear relation between different variables using Spearsman’s correlation co-efficient. Significant level measured according to P-value (Probability), P > 0.05 insignificant, P < 0.05 significant and P < 0.01 highly significant.
3. RESULTS
The clinical characteristics of the study sample are presented in Table 1; frail group had significantly lower levels of triglycerides and LDL. Hs-CRP level and glycated haemoglobin (HBA1C) were significantly higher among the frail group (P < 0.001). BMD and IGF-1 levels were significantly lower in frail group compared with the control group (P < 0.00*, P = 0.05) respectively.
However there is no difference between the two studied groups regarding age, smoking status, total cholesterol, HDL level and the presence of chronic diseases.
Table 2 shows the Pearson correlation analysis between BMD as measured by Femoral and Lumber Tscores with age, BMI, lipid profile, IGF, and hs-CRP level among frail group. We also found that BMD had a significant positive linear correlation with IGF and negative correlation with age.
4. DISCUSSION
The current study assesses the relationship between bone mineral density, inflammation, IGF-1, and coronary risk factors in frail elderly. The findings of this study demonstrated that frail group had lower BMD, as measured by Femoral and Lumber T-scores, than the control group. This finding was consistent with previous studies [19,20].
As anticipated, we found that frail participants had significantly higher levels of hs-CRP in comparison to their healthy counterparts. Studies done by Walston et al. & Hubbard et al. had comparable results [21,22]. The results of the current study showed that frail elderly had higher HBA1C levels. The results of Blaum et al. revealed that a HbA1c level of 6.5% or greater in older women was significantly associated with higher likelihood of prefrail and frail status and that BMI, inflammation, and comorbidities did not explain the association [23].
It was found that LDL and TG levels were significantly lower in the frail participants, and there was no association with total cholesterol and HDL levels. A study conducted by Walston et al. had similar results.
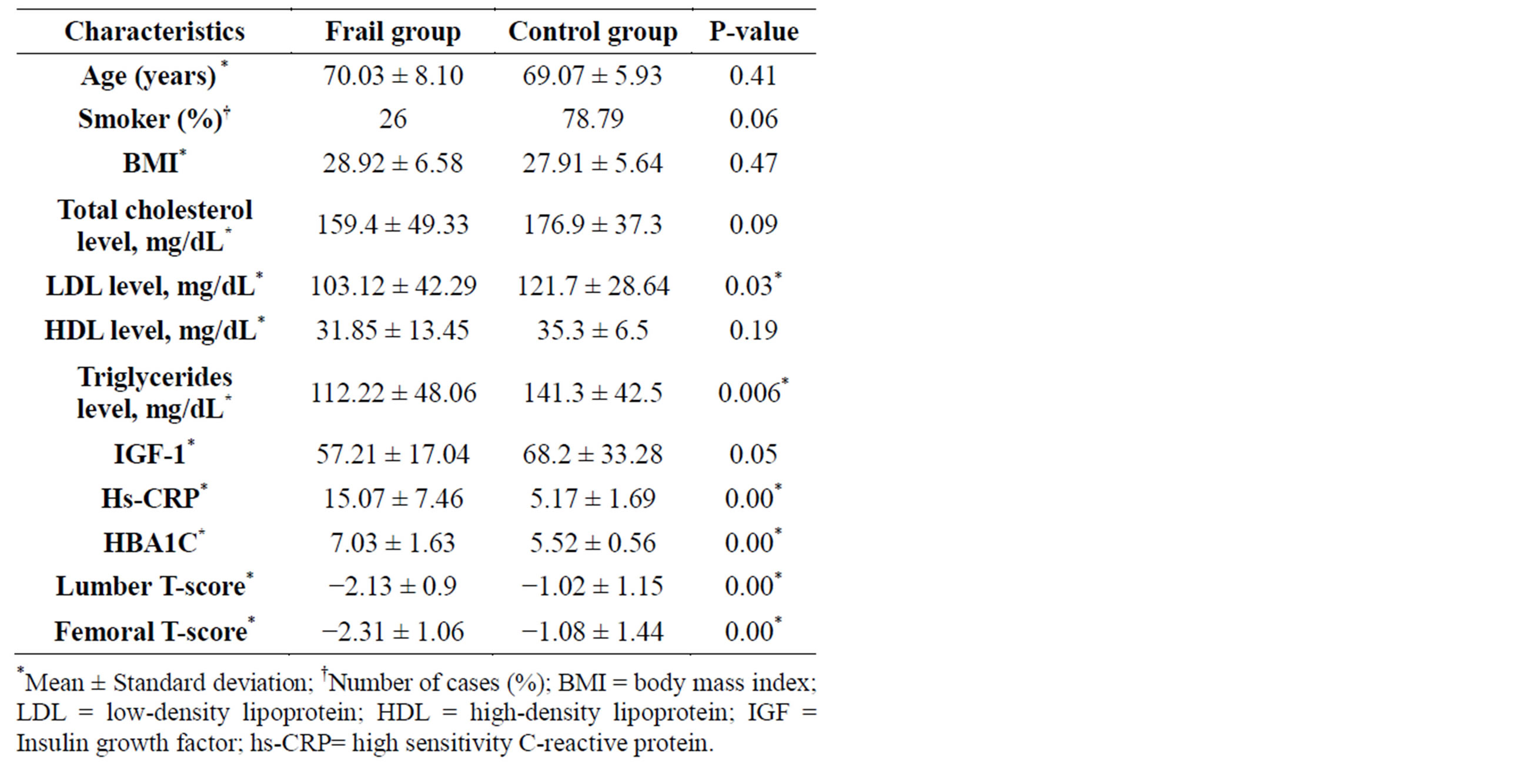
Table 1. Clinical characteristics of study participants.
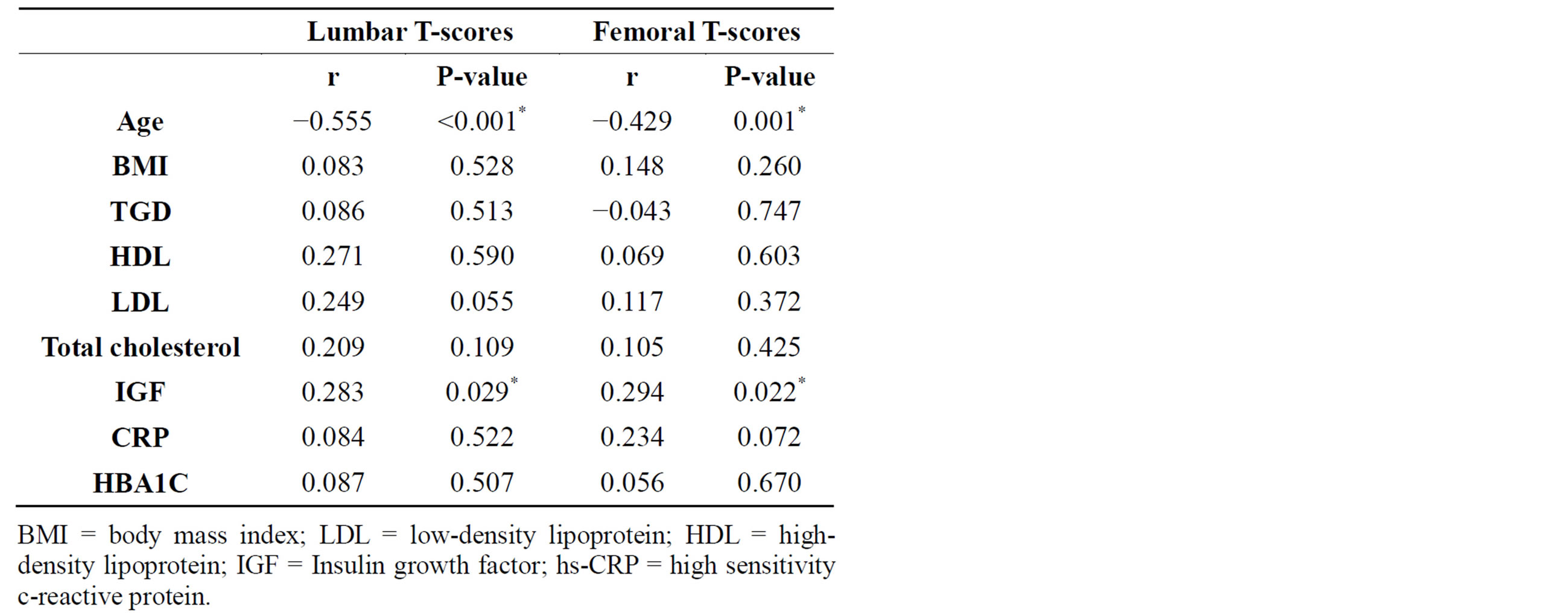
Table 2. Correlation between lumbar & femoral T-scores in the frail group with age, BMI, lipid profile, IGF, CRP & HBA1C.
They found that low-density lipoprotein cholesterol levels were lower in the frail group (with and without diabetes or CVD), and that the relationships of mean total cholesterol, high-density lipoprotein (HDL), and triglyceride levels with frailty level were weak. This was inconsistent with traditional views of CVD risk factors, but most consistent with the lower lipid levels reflecting and associating with frailty through an inflammation mechanism [21].
The findings of this study showed that frail participants had lower IGF-1 levels; which denotes that frail elderly tend to have lower levels of anabolic hormones than similarly aged non-frail counterparts. This has also been demonstrated in several other studies [24,25]. On the other hand, VanItallie stated that there was no association between frailty and low IGF-1 levels, and suggested that this maybe because it is not yet clear whether, in comparison with their non-frail counterparts, frail individuals consistently manifest larger reductions in IGF- 1 [5].
The results of the current study showed a positive correlation between IGF-1 levels and BMD in the frail group. Serum IGF-1 levels were found to be independent predictors of total bone density [26]. In fact, Giustina et al. found that both Growth Hormone and IGF-1 are essential for the development and growth of the skeleton and maintenance of bone mass [27]. Conversely Van den Beld et al., found no correlation between serum IGF-1 concentrations on the one hand and measures of bone mass and body composition on the other [28].
No linear correlation between hs-CRP and other coronary risk factors with the BMD among frail elderly was found in this study. Because frailty is multifactorial and frail elderly display heterogeneous characteristics it is difficult to establish a correlation between various markers of inflammation and frail status, especially in the presence of underlying chronic inflammation.
5. CONCLUSION
This study found that frailty was significantly associated with low BMD, lower TG & LDL levels, higher CRP levels, and higher HbA1c levels. A borderline significance was found between IGF-1 levels and frailty. A positive correlation was found between bone mineral density and IGF-1 in frail. These findings may support the hypothesis that frailty is associated and overlaps with disability. The results of this study also reflect the role that inflammation has to play in the pathogenesis of frailty.
FUNDING
This paper was funded by Ain Shams University, there were no sponsors.
ACKNOWLEDGEMENTS
The authors would like to thank Ain Shams University, faculty of medicine for funding this paper.
REFERENCES
- Fried, L., Walston, J. and Ferrucci, L. (2009) Frailty. In: Halter, J., Ouslander, J., Tinetti, M., et al., Eds., Hazzard’s Geriatric Medicine and Gerontology, 6th Edition, McGraw-Hill, New York, 631-646.
- Topinková, E. (2008) Aging, disability and frailty. Getting older, facts about a biological process. Annals of Nutrition and Metabolism, 52, 6-11.
- Salminen, H., Saaf, M., Johansson, S., Ringertz, H. and Strender, E. (2006) Nutritional status, as determined by the mini-nutritional assessment, and osteoporosis: A crosssectional study of an elderly female population. European Journal of Clinical Nutrition, 60, 486-493. http://dx.doi.org/10.1038/sj.ejcn.1602341
- Packard, P. and Heaney, R. (1997) Medical nutrition therapy for patients with osteoporosis. Journal of the American Dietetic Association, 97, 414-417. http://dx.doi.org/10.1016/S0002-8223(97)00103-X
- Vanitallie, T. (2003) Frailty in the elderly: Contributions of sarcopenia and visceral protein depletion. MetabolismClinical and Experimental, 52, 22-26. http://dx.doi.org/10.1016/S0026-0495(03)00297-X
- Afilalo, J. (2011) Frailty in patients with cardiovascular disease: Why, when, and how to measure. Current Cardiovascular Risk Reports, 5, 467-472. http://dx.doi.org/10.1007/s12170-011-0186-0
- Newman, A., Gottdiener, J., McBurnie, M., Hirsch, C., Kop, W., Tracy, R., et al. (2001) Associations of subclinical cardiovascular disease with frailty. Cardiovascular Health Study Research Group. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56, M158-M166. http://dx.doi.org/10.1093/gerona/56.3.M158
- Fried, L., Kronmal, R., Newman, A., Bild, D., Mittelmark, M., Polak, J., et al. (1998) Risk factors for 5 year mortality in older adults: The cardiovascular health study. The Journal of the American Medical Association, 279, 585- 592. http://dx.doi.org/10.1001/jama.279.8.585
- Avila-Funes, J., Helmer, C., Amieva, H., Barberger-Gateau, P., Le Goff, M., Ritchie, K., et al. (2008) Frailty among community dwelling elderly people in France: The three city study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63, 1089- 1096.
- Folstein, M., Folstein, S. and McHug, P. (1975) Minimental state. A practical method for gradient the cognitive state of patients for clinicians. Journal of Psychiatric Research, 12, 189-198.
- El-Okl, M. (2002) Prevalence of Alzheimer dementia and other causes of dementia in Egyptian elderly. Dissertation, Ain Shams University, Cairo.
- Katz, S., Ford, A., Moswowitz, R., Jackson, B. and Jaffe, M. (1963) Studies of illness in the aged. The index of ADL: Standardized measure of biological and psychological function. The Journal of the American Medical Association, 185, 914-919.
- El-Sherpiny, M., Mortagy, A. and Fahy, H. (2000) Prevalence of hypercholesterolemia among elderly people living in nursing houses in Cairo. Geriatric Department Library, Ain Shams University, Cairo, 6.
- Lawton, M. and Brody, E. (1969) Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9, 179-86. http://dx.doi.org/10.1093/geront/9.3_Part_1.179
- Fillenbaum, G. (1986) The wellbeing of the elderly: Approaches to multi-dimensional assessment. World Health Organization, WHO Offset Publication No. 84, Arabic translation Distributed by the Eastern Mediterranean Regional Office.
- Sheikh, J. and Yesavage, J. (1986) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology, 5, 165-173.
- Shehta, A.S., El-Banouby, M.H., Mortagy, A.K., et al. (1998) Prevalence of depression among Egyptian geriatric community. Geriatric Department Library, Ain Shams University, Cairo, 3-5.
- Orme, J., Reis, J. and Herz, E. (1986) Factorial and discriminate validity of the Center for Epidemiological Studies depression (CES-D) scale. Journal of Clinical Psychology, 42, 28-33. http://dx.doi.org/10.1002/1097-4679(198601)42:1<28::AID-JCLP2270420104>3.0.CO;2-T
- Kenny, A., Waynik, I., Smith, J., Fortinsky, R., Kleppinger, A. and McGee, D. (2006) Association between level of frailty and bone mineral density in communitydwelling men. Journal of Clinical Densitometry, 9, 309- 314. http://dx.doi.org/10.1016/j.jocd.2006.03.010
- Elffors, L. (1998) Are osteoporotic fractures due to osteoporosis? Impacts of a frailty pandemic in an aging world. Aging (Milano), 10, 191-204. http://dx.doi.org/10.1007/BF03339653
- Walston, J., McBurnie, M., Newman, A., Tracy, R., Kop, W., Hirsch, C., et al. (2002) Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities, results from the cardiovascular health study. Archives of Internal Medicine, 162, 2333- 2341. http://dx.doi.org/10.1001/archinte.162.20.2333
- Hubbard, R., Searle, S., Mitnitski, A. and Rockwood, K. (2009) Effect of smoking on the accumulation of deficits, frailty and survival in older adults: A secondary analysis from the Canadian study of health and aging. The Journal of nutrition, Health & Aging, 13, 468-472. http://dx.doi.org/10.1007/s12603-009-0085-y
- Blaum, C., Xue, Q., Tian, J., Semba, R., Fried, L. and Walston, J. (2009) Is hyperglycemia associated with frailty status in older women? Journal of the American Geriatrics Society, 57, 840-847. http://dx.doi.org/10.1111/j.1532-5415.2009.02196.x
- Leng, S., Cappola, A., Andersen, R., Blackman, M., Koenig, K., Blair, M., et al. (2004) Serum levels of insulinlike growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clinical and Experimental Research, 16, 153-157. http://dx.doi.org/10.1007/BF03324545
- Cappola, A., Xue, Q. and Fried, L. (2009) Multiple hormonal deficiencies in anabolic hormones are found in frail older women: The women’s health and aging studies. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 64, 243-248. http://dx.doi.org/10.1093/gerona/gln026
- Manuel Gomez, J. (2006) The role of insulin-like growth factor I components in the regulation of vitamin D. Current Pharmaceutical Biotechnology, 7, 125-132. http://dx.doi.org/10.2174/138920106776597621
- Giustina A, Mazziotti G & Canalis E. (2008) Growth hormone, insulin-like growth factors, and the skeleton. Endocrine Reviews, 29, 535-559. http://dx.doi.org/10.1210/er.2007-0036
- Van den Beld, A., Blum, W., Pols, H., Grobbee, D. and Lamberts, S. (2003) Serum insulin-like growth factor binding protein-2 levels as an indicator of functional ability in elderly men. European Journal of Endocrinology, 148, 627-634. http://dx.doi.org/10.1530/eje.0.1480627

