Open Journal of Applied Sciences
Vol.05 No.07(2015), Article ID:58509,9 pages
10.4236/ojapps.2015.57039
Evaluation of Nutritional Value for Four Kinds of Wild Plants in Northern Sector of Nile Delta, Egypt
Yasser Ahmed El-Amier, Thaer Jameel Abdullah
Faculty of Science, Department of Botany, Mansoura University, Mansoura, Egypt
Email: yasran@mans.edu.eg
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 July 2015; accepted 28 July 2015; published 31 July 2015
ABSTRACT
Wild plants play an essential role in non-traditional fodder producing plants. The aim of the study is to evaluate nutritional composition of wild plants. These plants are Calligonum polygonoides, Cakile maritima, Senecio glaucus (Psammophytes) and Zygophullum album (halophyte) naturally growing in Deltaic Mediterranean coastal desert of Egypt. These plants are rich with their nutritive values and their water requirements are low. The protein content of the investigated wild species ranges from 5.90% to 19.63% and the fat content ranges from 0.75% to 1.93%. The amount of nitrogen and phosphorus are higher in all plants followed by potassium, calcium, magnesium and sodium. On the other hand, the microelements levels in the studied wild plants exist as Fe > Mn > Zn > Cu > Ni ˃ Pb > Co ˃ Cd. Finally, the phytochemical analysis of the studied wild plants indicates that the four wild plants are important and there is a need to develop new techniques for cultivation as well as processing of such plants for future use.
Keywords:
Proximate Composition, Minerals, Antioxidant, Wild Species, Costal Desert

1. Introduction
The population density of the Egyptian desert is generally, low, and hundreds of square kilometers may be devoid of human habitation. The reverse is true in the River Nile region which covers only less than 4% of Egypt’s lands. In the same time it has very dense population that is expected to be hundred millions by 2020. Therefore, areas of desert vacant to-day should be habituated. Halophytic plants differ in their nutritive values from one species to another [1] .
The mineral nutrition is an important aspect and it plays an essential role in organisms’ life for healthy growth. Such type of mineral is easily available in wild edible plants [2] . The World Health Organization (1982) had reported that chronic under-nutrition affects some 200 million people, or 42% of the population, in sub-Saharan Africa.
The vegetation of the Mediterranean coastal region of Egypt is considered to be one of its major natural resources. Its proper utilization plays a key role in the sound of this region which is known to have enjoyed prosperity during the Graeco-Romoan times [3] [4] .
The narrow areas of the fertile lands of the River Nile Region (Nile Valley and Nile Delta), though occupy less than 5% of Egypt’s area they represent the backbone of the cultivation of the traditional crops that are fully utilizing the freshwater of the River Nile. Thus, there is no surplus of Nile water that can be used for any agro-industrial and social activities in Egypt’s deserts which should depend mainly upon their own natural resources of water to grow and establish non-conventional crops having low water requirements to be selected from the desert plants.
Therefore, the communities in Egypt have a long history in directed towards the utilization of the renewable resources of the non-cultivated areas to produce more food for people, forage for animals and industrialization our local raw materials [5] .
In addition to some earlier works on this topic [6] -[11] , recent publications [1] [12] -[15] have investigated the nutritional value of non-traditional wild plants and utilization of these plants as renewable resources for raw materials.
The plants under investigation are found in Egypt in abundance and there for I think to throw the light on the importance of these plants. We are in Egypt caring about finding a source of plants as fodder because we have an abroad plan for animal breeding. The purpose of this study is to conduct a preliminary assessment of the nutritive value of some common wild plants as non-traditional fodder producing plants. These plants are Calligonum polygonoides, Cakile maritima, Senecio glaucus and Zygophullum album.
2. Materials and Methods
2.1. Study Area
The middle sections of Mediterranean coastal desert of Egypt (the Deltaic Coast) runs from Abu Qir to Port Said for about 180 km, has a width from sea landward (southwards) for about 15 km (Figure 1). It is characterized by 3 shallow natural lakes: Manzala (in the east), Burullus (in the middle) and Idku (in the west). These lakes receive the main bulks of the drainage waters from the Nile Delta lands [16] .
Figure 1. Map of the Nile Delta region showing different localities (*) of the studied area.
The climate of the whole stretch of the Mediterranean coastal desert is generally, less arid than the remaining southern parts of Egypt. According to the Bioclimatic Map of (UNESCO/FAO, 1963), it belongs to the subdesertic warm climate. In the deltaic Mediterranean section annual rainfall ranges between 160 mm in Rosetta and 102.3 mm in Damietta.
Maximum air temperature in both coastal cities ranges between 18.1˚C to 30.2˚C and 18.4˚C to 31.3˚C whereas minimum temperatures range between 11.3˚C to 23˚C and 8.2˚C to 21.5˚C in January and July respectively. Relative humidity does not vary widely between the two cities being 76% and 74% and 83% and 75% in January and July respectively [16] .
2.2. Samples
Nutritional analyses (moisture, protein, fat, fibre, ash and minerals) were conducted using shoot system of fourwild plants (Calligonum polygonoides, Cakile maritima, Senecio glaucus and Zygophullum album) growing naturally in Deltaic Mediterranean coast, Egypt.
Approximately, 2 kg of shoot material were selected from several plants of each variety. Local floristic keys were used for determining the species. Data on different species and vernacular name have been sourced from publications on the flora of Egypt [17] see (Table 1).
The plants were processed on the same day of harvest. Died, yellow leaves were removed from the stems, shoot system washed thoroughly with distilled water until no foreign material remained, and air dried at room temperature for 2 h. Finally, the dried samples were stored in a dark cupboard in capped bottles in desiccators and used within 1 month after harvesting.
2.3. Chemical Analysis
Moisture content, total ash content, fat and fiber were analyzed by the methods described in AOAC [18] . The total nitrogen was determined by the Kjeldahl method [19] . Total protein was calculated by multiplying the total nitrogen by the factor 6.25 [18] . Glucose was determined based on the method of Feteris [20] . Sucrose was determined according to [21] . Na+ and K+ were determined photometrically whereas Ca2+, Mg2+, Fe2+, Mn2+, Zn2+, Cu2+, Cd2+, Co2+, Ni2+ and Pb2+ were estimated by using Atomic Absorption Spectrometer (A Perkin-Elemer, Model 2380, U.S.A.). The elements were expressed as mg/g according to Allen et al. [22] . The calculation of energy (kcal/100 g fresh weight) was carried out using the Atwater system as described by the World Health Organization (1985) by multiplying the values obtained for protein, carbohydrates and fat by 4.00, 3.75 and 9.00, respectively; the results are expressed in kcal. The total digestible nutrient was estimated according to the equation described by Abu El-Naga and EL-Shazly [23] .
2.4. Determination of Antioxidant Activity Scavenging Activity of DPPH Radical
In order to measure antioxidant activity, DPPH free radical scavenging assay was used according to a procedure
Table 1. Species, habitat, duration, life form, chorotype and occurrence of studied wild plants naturally growing in Deltaic Mediterranean coast, Egypt. Th, Therophytes; Nph, Nanophanerophytes; Ch, Chamaephytes; ME, Mediterranean; IR-TR, Irano-Turanian; ER-SR, Euro-Siberian; SA-SI, Saharo-Sindian.
based on Lim and Quah [24] as follows: two milliliters of 0.15 mM DPPH was added to 1 ml of various plant extracts in different concentrations. A control was prepared by adding 2 ml of DPPH to 1 ml solvent (methanol 50 %). The contents of the tubes were mixed and allowed to stand for 30 min., the absorbance was measured at 517 nm. The antioxidant activity was expressed as:

2.5. Statistical Analyses
One-way analysis of variance (ANOVA) was applied to assess the significance of variation in the nutritive values with equal replication using the COSTAT program.
3. Results
3.1. Proximate Composition
The composition of the four wild studied species is presented (Table 2). Shoot of Cakile maritima and Calligonum polygonoides yielded the highest energy levels of 353.74 kcal/100 g and 343.71 kcal/100 g, respectively.
Other parts of wild species have significant energy values ranging from 322.49 to 333.60 kcal/100 g. The total digestible nutrient ringing from 46.66 (shoot of Cakile maritima) to 57.13 (root of Senecio glaucus). All samples contained between 5.21% and 11.63% moisture. There was a slight variation in the fat content of the investigated wild species, ranging from 0.75% (root of Zygophyllum album) to 1.93% (shoot of Cakile maritima).
Shoot of Cakile maritima (19.63%), Calligonum polygonoides (18.94%) and Zygophyllum album (17.96%) stand out as being good sources of protein. Ash content ranged from 5.99% in shoot of Zygophyllum album to 9.27% in shoot of Senecio glaucus .The root of Cakile maritima and Zygophyllum album are good source of fibre (9.26% and 10.11%, respectively), while the lowest quantity of fibre was found in the shoot of Cakile maritima (5.52%).
Glucose and sucrose contentsattained the lowest value (1.56 and 2.31 mg/g, respectively) in root of Zygophyllum album, while the highest value (5.01 and 7.40 mg/g, respectively) in shoot of Cakile maritima. There was a slightly variation in carbohydrate of the studied species, ranging from 66.94 mg/g for Calligonum polygonoides to 78.77 for Zygophyllum album.
3.2. Mineral Levels
The concentrations of the macrominerals (N, P, K⁺, Ca⁺⁺, Mg⁺⁺ and Na⁺) estimated in the four studied species
Table 2. Moisture and ash contents as well as the constituents of studied plants. Car, carbohydrates; TDN, Total digestable nutrients.
are presented in Table 3. The nitrogen and potassium content of the species analyzed in this study showed remarkably high amounts in shoot than root. The highest value (31.40 and 28.10 mg/g dry weight, respectively) was observed in shoot of Cakile maritima, while the lowest value (8.95and 8.21mg/g dry weight, respectively) was recorded for Senecio glaucus roots. The phosphorus content of the wild species varied greatly and rangedfrom 1.67 mg/g (root of Zygophyllum album) to 4.02 mg/ g (shoot of Cakile maritima).
Sodium concentration showed little variation and ranged from 2.20 mg/g (shoot of Calligonum polygonoides) to 5.33 mg/g (root of Senecio glaucus), while calcium and magnesium concentration showed remarkably high amounts in root than shoot. The highest value (28.60 and 11.17 mg/g dry weight, respectively) was estimated in root of Zygophyllum album, while the lowest value (10.91 and 5.89 mg/g dry weight, respectively) was attained in shoot of Senecio glaucus.
On the other hand, the highest concentrations of the microminerals were recorded for F2+, Mn2+ and Zn2+ (4.521, 2.753 and 1.827 mg/100 g dry weight) in shoot of Senecio glaucus and Cu2+ (3.377 mg/100g dry weight) in shoot of Calligonum polygonoides. While, the lowest value (0.990, 0.510, 0.424 and 0.166 mg/100 g dry weight, respectively) were attained in root of Zygophyllum album. The highest concentrations of Co2+ and Ni2+ (0.148 and 0.291 mg/g dry weight, respectively) were recorded in root of Senecio glaucus, while Pb2+ and Cd2+ (0.866 and 0.018 mg/100 g dry weight, respectively) were observed in Senecio glaucus shoots. The lowest concen trations (0.062, 0.128, 0.349 and 0.007 mg/100g dry weight) were recorded in Cakile maritima shoots (Table 4).
3.3. Antioxidant
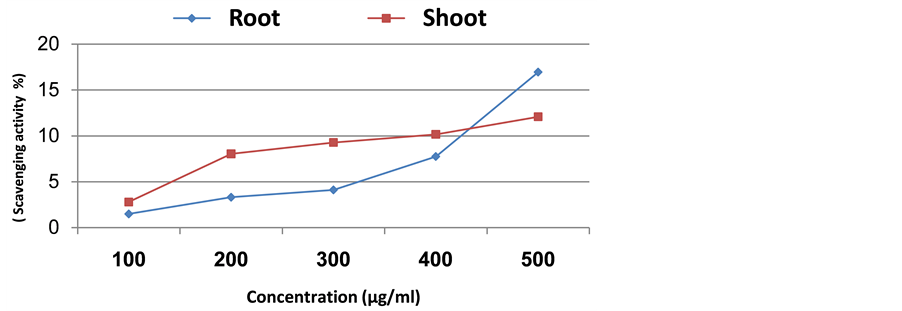
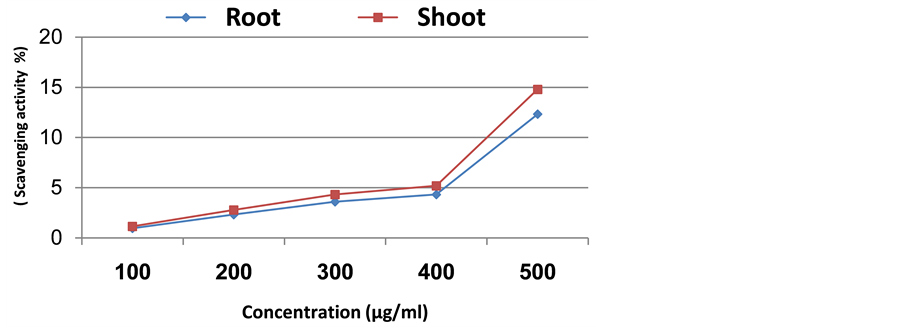
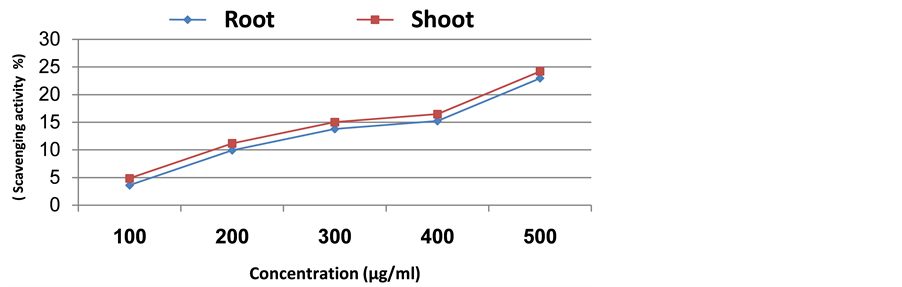
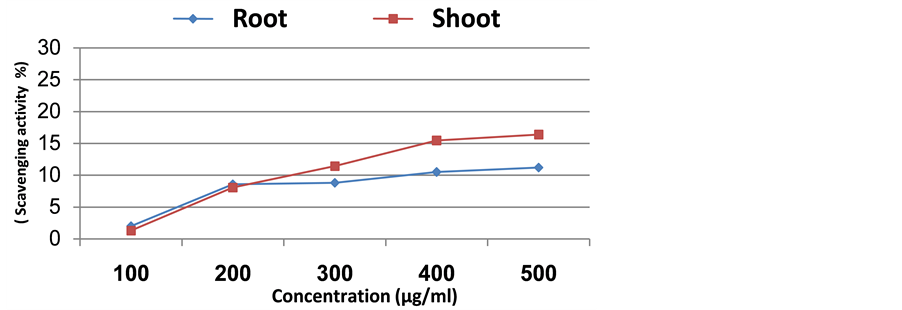
By increasing the plant extract concentration there was a corresponding continuous increase in scavenging activity (Figures 2-5). In case of Senecio glaucus shoot and root extract the increase was up to 500 µg/ml where the scavenging activity was 24.22% and 22.97%, respectively. For Calligonum polygonoides the scavenging activity was 16.96% for root and 12.08% for shoot. However, in case of Zygophyllum album the scavenging activity was 16.36% for shoot and 11.20% for root. Finally, in case of Cakile maritima attained high scavenging activity in shoot extract (14.78%), while in root extract attained 12.32%.
4. Discussion
The natural vegetation of Egypt’s deserts comprises considerable number of palatable species and/or species rich with their nutritive values that may be considered reliable natural resources for fodder production [25] .
Phytochemical analysis of the wild plants indicated that almost all plants are important and there is a need to develop techniques for cultivation and processing of such plants for future use. In our present study, most com-
Table 3. Analysis of macrominerals in plants under study.
Table 4. Analysis of microminerals in plants under study.
Figure 2. Scavenging activity of methanolic extract of Calligonum polygonoides.
Figure 3. Scavenging activity of methanolic extract of Cakile maritima.
monly used plants were evaluated for the nutritional values with special references to fiber, protein and fat content, reducing sugars, carbohydrates and energy.
Figure 4. Scavenging activity of methanolic extract of Senecio glaucus.
Figure 5. Scavenging activity of methanolic extract of Zygophyllum album.
Proteins and carbohydrates are the building blocks of the body and so are required in high amounts in regular diet. Similarly, lipids are the integral part in the cellular membranes and are required for various body functions [26] .
Presence of high crude fiber in food material is reported to decrease dry matter digestibility in animals. The high crude fiber content therefore provides a good indication of nutritive value of the feed material [27] .
The protein content of the investigated wild species ranged from 5.90% to 19.63% and fat in between 0.75% to 1.93%. Van Wyk [28] and Odhav et al. [29] reported the protein and fat values for Chenopodium album (4.4% and 0.3%), Amaranthus hybridus (3.5% and 0.3%) and Galinsoga parviflora (3.29% and 2.06%). The protein and fat contents of the wild plants in this study are relatively comparable to that obtained by El-Amier and Ejgholi [15] on Atriplex halimus (14.79 and 1.05%), Limonium pruinosum (12.38% and 0.92%) and Limoniastrum monopetalum (14.15% and 1.16%), but lower than those reported by Zahran and El-Amier [1] on Bassia indica (9.25 and 2.9%), Arthrocnemum macrostachyum (5.34% and 1.2%) and Halocnemum strobilaceum (9.44% and 2.13%) and Stanaćev et al. [30] on Medicago sativa and Trifolium alexandrinum.
The total digestible nutrient (TDN) is only an approximate measure of the food energy available to animals after digestion losses have been deduced [31] . Abd-El-Razik et al. [32] reported that the annual average of TDN value was 75% DM. In the studied species, the annual average of TDN is about 52.45% these results are comparable to those of clover (56%), barley (64%) and corn (68%) as reported by Soliman and EL-Shazly [33] .
The findings of the moisture, ash and fiber content of most of the wild plants analysed in this study conform the previous published literature for wild species of Atriplex halimus, Limonium pruinosum and Limoniastrum monopetalum [15] , on Bassia indica, Arthrocnemum macrostachyum and Halocnemum strobilaceum [1] , Atriplex halimus [34] , Medicago sativa [30] and Trifolium alexandrinum [35] .
Macro- and micro-minerals are very important in a required amount to achieve maximum health and production, as excess or lower amount is harmful in plants and animals [36] . Also, the results of the present study show that the concentrations of macro-elements (sodium, potassium and calcium) in the selected species are enough for the requirements of animals which reflects the findings of Mayland and Hankins [37] .
Generally, the four studied species contained concentrations of Na⁺, K⁺, Ca2⁺ and Mg2⁺, are in harmony with that those reported in Medicago sativa [38] and Trifolium alexandrinum [39] , but lower than those reported by Otal et al. [40] , Zahran and El Amier [1] on halophytic vegetation as well as Okon and James [41] on woody leafy vegetables (Heinsia crinata, Lasienthera africana and Pterocarpus mildbraedii). Nitrogen and phosphorus contents of the investigated wild species ranging from 9.43 - 31.40 mg/g dry wt. and 1.67 - 4.02 mg/g dry wt. in this study higher than those reported by Rathod et al. [2] on some selected wild edible plants (Ensete superbum, Oroxylum indicum, Smilax zeylanica, etc.) and Kalita et al. [42] on edible food plants (Solanum spirale and Cyathea spinulosa) as good source of mineral. Variations in the chemical composition of wild species, including the quantities of compounds are influenced by prevailing environmental conditions, the age of plants at harvest, which affects their genetic composition [29] [43] .
The results of the present work seem to be promise compared with the results reported previously by other investigates in the field of fodder and food plants. For example, the contents of protein and fat were19.63% and 1.93% which are higher than those reported by Zahran and El-Amier [1] on halophytic plants as fodder. Van Wyk [28] has reported the availability of some wild plants as food; however the reported protein and fat contents were lower than that in the present investigation.
5. Conclusions
Land use with shortage of fresh water in the deserts may limit agriculture and farming. Conventional crops need lots of fresh water and their cultivation in the deserts will, certainly, cause quick depletion of the ground water [1] .
It is worth to state that, according to the obtained results, the four species, namely: Calligonum polygonoides, Cakile maritima, Senecio glaucus (Psammophytes) and Zygophullum album (halophyte) naturally growing in the Deltaic Mediterranean coastal desert of Egypt are of high potentialities as fodder producing plants. They are rich with their nutritive values and their water requirements are low. Thus, mass production of their vegetative yields will certainly help to maintain reasonable quantities of raw materials for fodder industries.
Cite this paper
Yasser AhmedEl-Amier,Thaer JameelAbdullah, (2015) Evaluation of Nutritional Value for Four Kinds of Wild Plants in Northern Sector of Nile Delta, Egypt. Open Journal of Applied Sciences,05,393-402. doi: 10.4236/ojapps.2015.57039
References
- 1. Zahran, M.A. and El-Amier, Y.A. (2013) Non-Traditional Fodders from the Halophytic Vegetation of the deltaic Mediterranean Coastal Desert, Egypt. Journal of Biological Sciences, 13, 226-233. http://dx.doi.org/10.3923/jbs.2013.226.233
- 2. Rathod, V., Mahadkar, S. and Valvi, S. (2012) Nutritional Assessment of Some Selected Wild Edible Plants as a Good Source of Mineral. Asian Journal of Plant Science & Research, 2, 468-472.
- 3. Kassas, M. (1972) A Brief History of Land-Use in Meareotis Region, Egypt. Minerva Biotecnologica, 1, 167-174.
- 4. Shaltout, K.H. (1983) An Ecological Study of Thymelaea hirsuta (L.) Endl. PhD Thesis, Faculty of Science, Tanta University, Tanta.
- 5. El-Shami, M.M. (1989) Autecological and Phytochemical Studies on Lygo sraetam (Forssk.) Haywood. M.Sc. Thesis, Faculty of Science, Mansoura University, Mansoura.
- 6. Hassan, F. (1980) Prehistoric Settlements along the Main Nile. In: Williams, M.A.J. and Faure, H., Eds., The Sahara and the Nile, Quarternary Environments and Prehistoric Occupation in Northern Africa, Balkema, Rotterdam, 421- 450.
- 7. El-Dingawy, A.A.I. (1990) Contribution to the Studies on Kochia Plants and Their Potentialities in Fodder Production in Egypt. Ph.D. Thesis, Faculty of Science, Mansoura University, Mansoura.
- 8. Abd-El-Hamid, A.M. and Gabr, A.A. (1991) Utilization of Water-Hyacinth in Comparison with Berseem Hay as Sole Feeds by Sheep with Emphasis on Its Hazardous Effects. Journal of Agricultural Science, 16, 506-517.
- 9. El-Shamy, M.M. (1995) Studies on Some Taxa of the Genus Tanta in Egypt. Ph.D. Thesis, Faculty of Science, Mansoura University, Mansoura.
- 10. Ashour, N.I., Serag, M.S., Abd-El-Halemm, A.K. and Mekki, B.B. (1997) Forage Production from Three Grass Species under Saline Irrigation in Egypt. Journal of Arid Environments, 37, 299-307. http://dx.doi.org/10.1006/jare.1997.0284
- 11. El-Din, A.S. (1998) Nutrient Status of Vegetation of the Elba Nature Reserve. Journal of Union of Arab Biologists Cairo, 6, 73-87.
- 12. Ramachurum, R., Dullul, Z.B., Ruggoo, A. and Ragoo, J. (2000) Effects of Feeding Star Grass (Cynodon plectostachyus) on Growth and Digestibility of Nutrients in the Domestic Rabbit. Livestock Research for Rural Development, University of Mauritius, Reduit.
- 13. El-Halawany, E.F. (2002) Characterization of the Wetlands Habitat, alongside the Fish Farms in the North Nile Delta, Egypt. Pakistan Journal of Agricultural Sciences, 5, 626-632. http://dx.doi.org/10.3923/pjbs.2002.626.632
- 14. Mashaly, I.A., El-Halawany, E.F., Abu-Ziada, M.E. and Abd-El-Aal, M. (2009) Economic Potentialities of Some Wild Flora in Dakahlyia Governorate, Egypt, Journal of Biology, 36, 1-20.
- 15. El-Amier, Y.A. and Ejgholi, A.A. (2014) Fodder Potentialities of Three Halophytes Naturally Growing in Egypt. Journal of Environmental Sciences (Mansoura University), 43, 647-662.
- 16. Zahran, M.A. and Willis, A.J. (2009) The Vegetation of Egypt. 2nd Edition, Springer Netherlands.
- 17. Boulos, L. (1999-2005) Flora of Egypt. Vols. 1, 2, 3, and 4, All Hadara Publishing, Cairo.
- 18. AOAC (1990) Official Methods of Analysis. 15th Edition, Association of Official Analytical Chemists, Arlington.
- 19. Pirie, N.W. (1955) Protein. In: Peack, K. and Tracey, M.V., Eds., Modern Methods of Plant Analysis IV, Vol. 23, Springer Verlage, Berlin, 23-68.
- 20. Feteris, A.W. (1965) A Serum Glucose Method without Protein Precipitation. American Journal Medical Technologists, 31, 17-21.
- 21. Handel, E.V. (1968) Direct Micro Determinations of Sucrose. Analytical Biochemistry, 22, 280-283. http://dx.doi.org/10.1016/0003-2697(68)90317-5
- 22. Allen, S.E., Grimshaw, H.M., Parkinson, J.A., Quarmby, C. and Roberts, J.D. (1974) Chemical Analysis of Ecological Materials. Blackwell Scientific Publications, Osney, Oxford and London.
- 23. Abu-El-Naga, M.A. and EL-Shazly, K. (1971) The Prediction of the Nutritive Value of Animal Feeds from Chemical Analyses. Journal of Agricultural Science, 77, 25-31.
- 24. Lim, Y.Y. and Quah, E.P.L. (2007) Antioxidant Properties of Different Cultivars of Portulaca oleracea. Food Chemistry, 103, 734-740.
http://dx.doi.org/10.1016/j.foodchem.2006.09.025 - 25. Zahran, M.A., El-Ameir, Y.A. and Hammad, M.E. (2014) Contribution to the Eco-Palynological Studies of the Mediterranean Coastal Desert of Egypt. Journal of Environmental Sciences (Mansoura University), 43, 1-22.
- 26. Norton, B.W. (2003) The Nutritive Value of Tree Legumes. In: Gutteridge, R.C. and Shelton, H.M., Eds., Forage Tree Legumes in Tropical Agriculture, CAB International, Wallingford, 1-10.
- 27. Devendera, C. (1995) Tropical Legume for Small Ruminants. In: D’Melo, J.P. and Devendera, C., Eds., Tropical Legumes in Animal Nutrition, CAB International, Wallingford, 231-246.
- 28. Van Wyk, B.E. (2005) Food Plants of the World. Identification, Culinary Uses and Nutritional Value. Briza Publications, Pretoria.
- 29. Odhav, B., Beekrum, S., Akula, U. and Baijnath, H. (2007) Preliminary Assessment of Nutritional Value of Traditional Leafy Vegetables in KwaZulu-Natal, South Africa. Journal of Food Composition and Analysis, 20, 430-435. http://dx.doi.org/10.1016/j.jfca.2006.04.015
- 30. Stana&cacuteev, V., Dukic, D., Kovcin, S., Drini&cacute, M., Puvaca, N. and Stana&cacuteev, V. (2010) Nutritive Value of the Genetically Divergent Genotypes of Lucerne (Medicago sativa L.). African Journal of Agricultural Research, 5, 1284-1287.
- 31. Lofgreen, G.P. (1951) The Uses of Digestible Energy in evaluation of Feeds. Journal of Animal Science, 10, 344-351.
- 32. Abd-El-Razik, M., Ayyad, M. and Heneidy, S. (1988) Preference of Grazing Mammals for Forage Species and Their Nutritive Value in a Mediterranean Desert Ecosystem (Egypt). Journal of Arid Environments, 15. 297-305.
- 33. Soliman, S.M. and EL-Shazly, K. (1978) Increasing the Productivity per Feddan from Total Digestible Nutrients. Alexandria Journal of Agricultural Research, 26, 551-556.
- 34. Shawket, S.M., Youssef, K.M. and Ahmed, M.H. (2010) Comparative Evaluation of Egyptian Clover and Atriplex halimus Diets for Growth and Milk Production in Camel. Animal Science Reporter, 4, 9-21.
- 35. Khan, A.D., Ejaz, N. and Gilani, A.H. (2002) The Use of Berseem Clover (Trifoliuma lexandrinum L.) Pulp Residue, after Juice Extraction, in Lamb Finishing Diets. Archivos de Zootecnia, 51, 291-301.
- 36. Alessandra, G. and Robert, H.C. (2005) The Crucial Role of Metal Ions in NEURO degeneration; the Basis for Promising Therapeutic Strategy. British Journal of Pharmacology, 146, 1041-1059.
- 37. Mayland, H.F. and Hankins, L.H. (2001) Mineral Imbalances and Animal Health: A Management Puzzle. In: Launchbaugh, K., Ed., Anti-Quality Factors in Rangeland and Pastureland Forages, Idaho Forest, Wildlife and Range Experiment Station, University of Idaho, Moscow.
- 38. Frame, J., Charlton, J.F.L. and Laidlaw, A.S. (1998) Lucerne. Temperate Forage Legumes, C.A.B International, Wallingford, 107-179.
- 39. Fernandes, A.P. and Waditake, S.K. (2006) Comparative Evaluation of Berseem (Trifoliuma lexandrinum) Varieties for Yield and Fodder Quality. Animal Nutrition and Feed Technology, 6, 301-306.
- 40. Otal, J., Orengo, J., Quiles, A., Hevia, M.L. and Fuentes, F. (2010) Characterization of Edible Biomass of Atriplex halimus L. and Its Effect on Feed and Water Intakes, and on Blood Mineral Profile in Non-Pregnant Manchega-Breed Sheep. Small Ruminant Research, 91, 208-214. http://dx.doi.org/10.1016/j.smallrumres.2010.03.016
- 41. Okon, I.E. and James, U.S. (2015) Comparative Evaluation of Nutritional Values of Some Wild Plants LEAFY vegetables in South Eastern Nigeria. International Journal of Research in Applied, Natural and Social Sciences, 3, 21-26.
- 42. Kalita, P., Tag, H., Sarma, H.N. and Das, A.K. (2014) Evaluation of Nutritional Potential of Five Unexplored Wild Edible Food Plants from Eastern Himalayan Biodiversity Hotspot Region (India). International Journal of Biological, Food, Veterinary Agricultural Engineering, 8, 207-210.
- 43. Nordeide, M.B., Hatloy, A., Folling, M., Lied, E. and Oshaug, A. (1996) Nutrient Composition and Nutritional Importance of Green Leaves and Wild Food Resources in an Agricultural District, Koutiala, in southern Mali. International Journal of Food Sciences and Nutrition, 47, 455-478. http://dx.doi.org/10.3109/09637489609031874