Neuroscience & Medicine
Vol.3 No.1(2012), Article ID:17750,6 pages DOI:10.4236/nm.2012.31008
Effect of Exercise Program on the Rehabilitation of Patients with Cervical Spondylotic Myelopathy
![]()
1Faculty of Therapeutic Sciences, Masterskill University College of Health Sciences, Cheras, Malaysia; 2PMR Department, Christian Medical College & Hospital, Vellore, India.
Email: *nsshakthi@gmail.com, senthil@masterskill.edu.my
Received December 11th, 2011; revised January 20th, 2012; accepted February 12th, 2012
Keywords: Cervical Spondylotic Myelopathy; Rehabilitation; Exercise
ABSTRACT
Study Design: A clinical trial of 30 consecutive patients with cervical spondylotic myelopathy (CSM). Objectives: To evaluate the effect of directed physical exercise in patients with CSM and to measure severity of myelopathy before and after an exercise program. Setting: Christian Medical College and Hospital, India. Participants: Thirty patients with CSM (mean age = 54.1 years) with Nuricks Grade 2 and 3. Background: Myelopathy of the spinal cord can be caused by degenerative process of the cervical vertebrae and it is the most common type of dysfunction of the spinal cord in adult population. CSM usually develops insidiously and the natural history is not well understood, there is debate over the indication for operative vs non operative management. Method: Patients participated in a 6-week exercise program, consisting of active exercises to upper and lower extremities, scapulothoracic muscles, and gentle stretches, sub maximal isometric exercises of the deep neck flexors, relaxation and immobilization with a cervical hard collar. Main Outcome Measures: The mJOA (modified Japanese orthopaedic association score) and ASIA motor and sensory scoring. The results were processed by using Wilcoxon sign rank test. Results: After comparing the values at the beginning and end of the program a satisfactory neurological result (sensorimotor/motor and sensory) was obtained in all thirty patients. Conclusion: The exercise program had a positive impact for most of the variables of the study. Exercise intervention with neck immobilization may be a treatment of choice in early stages of CSM. Future randomized controlled studies would provide insight into the effectiveness and clinical relevance of this intervention.
1. Introduction
Myelopathy of the spinal cord can be caused by degenerative processes of the cervical vertebrae [1-4]. This disease is called cervical spondylotic myelopathy (CSM), and it is the most common type of dysfunction of the spinal cord in adult population [5,6]. White and Panjabi divide the mechanical factors involved in the pathogenesis of cervical spondylotic myelopathy into two groups: static and dynamic [7].
Static factors include congenital spinal canal stenosis (<13 mm anterior-posterior diameter), disc herniation, osophyte formation in the vertebral bodies, degenerative osteophytosis of the uncovertebral and facet joints, hypertrophy of the ligamentum flavum and posterior longitudinal ligaments [8-10]. Dynamic factors are abnormal forces placed on the spinal column and spinal cord during flexion and extension of the cervical spine under normal physiologic loads [8-10].
Cervical spondylotic myelopathy usually develops insidiously and the natural history of which is not well understood, hence there is debate over the indications for operative treatment due to long term hospitalization, medication, medical and surgical cost and complication involved [11-14]. Non-operative treatment can be helpful in the early period but current literature offers little research in physiotherapy treatment intervention [15]. There is a dearth of conclusive studies demonstrating the effect of exercise in CSM.
2. Methods
2.1. Case Description
Consecutive patients referred by their neurologist to Physiotherapy services of Christian medical college and hospital, diagnosed with CSM, were examined for eligibility criteria. Inclusion criteria for this case series included Nuricks grade of 2 and 3 presenting with walking difficulty (Table 1). Exclusion criteria included the presence of any medical red flags (i.e., tumor, fracture, metabolic diseases, rheumatoid arthritis, osteoporosis, neoplastic disease), sustained major trauma, evidence of central nervous system involvement (positive Hoffman’s sign), and prior surgery for the condition. The study protocol was approved by the research committee of the Institution. Post intervention data were collected for 30 subjects and one patient had fever with recurrent infection hence left out from study. The data were collected by a single observer blind to the clinical details. The study comprised 21 men and 9 women (N = 30; mean age = 54 years, SD = 10.01).

Table 1. Grading of cervical spondylotic myelopathy (Nuricks, 1972).
According to the baseline neurological analysis of the symptoms and signs, 28 patients had only myelopathy without radiculopathy, while 3 had radiculomyelopathy.
Subtle movements of the hand were affected to various degrees in 29 (96.6%) subjects. The patients with myelopathy had difficulty with finger escape and grip release. All 30 (100%) patients had hyperreflexia of the lower extremities, as well as a mild to moderate disturbance in gait. The decrease in motor function of the lower extremities was mild to moderate (Nuricks grade 2 in 18 (60%) patients, grade 3 in 12 (40%) patients). Sensory loss in the upper extremities was found in all 30 (100%) patients, 26 (86.6%) patients noted sensory loss in the trunk and lower extremities.
2.2. Outcome Measures
All subjects underwent a standardized examination with ASIA motor and sensory scoring and completed the mJOA (severity of myelopathy scale) at baseline and at 6 weeks after the intervention. As the traditional custom in our culture are different as people do not usually eat with forks and knife hence we adapted the Modification of Modified Japanese orthopaedic association scoring [16] (William et al.) as shown in Table 2.


Table 2. Modification of modified JOA scoring (William et al.)
2.3. Intervention
Training session lasted for 45 minutes ± 10 min per day, 4 days per week for 6 weeks with adequate warm up and cold down periods. We selected a 3-day rest in order to avoid over-stressing. Patients were instructed and performed exercises focusing on strengthening of the deep neck flexors, scapulothoracic muscles, upper and lower limb muscles depending of their strength levels. The patients performed deep neck flexor strengthening exercises as described by Petersen [17], without the use of a biofeedback unit. The patient was supine, with the cervical spine in neutral, and instructed to flatten the curve of the neck by nodding the head. This position was held for 10 seconds and repeated for 10 times. Scapulothoracic exercise includes serratus anterior and both middle and lower trapezius muscle strengthening, as described by Flynn et al. [18]. All patients were instructed to perform all strengthening exercises at home. Depending on individual tolerance, the length of the sessions was progressively increased to one hour of exercise and locomotor training. Each locomotor training session was 10 - 15 min in duration and the subject walked on a motorized treadmill. Depending on the need, one therapist was positioned near to the patient to help stabilize the patient posture. Subjects were encouraged to arm swing when possible (1 or 2) and horizontal bars positioned at chest level were used to aid in balance control. Subjects walked at own pace enabling them to concentrate on voluntarily activating their muscles during walking. Rest periods were taken when needed.
3. Analysis and Results
3.1. Statistical Analysis
The Wilcoxon sign rank test was used to compare the numeric variables obtained from pre and post intervention. The level of significance was set at p < 0.05. The analysis was performed using SPSS.
3.2. Results
Over-all, as assessed by the classification of the mJOA and ASIA scoring, all patients were improved neurologically. The average scores of mJOA improved from 9.60 at baseline to 14.90 post intervention and ASIA motor scores improved from 75.10 to 83.60, sensory score (pin prick) from 61.23 to 98.23, sensory score(light touch) from 66.47 to 102.46. There is life-altering impact, associated with recovery of motor and sensory improvements which could have markedly alter the daily functional abilities (See Table 3).
Exercise intervention increases the mJOA score, motor and sensory score and thus the quality of life and activeties of daily living. The over-all results were evaluated according to the rate of recovery and were graded according to the criteria outlined by Hirabayashi et al. with use of the following equation [19]: rate of recovery (percent) = post intervention score – pre intervention score ÷ 18 (full recovery) – pre intervention score × 100. Recovery rates in our study were 63.09%.
4. Discussion and Conclusions
4.1. Discussion
Concurrent with previous findings, the results of this study indicate that upper and lower extremity muscle strength scores are significantly associated with myelopathic scores.
Firstly the results presented here indicate that voluntary physical activity can prime adult sensory and motor neurons for enhanced axonal regeneration after subsequent injury [20]. Previous works have showed that exercise regulates gene expression in the spinal cord [21-24]. The increased motor and sensory scores after exercise in the current study now demonstrates a direct functional outcome of exercise-dependent change. Activity-dependent increase in BDNF and NT3 is one means by which exercise could trigger the increased axonal growth potential in neurons thereby facilitating recovery [25-27].
Secondly, maximum weight bearing of the lower limbs is important during stance [28]. Several animal studies have demonstrated that an increase in loading increases extensor motoneuron activity and facilitates locomotion; a response that can be mediated by the lumbosacral spinal cord in isolation of descending drive [29-31]. Similar results have been demonstrated in humans during locomotion [32]. The locomotor training we conducted is based on 2 assumptions. First, the spinal cord has the ability to respond to appropriate afferent information to generate
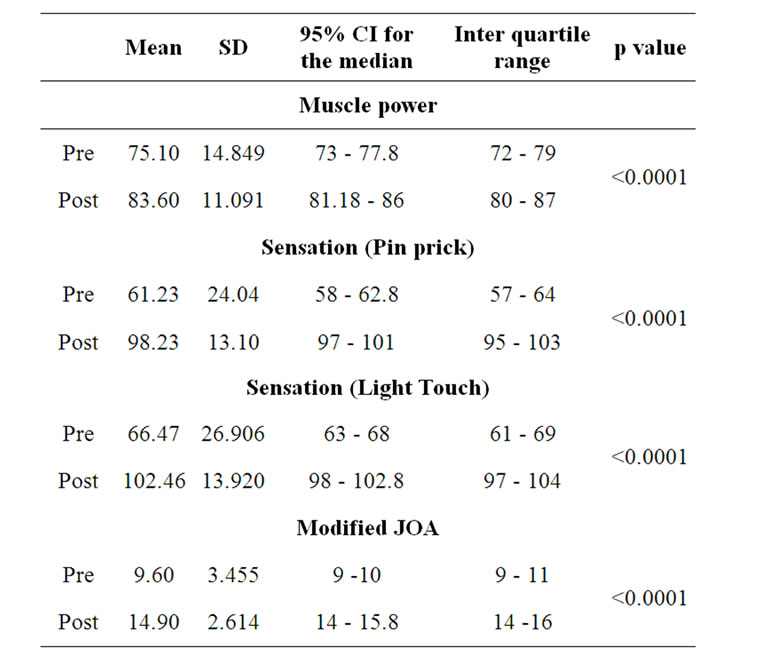
Table 3. Outcome measure at pre and post intervention levels.
stepping. Second, activity-dependent plasticity occurs in the neural circuitry responsible for locomotion at both spinal and supraspinal levels [33-42]. Using these princeples as the foundation for locomotor rehabilitation hypothesizes that the nervous system adapts to specific activity and that recovery requires relearning the task of walking by providing the spinal cord with the appropriate sensory information.
Thirdly, perhaps the most dramatic demonstration of reversing “learned non-use” has come from the results of training people with SCI. In 1992, Wernig and Muller reported that treadmill locomotion improved walking in people after severe SCI [43]. Dietz and colleagues suggest that two forms of adaptations occur after injury that may contribute to improved locomotor function: development of spastic muscle tone and activation of spinal locomotor centers induced by treadmill training [44]. Building on this work, additional groups have shown that a distal disconnected region of the spinal cord is capable of “learning” and that early gait training might promote improved gait, endurance, and energy consumption, enhanced ground speed walking, and, in a minority of individuals with incomplete SCI, improved over ground walking [45,46]. Thus, a growing body of work supports the activity-based recovery hypothesis.
Study findings have divided the possible benefits of patterned neural activity into two categories: physical and regeneration/recovery of function. This study suggested that the physical benefits provide sufficient reason for participating in an activity-based recovery program. The subjects experienced a number of physical benefits, including increased motor and sensory recovery, improvements in hand functions and gait and an improved overall quality of life. The benefits associated with these changes are overwhelming based on reduction of sick days, improved gainful employment days, reduction of prolonged hospitalisation, reduced medication and medical costs; however, the psychological benefits of feeling predictably well are perhaps even more meaningful. The limitations of our study were the lack of a control group. Future randomized control studies would provide further insight into the effectiveness and clinical relevance of exercise program on CSM.
4.2. Conclusions
CSM can be treated effectively with physiotherapy intervention. Nevertheless, careful patient selection remains one of the most critical factors to physiotherapy management as older individuals with prohibitive co morbidities or fixed long-term neurological deficits may not be benefited by the same.
Our directed exercise program had a positive impact in most of the measures of physical function in this study. Therefore, we recommend that this type of activity be included in conventional rehabilitation programs in CSM. Future long term research is needed to determine whether exercise indeed is the treatment of choice for early CSM, and if so under which circumstances.
5. Acknowledgements
The authors would like to thank Dr. Ari Chacko and Dr. Mathew Alexander, Christian Medical College and Hospital for referring patients for this study. In addition, we would like to graciously thank Masterskill University College of Health sciences for its continued support in research endeavours.
REFERENCES
- C. B. Adams and V. Logue, “The Movement and Contour of the Spine in Relation to the Neural Complications of Cervical Spondylosis,” Brain, Vol. 94, No. 3, 1971, pp. 569-586. doi:10.1093/brain/94.3.569
- J. G. Arnold Jr., “The Clinical Manifestations of Spondylochondrosis (Spondylosis) of the Cervical Spine,” Annuals of Surgery, Vol. 141, No. 6, 1955, pp. 872-889.
- D. M. Basso, “Neuroanatomical Substrates of Functional Recovery after Experimental Spinal Cord Injury: Implications of Basic Science Research for Human Spinal Cord Injury,” Physical Therapy, Vol. 80, No. 8, 2000, pp. 808- 817.
- A. Breig, I. Turnbull and O. Hassler, “Effects of Mechanical Stresses on the Spinal Cord in Cervical Spondylosis. A Study on Fresh Cadaver Material,” Journal of Neurosurgery, Vol. 25, No. 1, 1966, pp. 45-56. doi:10.3171/jns.1966.25.1.0045
- J. F. Cusick, “Monitoring of Cervical Spondylotic Myclopathy,” Spine, Vol. 13, No. 7, 1988, pp. 877-880. doi:10.1097/00007632-198807000-00033
- W. F. Young, “Cervical Spondylotic Myelopathy: A Common Cause of Spinal Cord Dysfunction in Older Persons,” American Family Physician, Vol. 62, No. 5, 2000, pp. 1064-1070, 1073.
- A. A. White and M. M. Panjabi, “Biomechanical Considerations in the Surgical Management of Cervical Spondylotic Myelopathy,” Spine, Vol. 13, No. 7, 1988, pp. 856- 860. doi:10.1097/00007632-198807000-00029
- F. Lees and J. W. Turner, “Natural History and Prognosis of Cervical Spondylosis,” British Medical Journal, Vol. 2, No. 5373, 1963, pp. 1607-1610.
- M. Panjabi and A. White 3rd, “Biomechanics of Nonacute Cervical Spinal Cord Trauma,” Spine, Vol. 13, No. 7, 1988, pp. 838-842. doi:10.1097/00007632-198807000-00024
- W. W. Parke, “Correlative Anatomy of Cervical Spondylotic Myelopathy,” Spine, Vol. 13, No. 7, 1988, pp. 831- 837. doi:10.1097/00007632-198807000-00023
- K. Fujiwara, K. Yonenobu, S. Ebara, K. Yamashita and K. Ono, “The Prognosis of Surgery for Cervical Compression Myelopathy. An Analysis of the Factors Involved,” The Journal of Bone and Joint Surgery, Vol. 71, No. 3, 1989, pp. 393-398.
- F. K. Gregonus, T. Estrin and P. H. Crandall, “Cervical Spondylotic Radiculopathy and Myelopathy. A LongTerm Follow-Up Study,” Archives of Neurology, Vol. 33 No. 9, 1976, pp. 618-625. doi:10.1001/archneur.1976.00500090024005
- D. Montgomery and R. S. Brower, “Cervical Spondylotic Myelopathy: Clinical Syndromes and Natural History,” The Orthopedic Clinics of North America, Vol. 23, No. 3, 1992, pp. 487-493.
- K. Yonenobu, K. Okada, T. Fuji, K. Fujiwara, K. Yamashita and K. Ono, “Causes of Neurologic Deterioration Following Surgical Treatment of Cervical Myelopathy,” Spine, Vol. 11, No. 8, 1986, pp. 818-823. doi:10.1097/00007632-198610000-00016
- M. E. Schwab and D. Bartholdi, “Degeneration and Regeneration of Axons in the Lesioned Spinal Cord,” Physiological Reviews, Vol. 76, No. 2, 1996, pp. 319-370.
- K. E. Williams, R. Paul and Y. Dewan, “Functional Outcome of Corpectomy in Cervical Spondylotic Myelopathy,” Indian Journal of Orthropaedics, Vol. 43, No. 2, 2009, pp. 205-209. doi:10.4103/0019-5413.50855
- S. M. Petersen, “Articular and Muscular Impairments in Cervicogenic Headache: A Case Report,” The Journal of Orthopedic and Sports Physical Therapy, Vol. 33, No. 1, 2003, pp. 21-30.
- T. W. Flynn, J. M. Whitman and J. Magel, “Orthopaedic Manual Physical Therapy Management of the CervicalThoracic Spine and Ribcage,” Manipulations, Inc., Fort Collins, 2000.
- K. Hirabayashi, K. Watanabe, K. Wakano, N. Suzuki, K. Satomi and Y. Ishii, “Expansive Open-Door Laminoplasty for Cervical Spinal Stenotic Myelopathy,” Spine, Vol. 8, No. 7, 1983, pp. 693-699. doi:10.1097/00007632-198310000-00003
- F. Gomez-Pinilla, Z. Ying, R. R. Roy, R. Molteni and V. R. Edgerton, “Voluntary Exercise Induces a BDNF-Mediated Mechanism That Promotes Neuroplasticity,” Journal of Neurophysiology, Vol. 88, No. 5, 2002, pp. 2187- 2195. doi:10.1152/jn.00152.2002
- F. Gómez-Pinilla, Z. Ying, P. Opazo, R. R. Roy and V. R. Edgerton, “Differential Regulation by Exercise of BDNF and NT-3 in Rat Spinal Cord and Skeletal Muscle,” The European Journal of Neurosciences, Vol. 13, No. 6, 2001, pp. 1078-1084. doi:10.1046/j.0953-816x.2001.01484.x
- V. R. Edgerton, J. K. Niranjala, Tillakaratne, A. J. Bigbee, R. D. de Leon and R. R. Roy, “Plasticity of the Spinal Neural Circuitry after Injury,” The Annual Review of Neuroscience, Vol. 27, 2004, pp. 145-167.
- S. A. Neeper, F. Gomez-Pinilla, J. Choi and C. W. Cotman, “Physical Activity Increases mRNA for Brain-Derived Neurotrophic Factor and Nerve Growth Factor in Rat Brain,” Brain Research, Vol. 726, No. 1-2, 1996, pp. 49-56. doi:10.1016/0006-8993(96)00273-9
- Z. Ying, R. R. Roy, V. R. Edgerton and F. Gómez-Pinilla, “Voluntary Exercise Increases Neurotrophin-3 and Its Receptor TrkC in the Spinal Cord,” Brain Research, Vol. 987, No. 1, 2003, pp. 93-99. doi:10.1016/S0006-8993(03)03258-X
- P. Carroll, G. R. Lewin, M. Koltzenburg, K. V. Toyka and H. Thoenen, “A Role for BDNF in Mechanosensation,” Nature Neuroscience, Vol. 1, No. 1, 1998, pp. 42- 46. doi:10.1038/242
- V. R. Edgerton, R. D. de Leon, S. J. Harkema, J. A. Hodgson, N. London, D. J. Reinkensmeyer, R. N. Roy, R. J. Talmadge, N. J. Tillakaratne, W. Timoszyk and A. Tobin, “Retraining the Injured Spinal Cord,” Journal of Physiology, Vol. 533, No. 1, 2001, pp 15-22.
- J. Widenfalk, K. Lundstromer, M. Jubran, S. Brene and L. Olson, “Neurotrophic Factors and Receptors in the Immature and Adult Spinal Cord after Mechanical Injury or Kainic Acid,” The Journal of Neuroscience, Vol. 21, No. 10, 2001, pp. 3457-3475.
- V. R. Edgerton, R. D. de Leon, N. Tillakaratne, M. R. Recktenwald, J. A. Hodgson and R. R. Roy, “Use-Dependent Plasticity in Spinal Stepping and Standing,” Advances in Neurology, Vol. 72, 1997, pp. 233-247.
- B. A. Conway, H. Hultborn and O. Kiehn, “Proprioceptive Input Resets Central Locomotor Rhythm in the Spinal Cat,” Experimental Brain Research, Vol. 68, No. 3, 1987, pp. 643-656. doi:10.1007/BF00249807
- R. D. De Leon, J. A. Hodgson, R. R. Roy and V. R. Edgerton, “Locomotor Capacity Attributable to Step Training versus Spontaneous Recovery after Spinalization in Adult Cats,” Journal of Neurophysiology, Vol. 79, No. 3, 1998, pp. 1329-1340.
- K. J. Hutchinson, J. K. Linderman and D. M. Basso, “Skeletal Muscle Adaptations Following Spinal Cord Contusion Injury in Rat and the Relationship to Locomotor Function: A Time Course Study,” Journal of Neurotrauma, Vol. 18, No. 10, 2001, pp. 1075-1089. doi:10.1089/08977150152693764
- S. J. Harkema, S. L. Hurley, U. K. Patel, P. S. Requejo, B. H. Dobkin and V. R. Edgerton, “Human Lumbosacral Spinal Cord Interprets Loading during Stepping,” Journal of Neurophysiology, Vol. 77, No. 2, 1997, pp. 797-811.
- O. Andersson, S. Grillner, M. Lindquist and M. Zomlefer, “Peripheral Control of the Spinal Pattern Generators for Locomotion in Cat,” Brain Research, Vol. 150, No. 3, 1978, pp. 625-630. doi:10.1016/0006-8993(78)90827-2
- O. Andersson and S. Grillner, “Peripheral Control of the Cat’s Step Cycle, II: Entrainment of the Central Pattern Generators for Locomotion by Sinusoidal Hip Movements during ‘Fictive Locomotion’,” Acta Physiologica Scandinavica, Vol. 118, No. 3, 1983, pp. 229-239. doi:10.1111/j.1748-1716.1983.tb07267.x
- H. Barbeau, D. A. McCrea, M. J. O’Donovan, S. Rossignol, W. M. Grill and M. A. Lemay, “Tapping into Spinal Circuits to Restore Motor Function,” Brain Research, Brain Research Reviews, Vol. 30, No. 1, 1999, pp. 27-51. doi:10.1016/S0165-0173(99)00008-9
- H. Barbeau and S. Rossignol, “Recovery of Locomotion after Chronic Spinalization in the Adult Cat,” Brain Research, Vol. 412, No. 1, 1987, pp. 84-95. doi:10.1016/0006-8993(87)91442-9
- B. H, Dobkin, S. J. Harkema, P. S. Requejo and V. R. Edgerton, “Modulation of Locomotor-Like EMG Activity in Subjects with Complete and Incomplete Spinal Cord Injury,” Journal of Neurologic Rehabilitation, Vol. 9, No. 4, 1995, pp. 183-190.
- H. Forssberg, “Stumbling Corrective Reaction: A PhaseDependent Compensatory Reaction during Locomotion,” Journal of Neurophysiology, Vol. 42, No. 4, 1979, pp. 936-953.
- S. Grillner, “Interaction between Central and Peripheral Mechanisms in the Control of Locomotion,” Progress in Brain Research, Vol. 50, 1979, pp. 227-235. doi:10.1016/S0079-6123(08)60823-7
- S. Grillner, “Neurobiological Bases of Rhythmic Motor Acts in Vertebrates,” Science, Vol. 228, No. 4696, 1985, pp. 143-149. doi:10.1126/science.3975635
- S. Grillner and S. Rossignol, “On the Initiation of the Swing Phase of Locomotion in Chronic Spinal Cats,” Brain Research, Vol. 146, No. 2, 1978, pp. 269-277. doi:10.1016/0006-8993(78)90973-3
- K. G. Pearson and S. Rossignol, “Fictive Motor Patterns in Chronic Spinal Cats,” Journal of neurophysiology, Vol. 66, No. 6, 1991, pp. 1874-1887.
- A. Wernig and S. Muller, “Laufband Locomotion with Body Weight Support Improved Walking in Persons with Severe Spinal Cord Injuries,” Paraplegia, Vol. 30, No. 4, 1992, pp. 229-238. doi:10.1038/sc.1992.61
- V. Dietz, “Spinal Cord Lesion: Effects of and Perspectives for Treatment,” Neural Plasticity, Vol. 8, No. 1-2, 2001, pp. 83-90. doi:10.1155/NP.2001.83
- A. L. Behrman and S. J. Harkema, “Locomotor Training after Human Spinal Cord Injury: A Series of Case Studies,” Physical Therapy, Vol. 80, No. 7, 2000, pp. 688-700.
- R. G. Lovely, R. J. Gregor, R. R. Roy and V. R. Edgerton, “Effects of Training on the Recovery of Full-WeightBearing Stepping in the Adult Spinal Cat,” Experimental Neurology, Vol. 92, No. 2, 1986, pp. 421-435. doi:10.1016/0014-4886(86)90094-4
NOTES
*Corresponding author.

